Bioactive Metabolites of the Stem Bark of Strychnos aff. darienensis and Evaluation of Their Antioxidant and UV Protection Activity in Human Skin Cell Cultures
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Plant Material
2.3. Isolation of Metabolites
2.4. Cells and Cell Culture Conditions
2.5. Assessment of Cytotoxicity
2.6. IntracellularReactive Oxygen Species (ROS) Assay
2.7. UV-Protection Assay
3. Results and Discussion
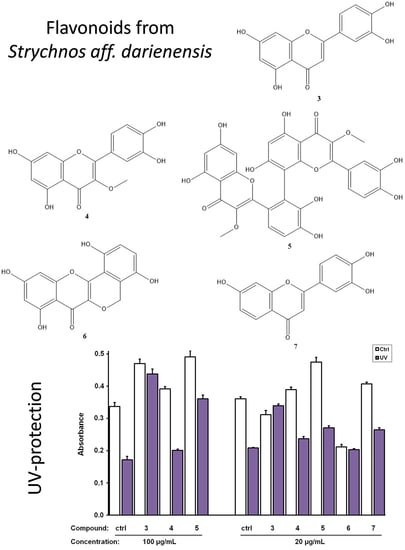
3.1. Isolation and Identification of Secondary Metabolites
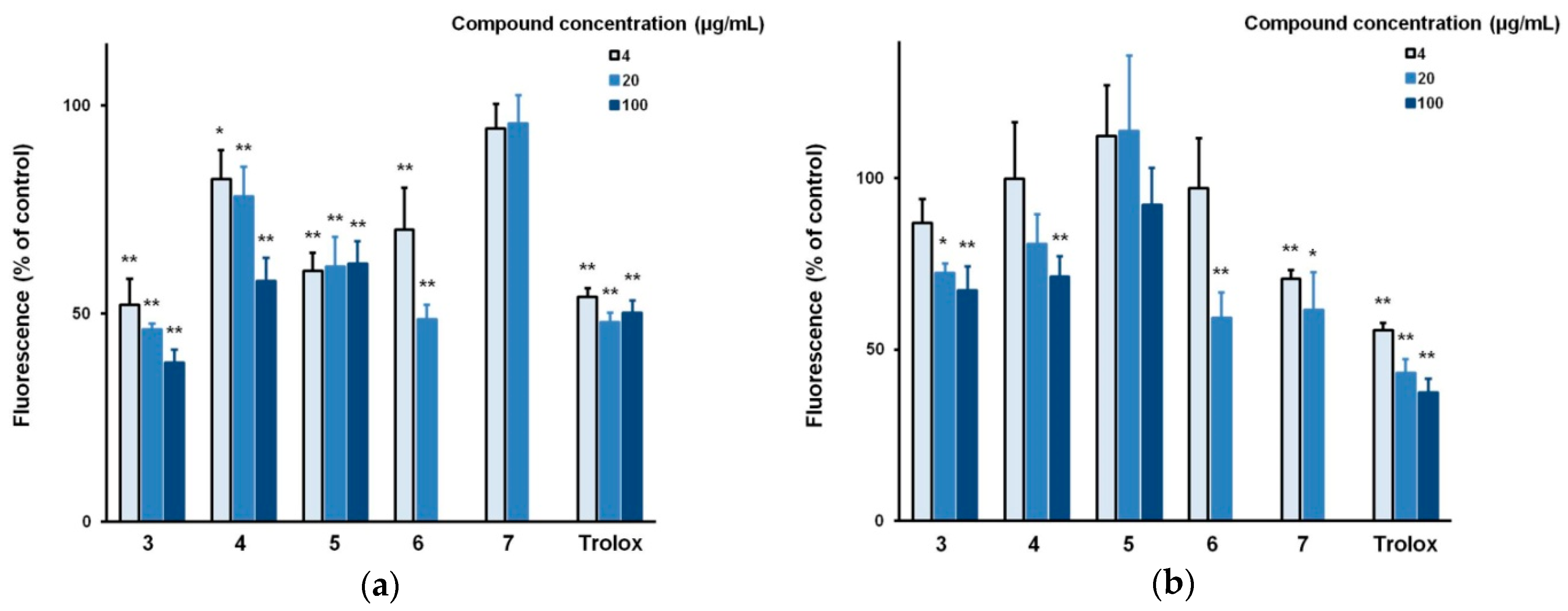
3.2. Biological Evaluation of The Isolated Flavonoids
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burlando, B.; Cornara, L. Revisiting Amazonian Plants for Skin Care and Disease. Cosmetics 2017, 4, 25. [Google Scholar] [CrossRef]
- Massiot, G.; Delaude, C. Chapter 5 African Strychnos Alkaloids. In The Alkaloids: Chemistry and Pharmacology; Brossi, A., Ed.; Academic Press: New York, NY, USA, 1989; Volume 34, pp. 211–329. [Google Scholar]
- Philippe, G.; Angenot, L.; Tits, M.; Frederich, M. About the toxicity of some Strychnos species and their alkaloids. Toxicon 2004, 44, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Valadeau, C.; Castillo, J.A.; Sauvain, M.; Lores, A.F.; Bourdy, G. The rainbow hurts my skin: Medicinal concepts and plants uses among the Yanesha (Amuesha), an Amazonian Peruvian ethnic group. J. Ethnopharmacol. 2010, 127, 175–192. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Wang, T.; Zhou, G.; Xu, M.; Yu, X.; Zhang, X.; Sui, F.; Li, C.; Tang, L.; Wang, Z. Botany, Phytochemistry, Pharmacology and Toxicity of Strychnos nux-vomica L.: A Review. Am. J. Chin. Med. 2018, 46, 1–23. [Google Scholar] [CrossRef] [PubMed]
- De Wet, H.; Nciki, S.; van Vuuren, S.F. Medicinal plants used for the treatment of various skin disorders by a rural community in northern Maputaland, South Africa. J. Ethnobiol. Ethnomed. 2013, 9, 51. [Google Scholar] [CrossRef] [Green Version]
- Vougogiannopoulou, K.; Fokialakis, N.; Aligiannis, N.; Cantrell, C.; Skaltsounis, A.L. The raputindoles: Novel cyclopentylbisindole alkaloids from Raputiasimulans. Organ. Lett. 2010, 12, 1908–1911. [Google Scholar] [CrossRef] [PubMed]
- Vougogiannopoulou, K.; Fokialakis, N.; Aligiannis, N.; Cantrell, C.; Skaltsounis, A.L. Simple indole alkaloids from the neotropical rutaceous tree Raputiasimulans. Planta Med. 2011, 77, 1559–1561. [Google Scholar] [CrossRef]
- Vougogiannopoulou, K.; Angelopoulou, M.T.; Pratsinis, H.; Grougnet, R.; Halabalaki, M.; Kletsas, D.; Deguin, B.; Skaltsounis, L.A. Chemical and Biological Investigation of Olive Mill Waste Water—OMWWSecoiridoid Lactones. Planta Med. 2015, 81, 1205–1212. [Google Scholar] [CrossRef]
- Guldbrandsen, N.; De Mieri, M.; Gupta, M.; Liakou, E.; Pratsinis, H.; Kletsas, D.; Chaita, E.; Aligiannis, N.; Skaltsounis, A.L.; Hamburger, M. Screening of Panamanian Plants for Cosmetic Properties, and HPLC-Based Identification of Constituents with Antioxidant and UV-B Protecting Activities. Sci. Pharm. 2015, 83, 177–190. [Google Scholar] [CrossRef] [Green Version]
- Collins, T.L.; Andrew, R.L.; Greatrex, B.W.; Bruhl, J.J. Reliable analysis of volatile compounds from small samples of Eucalyptus magnificata (Myrtaceae). Aust. Syst. Bot. 2018, 31, 232–240. [Google Scholar] [CrossRef]
- Kao, D.; Henkin, J.M.; Soejarto, D.D.; Kinghorn, A.D.; Oberlies, N.H. Non-destructive chemical analysis of a Garcinia mangostana L. (Mangosteen) herbarium voucher specimen. Phytochem. Lett. 2018, 28, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Phillipson, J.D. Chemical investigations of herbarium material for alkaloids. Phytochemistry 1982, 21, 2441–2456. [Google Scholar] [CrossRef]
- Lee, K.H.; Whang, W.K. Inhibitory Effects of Bioassay-Guided Isolation of Anti-Glycation Components from Taraxacumcoreanum and Simultaneous Quantification. Molecules 2018, 23, 2148. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Guo, S.; He, K.; Roller, M.; Yang, M.; Liu, Q.; Zhang, L.; Ho, C.T.; Bai, N. Qualitative and quantitative analysis of chemical constituents of Ptychopetalumolacoides Benth. Nat. Prod. Res. 2018, 32, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, M.; Goulart, M.O.; de Lima, R.A.; Goulart, A.E.; DelleMonache, F.; Marini Bettolo, G.B. Flavonoids and alkaloids from Strychnos pseudoquina. J. Nat. Prod. 1984, 47, 953–957. [Google Scholar] [CrossRef]
- Xu, N.; Xu, X.D.; Ma, L.Y.; Yuan, J.Q.; Miao, J.H.; Cheng, Y.; Yang, J.S. A new homoflavonoid from the seed of Caesalpinia minax Hance. Chin. Chem. Lett. 2010, 21, 696–698. [Google Scholar] [CrossRef]
- Haruna, M.; Koube, T.; Ito, K.; Murata, H. Balanophonin, A New Neo-Lignan from Balanophora japonica Makino. Chem. Pharm. Bull. 1982, 30, 1525–1527. [Google Scholar] [CrossRef]
- Chen, G.-Y.; Dai, C.-Y.; Wang, T.-S.; Jiang, C.-W.; Han, C.-R.; Song, X.-P. A new flavonol from the stem-bark of Premnafulva. Arkivoc 2010, 2010, 179–185. [Google Scholar]
- Lami, N.; Kadota, S.; Kikuchi, T.; Momose, Y. Constituents of the roots of Boerhaavia diffusa, L. III. Identification of Ca2+ channel antagonistic compound from the methanol extract. Chem. Pharm. Bull. 1991, 39, 1551–1555. [Google Scholar] [CrossRef]
- Ray, A.B.; Chatterjee, A. Venoterpine—A new monoterpenoid alkaloid from the fruits of Alstonia venenata R. Br. Tetrahedron Lett. 1968, 9, 2763–2766. [Google Scholar] [CrossRef]
- Thepenier, P.; Jacquier, M.-J.; Massiot, G.; Le Men-olivier, L.; Delaude, C. Alkaloids from Strychnos staudtii. Phytochemistry 1988, 27, 657–659. [Google Scholar] [CrossRef]
- Wu, J.H.; Tung, Y.T.; Chien, S.C.; Wang, S.Y.; Kuo, Y.H.; Shyur, L.F.; Chang, S.T. Effect of phytocompounds from the heartwood of Acacia confusa on inflammatory mediator production. J. Agric. Food Chem. 2008, 56, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Kuo, Y.H. Four new compounds, ficusal, ficusesquilignan A, B, and ficusolidediacetate from the heartwood of Ficus microcarpa. Chem. Pharm. Bull. 2000, 48, 1862–1865. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, T.; Xu, Y.; Takita, R.; Shibasaki, M. Enantioselective total synthesis of (−)-strychnine: Development of a highly practical catalytic asymmetric carbon–carbon bond formation and domino cyclization. Tetrahedron 2004, 60, 9569–9588. [Google Scholar] [CrossRef]
- Martinez, S.E.; Davies, N.M.; Reynolds, J.K. Toxicology and Safety of Flavonoids. In Flavonoid Pharmacokinetics: Methods of Analysis, Preclinical and Clinical Pharmacokinetics, Safety, and Toxicology; Davies, N.M., Yáñez, J.A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013; Chapter 6; pp. 249–280. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.A.; York, B.M. The toxicity of plant alkaloids: An Ecogeographic perspective. Biochem. Syst. Ecol. 1978, 6, 61–76. [Google Scholar] [CrossRef]
- Lien, E.J.; Ren, S.; Bui, H.-H.; Wang, R. Quantitative structure-activity relationship analysis of phenolic antioxidants. Free Radic. Biol. Med. 1999, 26, 285–294. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, J. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Wolfle, U.; Esser, P.R.; Simon-Haarhaus, B.; Martin, S.F.; Lademann, J.; Schempp, C.M. UVB-induced DNA damage, generation of reactive oxygen species, and inflammation are effectively attenuated by the flavonoid luteolin in vitro and in vivo. Free Radic. Biol. Med. 2011, 50, 1081–1093. [Google Scholar] [CrossRef]
- Kumar, A.D.; Bevara, G.B.; Kaja, L.K.; Badana, A.K.; Malla, R.R. Protective effect of 3-O-methyl quercetin and kaempferol from Semecarpus anacardium against H2O2 induced cytotoxicity in lung and liver cells. BMC Complement. Altern. Med. 2016, 16, 376. [Google Scholar] [CrossRef]
- Wolfle, U.; Haarhaus, B.; Schempp, C.M. The photoprotective and antioxidative properties of luteolin are synergistically augmented by tocopherol and ubiquinone. Planta Med. 2013, 79, 963–965. [Google Scholar] [CrossRef] [PubMed]
- Bieski, I.G.C.; Rios Santos, F.; de Oliveira, R.M.; Espinosa, M.M.; Macedo, M.; Albuquerque, U.P.; de Oliveira Martins, D.T. Ethnopharmacology of Medicinal Plants of the Pantanal Region (Mato Grosso, Brazil). Evid. Based Complement. Altern. Med. 2012, 2012, 36. [Google Scholar] [CrossRef] [PubMed]
- Bonamin, F.; Moraes, T.M.; Kushima, H.; Silva, M.A.; Rozza, A.L.; Pellizzon, C.H.; Bauab, T.M.; Rocha, L.R.; Vilegas, W.; Hiruma-Lima, C.A. Can a Strychnos species be used as antiulcer agent? Ulcer healing action from alkaloid fraction of Strychnos pseudoquina St. Hil. (Loganiaceae). J. Ethnopharmacol. 2011, 138, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.V.; Colus, I.M.; Silva, M.A.; Vilegas, W.; Varanda, E.A. Assessment of DNA damage by extracts and fractions of Strychnos pseudoquina, a Brazilian medicinal plant with antiulcerogenic activity. Food Chem. Toxicol. 2006, 44, 1585–1589. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Travasarou, A.; Angelopoulou, M.T.; Vougogiannopoulou, K.; Papadopoulou, A.; Aligiannis, N.; Cantrell, C.L.; Kletsas, D.; Fokialakis, N.; Pratsinis, H. Bioactive Metabolites of the Stem Bark of Strychnos aff. darienensis and Evaluation of Their Antioxidant and UV Protection Activity in Human Skin Cell Cultures. Cosmetics 2019, 6, 7. https://doi.org/10.3390/cosmetics6010007
Travasarou A, Angelopoulou MT, Vougogiannopoulou K, Papadopoulou A, Aligiannis N, Cantrell CL, Kletsas D, Fokialakis N, Pratsinis H. Bioactive Metabolites of the Stem Bark of Strychnos aff. darienensis and Evaluation of Their Antioxidant and UV Protection Activity in Human Skin Cell Cultures. Cosmetics. 2019; 6(1):7. https://doi.org/10.3390/cosmetics6010007
Chicago/Turabian StyleTravasarou, Aikaterini, Maria T. Angelopoulou, Konstantina Vougogiannopoulou, Adamantia Papadopoulou, Nektarios Aligiannis, Charles L. Cantrell, Dimitris Kletsas, Nikolas Fokialakis, and Harris Pratsinis. 2019. "Bioactive Metabolites of the Stem Bark of Strychnos aff. darienensis and Evaluation of Their Antioxidant and UV Protection Activity in Human Skin Cell Cultures" Cosmetics 6, no. 1: 7. https://doi.org/10.3390/cosmetics6010007
APA StyleTravasarou, A., Angelopoulou, M. T., Vougogiannopoulou, K., Papadopoulou, A., Aligiannis, N., Cantrell, C. L., Kletsas, D., Fokialakis, N., & Pratsinis, H. (2019). Bioactive Metabolites of the Stem Bark of Strychnos aff. darienensis and Evaluation of Their Antioxidant and UV Protection Activity in Human Skin Cell Cultures. Cosmetics, 6(1), 7. https://doi.org/10.3390/cosmetics6010007