Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives
Abstract
:1. Introduction
2. Antioxidants
3. Natural Antioxidants in Cosmetics
4. Vitamins
5. Vitamin A
6. Vitamin B
7. Vitamin C
8. Vitamins E and K
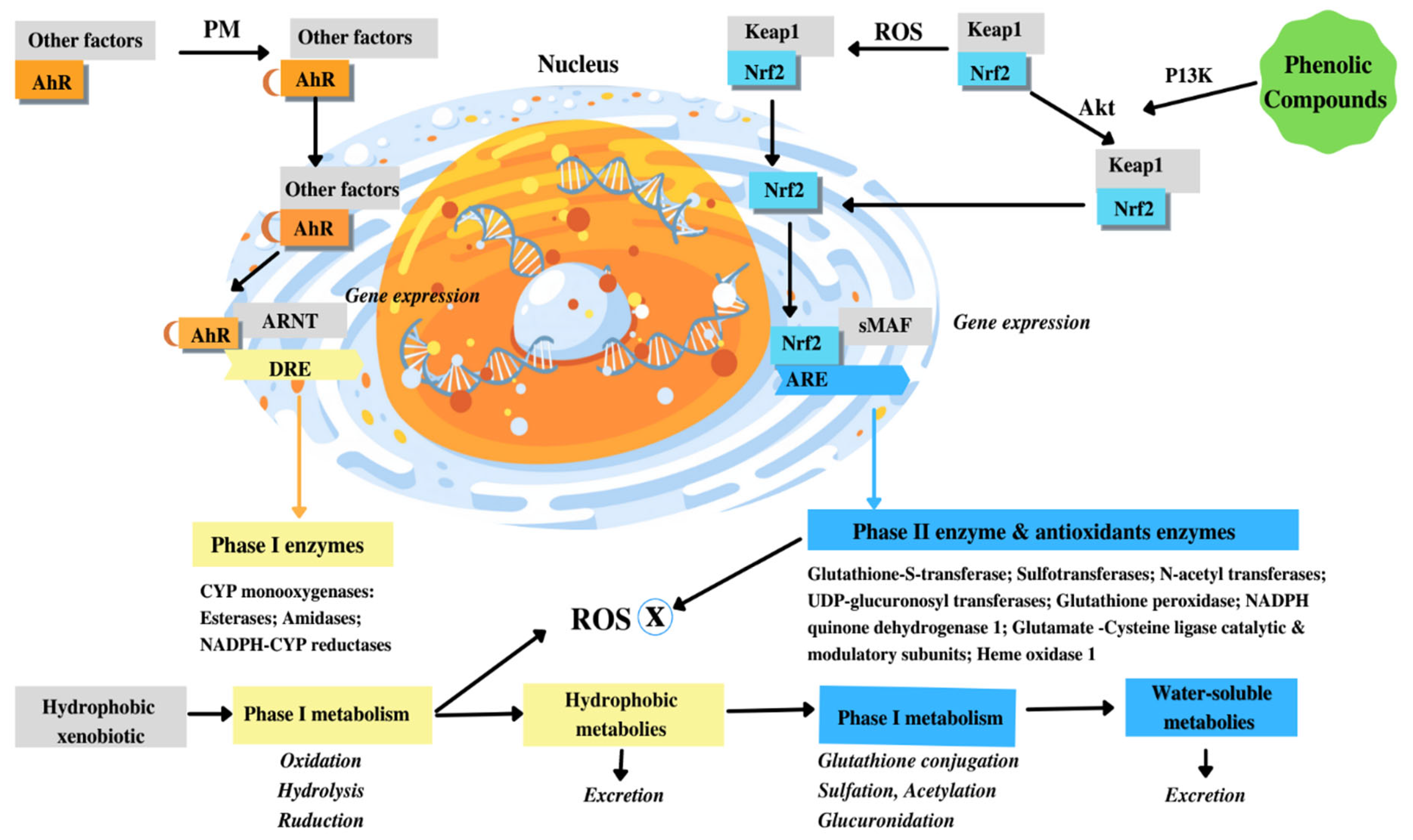
9. Polyphenols
Cosmetic Nanoformulation Containing Natural Antioxidants
| Plant | Active Compound | System | Application | Reference |
|---|---|---|---|---|
| White tea | Phenolic compounds | Polymeric nanoparticle | Protect bioactive compounds, enhance subsequent bioactivity and bioavailability | [210] |
| Centella asiatica | Asiaticoside Madecassoside Asiatic acid Madecassic acid | Nanoencapsulation | Enhance skin protection activity | [211] |
| Camellia sinensis | Phenolic compounds | Nanoemulsion | Improve emulsion stability | [212] |
| Hibiscus sabdariffa | Polyphenolic compounds | Liposome | Protect and deliver water-soluble functional compounds | [213] |
| Curcuma longa | Phenolic compounds (curcumin) | Liposome, ethosome, transferosome | Better skin penetration and protect skin from hydration | [214] |
| Fraxinus angustifolia | Phenolic compounds | Ethosome | Increase intracellular antioxidant activity | [215] |
| Aloe vera | Phenolic compounds | Liposomes | Enhance bioavailability and increase the collagen synthesis | [216] |
| Orthosiphon Stamineus | Phenolic compounds (rosmarinic acid, eupatorin) | Liposome (lecithin) | Improve the extract’s solubility and permeability | [217] |
| Vitis vinifera | Phenolic compounds | Nanoemulsion | Improve solubility and antioxidant efficiency | [218] |
| Panax quinquefolius | Saponin (Ginsenoside) | Liposome | Increase intracellular antioxidant activities | [219] |
| Polygonum aviculare | Phenolic compounds (quercetin and myricetin) | Liposome | Improve transdermal drug delivery | [220] |
| Phyllanthus urinaria | Phenolic flavonoids, saponins compounds | Nanoemulsion | Improve drug delivery to the skin | [221] |
| Achyrocline satureioides | Flavonoid compound (quercetin) | Nanoemulsion | Increase in drug absorption on skin | [222] |
10. Limitations of Natural Antioxidants in Skincare
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Abels, C.; Angelova-Fischer, I. Skin Care Products: Age-Appropriate Cosmetics. Curr. Probl. Dermatol. 2018, 54, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Nilforoushzadeh, M.A.; Amirkhani, M.A.; Zarintaj, P.; Ms, A.S.M.; Mehrabi, T.; Alavi, S.; Sisakht, M.M. Skin care and rejuvenation by cosmeceutical facial mask. J. Cosmet. Dermatol. 2018, 17, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Misery, L.; Proksch, E.; Metz, M.; Ständer, S.; Schmelz, M. Skin Barrier Damage and Itch: Review of Mechanisms, Topical Management and Future Directions. Acta Derm. Venereol. 2019, 99, 1201–1209. [Google Scholar] [CrossRef] [Green Version]
- Husein el Hadmed, H.; Castillo, R.F. Cosmeceuticals: Peptides, proteins, and growth factors. J. Cosm. Dermatol. 2016, 15, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Thiyagarasaiyar, K.; Goh, B.-H.; Jeon, Y.-J.; Yow, Y.-Y. Algae Metabolites in Cosmeceutical: An Overview of Current Applications and Challenges. Mar. Drugs 2020, 18, 323. [Google Scholar] [CrossRef] [PubMed]
- Veeresham, C. Natural products derived from plants as a source of drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200–201. [Google Scholar] [CrossRef]
- Fowler, J.F., Jr.; Woolery-Lloyd, H.; Waldorf, H.; Saini, R. Innovations in natural ingredients and their use in skin care. J. Drugs Dermatol. 2010, 9 (Suppl. S6), S72–S81. [Google Scholar] [PubMed]
- Jesumani, V.; Du, H.; Aslam, M.; Pei, P.; Huang, N. Potential Use of Seaweed Bioactive Compounds in Skincare—A Review. Mar. Drugs 2019, 17, 688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowe, W.P. Advances in natural ingredients and their use in skin care. Introduction. J. Drugs Dermatol. 2013, 12, s122. [Google Scholar] [PubMed]
- Speit, G. How to assess the mutagenic potential of cosmetic products without animal tests? Mutat. Res. Toxicol. Environ. Mutagen. 2009, 678, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Hameury, S.; Borderie, L.; Monneuse, J.; Skorski, G.; Pradines, D. Prediction of skin anti-aging clinical benefits of an association of ingredients from marine and maritime origins: Ex vivo evaluation using a label-free quantitative proteomic and customized data processing approach. J. Cosmet. Dermatol. 2018, 18, 355–370. [Google Scholar] [CrossRef] [Green Version]
- Sumpio, B.E.; Cordova, A.C.; Berke-Schlessel, D.W.; Qin, F.; Chen, Q.H. Green tea, the “Asian paradox,” and cardiovascular disease. J. Am. Coll. Surg. 2006, 202, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Trovato, M.; Ballabio, C. Botanical products: General aspects. In Food Supplements Containing Botanicals: Benefits, Side Effects and Regulatory Aspects; Springer: Cham, Switzerland, 2018; pp. 3–26. [Google Scholar]
- Koch, W.; Zagórska, J.; Marzec, Z.; Kukula-Koch, W. Applications of Tea (Camellia sinensis) and its Active Constituents in Cosmetics. Molecules 2019, 24, 4277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinrich, U.; Moore, C.E.; De Spirt, S.; Tronnier, H.; Stahl, W. Green Tea Polyphenols Provide Photoprotection, Increase Microcirculation, and Modulate Skin Properties of Women. J. Nutr. 2011, 141, 1202–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kottner, J.; Lichterfeld, A.; Blume-Peytavi, U. Maintaining skinintegrity in the aged: A systematic review. Br. J. Dermatol. 2013, 169, 528–542. [Google Scholar] [CrossRef] [PubMed]
- De Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Ferreira, M.; Oliveira, A.S.; Magalhaes, C.; Sousa, M.E.; Pinto, M.; Sousa Lobo, J.M.; Almeida, I.F. Evolution of the use of anti-oxidants in anti-ageing cosmetics. Int. J. Cosmet. Sci. 2019, 41, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Babbush, K.; Babbush, R.; Khachemoune, A. The Therapeutic Use of Antioxidants for Melasma. J. Drugs Dermatol. 2020, 19, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Augustyniak, A.; Bartosz, G.; Čipak, A.; Duburs, G.; Horáková, L.U.; Łuczaj, W.; Majekova, M.; Odysseos, A.D.; Rackova, L.; Skrzydlewska, E.; et al. Natural and synthetic antioxidants: An updated overview. Free Radic. Res. 2010, 44, 1216–1262. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Kim, K.S.; Yu, B.J. Optimization of Antioxidant and Skin-Whitening Compounds Extraction Condition from Tenebrio molitor Larvae (Mealworm). Molecules 2018, 23, 2340. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Li, A.; Li, S.; Tang, J.; Li, L.; Xiong, L. Natural components in sunscreens: Topical formulations with sun protection factor (SPF). Biomed. Pharmacother. 2020, 134, 111161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Duan, E. Fighting against skin aging: The way from bench to bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef] [PubMed]
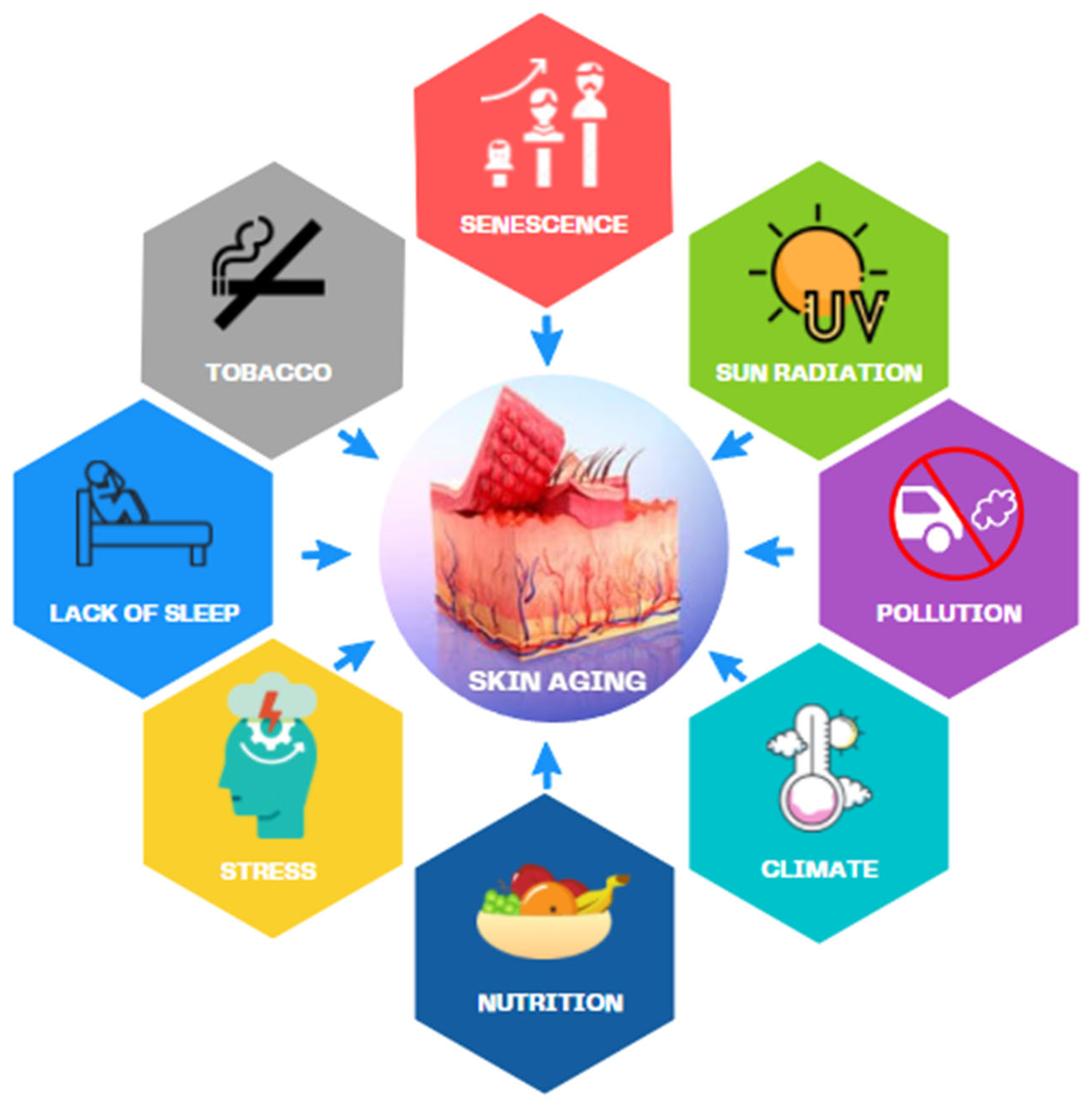
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.L. The Genetics of Sun Sensitivity in Humans. Am. J. Hum. Genet. 2004, 75, 739–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krutmann, J.; Schikowski, T.; Morita, A.; Berneburg, M. Environmentally-Induced (Extrinsic) Skin Aging: Exposomal Factors and Underlying Mechanisms. J. Investig. Dermatol. 2021, 141, 1096–1103. [Google Scholar] [CrossRef]
- Coppé, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppres-sion. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 99–118. [Google Scholar] [CrossRef] [Green Version]
- Flament, F.; Bazin, R.; Qiu, H.; Ye, C.; Laquieze, S.; Rubert, V.; Decroux, A.; Simonpietri, E.; Piot, B. Solar exposure(s) and facial clinical signs of aging in Chinese women: Impacts upon age perception. Clin. Cosmet. Investig. Dermatol. 2015, 8, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Morais, M.L.; Silva, A.C.; Araújo, C.R.; Esteves, E.A.; Dessimoni-Pinto, N.A. Determinação do potencial antioxidante in vitro de frutos do cerrado brasileiro. Rev. Bras. Fruticult. 2013, 35, 355–360. [Google Scholar] [CrossRef] [Green Version]
- Bose, B.; Choudhury, H.; Tandon, P.; Kumaria, S. Studies on secondary metabolite profiling, anti-inflammatory potential, in vitro photoprotective and skin-aging related enzyme inhibitory activities of Malaxis acuminata, a threatened orchid of nutraceutical importance. J. Photochem. Photobiol. B Biol. 2017, 173, 686–695. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Lin, T.-K.; Zhong, L.; Santiago, J.L. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajaram, S.; Jones, J.; Lee, G.J. Plant-based dietary patterns, plant foods, and age-related cognitive decline. Adv. Nutr. 2019, 10 (Suppl. S4), S422–S436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavinato, M.; Waltenberger, B.; Baraldo, G.; Grade, C.V.; Stuppner, H.; Jansen-Dürr, P. Plant extracts and natural compounds used against UVB-induced photoaging. Biogerontology 2017, 18, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Petruk, G.; Del Giudice, R.; Rigano, M.M.; Monti, D.M. Antioxidants from Plants Protect against Skin Photoaging. Oxid. Med. Cell. Longev. 2018, 2018, 1454936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisoschi, A.M.; Pop, A. The Role of Antioxidants in the Chemistry of Oxidative Stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Aune, D. Plant Foods, Antioxidant Biomarkers, and the Risk of Cardiovascular Disease, Cancer, and Mortality: A Review of the Evidence. Adv. Nutr. 2019, 10, S404–S421. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Jenab, M.; Riboli, E.; Ferrari, P.; Sabate, J.; Slimani, N.; Norat, T.; Friesen, M.; Tjonneland, A.; Olsen, A.; Overvad, K.; et al. Plasma and dietary vitamin C levels and risk of gastric cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST). Carcinogenesis 2006, 27, 2250–2257. [Google Scholar] [CrossRef] [Green Version]
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef]
- Salomone, F.; Godos, J.; Zelber-Sagi, S. Natural antioxidants for non-alcoholic fatty liver disease: Molecular targets and clinical perspectives. Liver Int. 2016, 36, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Balmus, I.M.; Ciobica, A.; Trifan, A.; Stanciu, C. The implications of oxidative stress and antioxidant therapies in Inflammatory Bowel Disease: Clinical aspects and animal models. Saudi J. Gastroenterol. 2016, 22, 3–17. [Google Scholar] [CrossRef]
- Khan, B.A.; Mahmood, T.; Menaa, F.; Shahzad, Y.; Yousaf, A.M.; Hussain, T.; Ray, S.D. New Perspectives on the Efficacy of Gallic Acid in Cosmetics & Nanocosmeceuticals. Curr. Pharm. Des. 2019, 24, 5181–5187. [Google Scholar] [CrossRef]
- Neha, K.; Haider, R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Nalimu, F.; Oloro, J.; Kahwa, I.; Ogwang, P.E. Review on the phytochemistry and toxicological profiles of Aloe vera and Aloe ferox. Futur. J. Pharm. Sci. 2021, 7, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Burke, K.E. Protection From Environmental Skin Damage With Topical Antioxidants. Cl. Pharmacol. Ther. 2018, 105, 36–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic Compounds in Brassica Vegetables. Molecules 2010, 16, 251–280. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Kurakula, M.; Mamidipalli, N.; Tiyyagura, P.; Patel, B.; Manne, R. Pharmacological Exploration of Phenolic Compound: Raspberry Ketone—Update 2020. Plants 2021, 10, 1323. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Raphaelli, C.; Azevedo, J.G.; dos Santos Pereira, E.; Vinholes, J.R.; Camargo, T.M.; Hoffmann, J.F.; Ribeiro, J.A.; Vizzotto, M.; Rombaldi, C.V.; Wink, M.R.; et al. Phenolic-rich apple extracts have photoprotective and anti-cancer effect in dermal cells. Phytomed. Plus. 2021, 1, 100112. [Google Scholar] [CrossRef]
- Rabelo, A.C.S.; Costa, D. A review of biological and pharmacological activities of Baccharis trimera. Chem. Interact. 2018, 296, 65–75. [Google Scholar] [CrossRef]
- Sikora, M.; Złotek, U.; Kordowska-Wiater, M.; Świeca, M. Effect of Basil Leaves and Wheat Bran Water Extracts on Antioxidant Capacity, Sensory Properties and Microbiological Quality of Shredded Iceberg Lettuce during Storage. Antioxidants 2020, 9, 355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, U.; Tudu, C.K.; Nandy, S.; Sunita, K.; Tripathi, V.; Loake, G.J.; Dey, A.; Proćków, J. Ethnodermatological use of medicinal plants in India: From ayurvedic formulations to clinical perspectives—A review. J. Ethnopharmacol. 2021, 284, 114744. [Google Scholar] [CrossRef] [PubMed]
- Curutchet, A.; Cozzano, S.; Tárrega, A.; Arcia, P. Blueberry pomace as a source of antioxidant fibre in cookies: Consumer’s ex-pectations and critical attributes for developing a new product. Food Sci. Technol. Int. 2019, 25, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Routray, W.; Orsat, V. Blueberries and Their Anthocyanins: Factors Affecting Biosynthesis and Properties. Compr. Rev. Food Sci. Food Saf. 2011, 10, 303–320. [Google Scholar] [CrossRef]
- Chaves-Gómez, J.L.; Cotes-Prado, A.M.; Gómez-Caro, S.; Restrepo-Díaz, H. Physiological Response of Cape Gooseberry Seedlings to Two Organic Additives and Their Mixture under Inoculation with Fusarium oxysporum f. sp. physali. HortScience 2020, 55, 55–62. [Google Scholar] [CrossRef]
- Hassanien, M.F.R.; Serag, H.M.; Qadir, M.S.; Ramadan, M.F. Cape gooseberry (Physalis peruviana) juice as a modulator agent for hepatocellular carcinoma-linked apoptosis and cell cycle arrest. Biomed. Pharmacother. 2017, 94, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Curaba, J.; Pottorff, M.; Ferruzzi, M.G.; Simon, P.; Cavagnaro, P.F. Carrot Anthocyanins Genetics and Genomics: Status and Perspectives to Improve Its Application for the Food Colorant Industry. Genes 2020, 11, 906. [Google Scholar] [CrossRef]
- Zerres, S.; Stahl, W. Carotenoids in human skin. Biochim. Biophys. Acta BBA–Mol. Cell Biol. Lipids. 2020, 1865, 158588. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Vieira, E.F.; Peixoto, A.F.; Freire, C.; Freitas, V.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Optimizing the extraction of phenolic antioxidants from chestnut shells by subcritical water extraction using response surface methodology. Food Chem. 2020, 334, 127521. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.S.; Estanqueiro, M.; Oliveira, M.B.; Lobo, J.M.S. Main Benefits and Applicability of Plant Extracts in Skin Care Products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, Â.; Colomban, S.; Azinheira, H.G.; Guerra-Guimarães, L.; Silva, M.D.C.; Navarini, L.; Resmini, M. Dietary Antioxidants in Coffee Leaves: Impact of Botanical Origin and Maturity on Chlorogenic Acids and Xanthones. Antioxidants 2019, 9, 6. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, É.M.; de Macedo, L.M.; Tundisi, L.L.; Ataide, J.A.; Camargo, G.A.; Alves, R.C.; Oliveira, M.B.; Mazzola, P.G. Coffee by-products in topical formulations: A review. Trends Food Sci. Technol. 2021, 111, 280–291. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Ferreira, A.L.A.; Rosa, G.B.; Azevedo, M.S.; Ferrareze, J.P.; Komatsu, R.A.; Nunes, M.R.; Da Rosa, C.G.; Schmit, R.; Costa, M.D.; et al. Feijoa [Acca sellowiana (Berg) Burret] accessions characterization and discrimination by chemometrics. J. Sci. Food Agric. 2020, 100, 5373–5384. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Denaro, M.; De Francesco, C.; Cornara, L.; Barreca, D.; Bellocco, E.; Ginestra, G.; Mandalari, G.; Trombetta, D. Feijoa Fruit Peel: Micro-morphological Features, Evaluation of Phytochemical Profile, and Biological Properties of Its Essential Oil. Antioxidants 2019, 8, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, J.; Wang, Z.; Wang, P.; Wang, M. Extraction, structure and bioactivities of the polysaccharides from Ginkgo biloba: A review. Int. J. Biol. Macromol. 2020, 162, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gong, X.; Zhang, H.; Zhu, W.; Jiang, Z.; Shi, Y.; Li, L. In vitro anti-aging activities of ginkgo biloba leaf extract and its chemical constituents. Food Sci. Technol. 2020, 40, 476–482. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.F.; Zhang, H.; Teh, S.S.; Wang, C.W.; Zhang, Y.; Hayford, F.; Wang, L.; Ma, T.; Dong, Z.; Zhang, Y.; et al. Goji Berries as a Potential Natural Antioxidant Medicine: An Insight into Their Molecular Mechanisms of Action. Oxid. Med. Cell. Longev. 2019, 2019, 2437397. [Google Scholar] [CrossRef] [Green Version]
- Medina, S.; Collado-González, J.; Ferreres, F.; Londoño-Londoño, J.; Jiménez-Cartagena, C.; Guy, A.; Durand, T.; Galano, J.M.; Gil-Izquierdo, Á. Potential of Physalis peruviana calyces as a low-cost valuable resource of phytoprostanes and phenolic com-pounds. J. Sci. Food Agric. 2019, 99, 2194–2204. [Google Scholar] [CrossRef]
- Nocetti, D.; Núñez, H.; Puente, L.; Espinosa, A.; Romero, F. Composition and biological effects of goldenberry byproducts: An overview. J. Sci. Food Agric. 2020, 100, 4335–4346. [Google Scholar] [CrossRef]
- Soto, M.L.; Falqué, E.; Domínguez, J. Relevance of natural phenolics from grape and derivative products in the formulation of cosmetics. Cosmetics 2015, 2, 259–276. [Google Scholar] [CrossRef] [Green Version]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an anti-cancer agent: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef] [PubMed]
- Bito, T.; Okumura, E.; Fujishima, M.; Watanabe, F. Potential of Chlorella as a Dietary Supplement to Promote Human Health. Nutrients 2020, 12, 2524. [Google Scholar] [CrossRef] [PubMed]
- Dantas Silva, R.P.; Machado, B.A.; Barreto, G.D.; Costa, S.S.; Andrade, L.N.; Amaral, R.G.; Carvalho, A.A.; Padilha, F.F.; Barbosa, J.D.; Umsza-Guez, M.A. Antioxidant, antimicrobial, antiparasitic, and cytotoxic properties of various Brazilian propolis extracts. PLoS ONE 2017, 12, e0172585. [Google Scholar] [CrossRef]
- Berthon, J.-Y.; Kappes, R.N.; Bey, M.; Cadoret, J.-P.; Renimel, I.; Filaire, E. Marine algae as attractive source to skin care. Free. Radic. Res. 2017, 51, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.G.; Silva, K.A.; Silva, L.O.; Costa, A.M.; Akil, E.; Coelho, M.A.; Torres, A.G. Jussara berry (Euterpe edulis M.) oil-in-water emulsions are highly stable: The role of natural antioxidants in the fruit oil. J. Sci. Food Agric. 2019, 99, 90–99. [Google Scholar] [CrossRef] [Green Version]
- Lupatini, N.R.J.; Danopoulos, P.; Swikidisa, R.; Alves, P.V. Evaluation of the Antibacterial Activity of Green Propolis Extract and Meadowsweet Extract Against Staphylococcus aureus Bacteria: Importance in Would Care Compounding Preparations. Int. J. Pharm. Compd. 2016, 20, 333–337. [Google Scholar]
- Costanzo, G.; Iesce, M.R.; Naviglio, D.; Ciaravolo, M.; Vitale, E.; Arena, C. Comparative studies on different citrus cultivars: A re-valuation of waste mandarin components. Antioxidants 2020, 9, 517. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.R.C.; Mesquita, L.M.D.S.; Martins, P.; Habu, S.; de Rosso, V.V. Lactobacillus fermentation of jussara pulp leads to the enzymatic conversion of anthocyanins increasing antioxidant activity. J. Food Compos. Anal. 2018, 69, 162–170. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Li, X.; Zhao, P.; Zhang, X.; Qiao, O.; Huang, L.; Guo, L.; Gao, W. A review of chemical constituents and health-promoting effects of citrus peels. Food Chem. 2021, 365, 130585. [Google Scholar] [CrossRef] [PubMed]
- Umamahesh, K.; Gandhi, A.D.; Reddy, O.V. Ethnopharmacological Applications of Mango (Mangifera indica L.) Peel-A Review. Curr. Pharm. Biotechnol. 2020, 21, 1298–1303. [Google Scholar] [CrossRef] [PubMed]
- Patravale, V.; Mandawgade, S. Formulation and evaluation of exotic fat based cosmeceuticals for skin repair. Indian J. Pharm. Sci. 2008, 70, 539–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safari, R.; Hoseinifar, S.H.; Van Doan, H.; Dadar, M. The effects of dietary Myrtle (Myrtus communis) on skin mucus immune parameters and mRNA levels of growth, antioxidant and immune related genes in zebrafish (Danio rerio). Fish. Shellfish Immunol. 2017, 66, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, O.; Ipekci, H.; Alev, B.; Ustundag, U.V.; Ak, E.; Sen, A.; Alturfan, E.E.; Sener, G.; Yarat, A.; Cetinel, S.; et al. Protective effect of Myrtle (Myrtus communis) on burn induced skin injury. Burns 2019, 45, 1856–1863. [Google Scholar] [CrossRef] [PubMed]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Gammazza, A.M.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danby, S.G.; AlEnezi, T.; Sultan, A.; Lavender, T.; Chittock, J.; Brown, K.; Cork, M.J. Effect of olive and sunflower seed oil on the adult skin barrier: Implications for neonatal skin care. Pediatr. Dermatol. 2013, 30, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Yap, J.Y.; Hii, C.L.; Ong, S.P.; Lim, K.H.; Abas, F.; Pin, K.Y. Effects of drying on total polyphenols content and antioxidant properties of Carica papaya leaves. J. Sci. Food Agric. 2020, 100, 2932–2937. [Google Scholar] [CrossRef]
- Sharma, A.; Bachheti, A.; Sharma, P.; Bachheti, R.K.; Husen, A. Phytochemistry, pharmacological activities, nanoparticle fabrication, commercial products and waste utilization of Carica papaya L.: A comprehensive review. Curr. Res. Biotechnol. 2020, 2, 145–160. [Google Scholar] [CrossRef]
- Drogoudi, P.; Gerasopoulos, D.; Kafkaletou, M.; Tsantili, E. Phenotypic characterization of qualitative parameters and antioxidant contents in peach and nectarine fruit and changes after jam preparation. J. Sci. Food Agric. 2017, 97, 3374–3383. [Google Scholar] [CrossRef]
- Noratto, G.; Porter, W.; Byrne, D.; Cisneros-Zevallos, L. Polyphenolics from peach (Prunus persica var. Rich Lady) inhibit tumor growth and metastasis of MDA-MB-435 breast cancer cells in vivo. J. Nutr. Biochem. 2014, 25, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Gurbuz, N.; Uluişik, S.; Frary, A.; Frary, A.; Doğanlar, S. Health benefits and bioactive compounds of eggplant. Food Chem. 2018, 268, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Topal, U.; Sasaki, M.; Goto, M.; Otles, S. Chemical compositions and antioxidant properties of essential oils from nine species of Turkish plants obtained by supercritical carbon dioxide extraction and steam distillation. Int. J. Food Sci. Nutr. 2008, 59, 619–634. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.; Hashim, N.; Aziz, S.A.; Lasekan, O. Pineapple (Ananas comosus): A comprehensive review of nutritional values, volatile compounds, health benefits, and potential food products. Food Res. Int. 2020, 137, 109675. [Google Scholar] [CrossRef]
- Ajayi, A.M.; Coker, A.I.; Oyebanjo, O.T.; Adebanjo, I.M.; Ademowo, O.G. Ananas comosus (L) Merrill (pineapple) fruit peel extract demonstrates antimalarial, anti-nociceptive and anti-inflammatory activities in experimental models. J. Ethnopharmacol. 2021, 282, 114576. [Google Scholar] [CrossRef] [PubMed]
- Vučić, V.; Grabež, M.; Trchounian, A.; Arsić, A. Composition and Potential Health Benefits of Pomegranate: A Review. Curr. Pharm. Des. 2019, 25, 1817–1827. [Google Scholar] [CrossRef]
- Cervi, V.F.; Saccol, C.P.; Sari, M.H.M.; Martins, C.C.; da Rosa, L.S.; Ilha, B.D.; Soares, F.Z.; Luchese, C.; Wilhelm, E.A.; Cruz, L. Pullulan film incorporated with nanocapsules improves pomegranate seed oil anti-inflammatory and antioxidant effects in the treatment of atopic dermatitis in mice. Int. J. Pharm. 2021, 609, 121144. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxid. Med. Cell. Longev. 2017, 2017, 1259510. [Google Scholar] [CrossRef]
- Abdellatif, M.M.; Elakkad, Y.E.; Elwakeel, A.A.; Allam, R.M.; Mousa, M.R. Formulation and characterization of propolis and tea tree oil nanoemulsion loaded with clindamycin hydrochloride for wound healing: In-vitro and in-vivo wound healing assessment. Saudi Pharm. J. 2021. [Google Scholar] [CrossRef]
- Vega, J.; Bonomi-Barufi, J.; Gómez-Pinchetti, J.L.; Figueroa, F.L. Cyanobacteria and Red Macroalgae as Potential Sources of Anti-oxidants and UV Radiation-Absorbing Compounds for Cosmeceutical Applications. Mar. Drugs 2020, 18, 659. [Google Scholar] [CrossRef]
- Aslam, A.; Bahadar, A.; Liaqat, R.; Saleem, M.; Waqas, A.; Zwawi, M. Algae as an attractive source for cosmetics to counter envi-ronmental stress. Sci. Total Environ. 2021, 772, 144905. [Google Scholar] [CrossRef] [PubMed]
- Septembre-Malaterre, A.; Stanislas, G.; Douraguia, E.; Gonthier, M.-P. Evaluation of nutritional and antioxidant properties of the tropical fruits banana, litchi, mango, papaya, passion fruit and pineapple cultivated in Réunion French Island. Food Chem. 2016, 212, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Al-Mqbali, L.R.A.; Hossain, M.A. Cytotoxic and antimicrobial potential of different varieties of ripe banana used traditionally to treat ulcers. Toxicol. Rep. 2019, 6, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Bonifácio-Lopes, T.; Boas, A.A.; Coscueta, E.R.; Costa, E.M.; Silva, S.; Campos, D.; Teixeira, J.A.; Pintado, M. Bioactive extracts from brewer’s spent grain. Food Funct. 2020, 11, 8963–8977. [Google Scholar] [CrossRef] [PubMed]
- Connolly, A.; Cermeño, M.; Alashi, A.M.; Aluko, R.E.; FitzGerald, R.J. Generation of phenolic-rich extracts from brewers’ spent grain and characterisation of their in vitro and in vivo activities. Innov. Food Sci. Emerg. Technol. 2021, 68, 102617. [Google Scholar] [CrossRef]
- Kocaadam, B.; Şanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2015, 57, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, V.; Mackonochie, M.; Mills, S.; MacLennan, E. Turmeric/curcumin and health outcomes: A meta-review of systematic reviews. Eur. J. Integr. Med. 2020, 40, 101252. [Google Scholar] [CrossRef]
- Oviedo-Solís, C.I.; Cornejo-Manzo, S.; Murillo-Ortiz, B.O.; Guzmán-Barrón, M.M.; Ramírez-Emiliano, J. Strawberry polyphenols decrease oxidative stress in chronic diseases. Gaceta Med. Mex. 2019, 154, 60–65. [Google Scholar] [CrossRef] [Green Version]
- Lan, W.; Zhang, R.; Ahmed, S.; Qin, W.; Liu, Y. Effects of various antimicrobial polyvinyl alcohol/tea polyphenol composite films on the shelf life of packaged strawberries. LWT 2019, 113, 108297. [Google Scholar] [CrossRef]
- Nguyen, H.; Chen, C.-C.; Lin, K.-H.; Chao, P.-Y.; Lin, H.-H.; Huang, M.-Y. Bioactive Compounds, Antioxidants, and Health Benefits of Sweet Potato Leaves. Molecules 2021, 26, 1820. [Google Scholar] [CrossRef]
- Krochmal-Marczak, B.; Zagórska-Dziok, M.; Michalak, M.; Kiełtyka-Dadasiewicz, A. Comparative assessment of phenolic content, cellular antioxidant, antityrosinase and protective activities on skin cells of extracts from three sweet potato (Ipomoea batatas (L.) Lam.) cultivars. J. King Saud Univ. Sci. 2021, 33, 101532. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, R.; Sharopov, F.; Namiesnik, J.; Roointan, A.; Kamle, M.; Kumar, P.; Martins, N.; Sharifi-Rad, J. Beneficial effects and potential risks of tomato consumption for human health: An overview. Nutrition 2019, 62, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, D.; Kumar, K.S.; Paswan, S.; Srivastava, S. Tomato-a natural medicine and its health benefits. J. Pharmacogn. Phytochem. 2012, 1, 33–43. [Google Scholar]
- Manuguerra, S.; Caccamo, L.; Mancuso, M.; Arena, R.; Rappazzo, A.C.; Genovese, L.; Santulli, A.; Messina, C.M.; Maricchiolo, G. The antioxidant power of horseradish, Armoracia rusticana, underlies antimicrobial and antiradical effects, exerted in vitro. Nat. Prod. Res. 2018, 34, 1567–1570. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Muhammad, G.; Hussain, M.A.; Altaf, M.; Bukhari, S.N. Withania somnifera L.: Insights into the phytochemical profile, therapeutic potential, clinical trials, and future prospective. Iran. J. Basic Med. Sci. 2020, 23, 1501. [Google Scholar] [PubMed]
- Kim, D.Y.; Kim, M.K.; Kim, B.-W. The Antioxidant and Skin Whitening Effect of Withania somnifera (Winter Cherry). J. Food Hyg. Saf. 2015, 30, 258–264. [Google Scholar] [CrossRef] [Green Version]
- Lupo, M.P. Antioxidants and vitamins in cosmetics. Clin. Dermatol. 2001, 19, 467–473. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Saliou, C. Role of vitamins in skin care. Nutrition 2001, 17, 839–844. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira Pinto, C.A.; Martins, T.E.; Martinez, R.M.; Freire, T.B.; Velasco, M.V.; Baby, A.R. Vitamin E in Human Skin: Functionality and Topical Products; InTech Open: London, UK, 2021. [Google Scholar]
- Tozer, S.; O’Mahony, C.; Hannah, J.; O’Brien, J.; Kelly, S.; Kosemund-Meynen, K.; Alexander-White, C. Aggregate exposure modelling of vitamin A from cosmetic products, diet and food supplements. Food Chem. Toxicol. 2019, 131, 110549. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, L.R.; Campos, P.M.B.G.M. A HPLC method to evaluate the influence of photostabilizers on cosmetic formulations containing UV-filters and vitamins A and E. Talanta 2010, 82, 1490–1494. [Google Scholar] [CrossRef] [PubMed]
- Zasada, M.; Budzisz, E. Retinoids: Active molecules influencing skin structure formation in cosmetic and dermatological treat-ments. Adv. Dermatol. Allergol. 2019, 36, 392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duester, G. Retinoic Acid Synthesis and Signaling during Early Organogenesis. Cell 2008, 134, 921–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varani, J.; Warner, R.L.; Gharaee-Kermani, M.; Phan, S.H.; Kang, S.; Chung, J.; Wang, Z.; Datta, S.C.; Fisher, G.J.; Voorhees, J.J. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen ac-cumulation in naturally aged human skin1. J. Investig. Dermatol. 2000, 114, 480–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, S.; Bardawil, T.; Stephan, C.; Darwiche, N.; Abbas, O.; Kibbi, A.G.; Nemer, G.; Kurban, M. Retinoids: A journey from the molecular structures and mechanisms of action to clinical uses in dermatology and adverse effects. J. Dermatol. Treat. 2017, 28, 684–696. [Google Scholar] [CrossRef]
- Alaluf, S.; Heinrich, U.; Stahl, W.; Tronnier, H.; Wiseman, S. Dietary Carotenoids Contribute to Normal Human Skin Color and UV Photosensitivity. J. Nutr. 2002, 132, 399–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köpcke, W.; Krutmann, J. Protection from Sunburn with β-Carotene—A Meta-analysis. Photochem. Photobiol. 2008, 84, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-J.; Taylor, A. Nutritional antioxidants and age-related cataract and maculopathy. Exp. Eye Res. 2007, 84, 229–245. [Google Scholar] [CrossRef]
- Zizola, C.F.; Frey, S.K.; Jitngarmkusol, S.; Kadereit, B.; Yan, N.; Vogel, S. Cellular Retinol-Binding Protein Type I (CRBP-I) Regulates Adipogenesis. Mol. Cell. Biol. 2010, 30, 3412–3420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darlenski, R.; Surber, C.; Fluhr, J.W. Topical retinoids in the management of photodamaged skin: From theory to evidence-based practical approach. Br. J. Dermatol. 2010, 163, 1157–1165. [Google Scholar] [CrossRef]
- Sorg, O.; Kuenzli, S.; Kaya, G.; Saurat, J.-H. Proposed mechanisms of action for retinoid derivatives in the treatment of skin aging. J. Cosmet. Dermatol. 2005, 4, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Sorg, O.; Saurat, J.-H. Topical Retinoids in Skin Ageing: A Focused Update with Reference to Sun-Induced Epidermal Vitamin A Deficiency. Dermatology 2014, 228, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; He, T.; Fisher, G.J.; Voorhees, J.J.; Quan, T. Molecular basis of retinol anti-ageing properties in naturally aged human skin in vivo. Int. J. Cosmet. Sci. 2016, 39, 56–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossetti, D.; Kielmanowicz, M.G.; Vigodman, S.; Hu, Y.P.; Chen, N.; Nkengne, A.; Oddos, T.; Fischer, D.; Seiberg, M.; Lin, C.B. A novel anti-ageing mechanism for retinol: Induction of dermal elastin synthesis and elastin fibre formation. Int. J. Cosmet. Sci. 2011, 33, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Kligman, A.M.; Grove, G.L.; Hirose, R.; Leyden, J.J. Topical tretinoin for photoaged skin. J. Am. Acad. Dermatol. 1986, 15, 836–859. [Google Scholar] [CrossRef]
- Bauer, E.A.; Seltzer, J.L.; Eisen, A.Z. Retinoic Acid Inhibition of Collagenase and Gelatinase Expression in Human Skin Fibroblast Cultures. Evidence for a Dual Mechanism. J. Investig. Dermatol. 1983, 81, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Michalak, M.; Pierzak, M.; Kręcisz, B.; Suliga, E. Bioactive Compounds for Skin Health: A Review. Nutrients 2021, 13, 203. [Google Scholar] [CrossRef]
- Everts, H.B. Endogenous retinoids in the hair follicle and sebaceous gland. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2012, 1821, 222–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, S.; Date, A.; Patravale, V.; Korting, H.C.; Roeder, A.; Weindl, G. Retinoids in the treatment of skin aging: An overview of clinical efficacy and safety. Clin. Interv. Aging 2006, 1, 327–348. [Google Scholar] [CrossRef]
- Dawson, M.I.; Hobbs, P.D.; Peterson, V.J.; Leid, M.; Lange, C.W.; Feng, K.C.; Chen, G.Q.; Gu, J.; Li, H.; Kolluri, S.K.; et al. Apoptosis induction in cancer cells by a novel analogue of 6-[3-(1-adamantyl)-4-hydroxyphenyl]-2-naphthalenecarboxylic acid lacking retinoid receptor transcriptional activation activity. Cancer Res. 2001, 61, 4723–4730. [Google Scholar]
- Duvic, M.; Nagpal, S.; Asano, A.T.; Chandraratna, R.A. Molecular mechanisms of tazarotene action in psoriasis. J. Am. Acad. Dermatol. 1997, 37, S18–S24. [Google Scholar] [CrossRef]
- Tanno, O.; Ota, Y.; Kitamura, N.; Katsube, T.; Inoue, S. Nicotinamide increases biosynthesis of ceramides as well as other stratum corneum lipids to improve the epidermal permeability barrier. Br. J. Dermatol. 2000, 143, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Forbat, E.; Al-Niaimi, F.; Ali, F.R. Use of nicotinamide in dermatology. Clin. Exp. Dermatol. 2017, 42, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Bains, P.; Kaur, M.; Kaur, J.; Sharma, S. Nicotinamide: Mechanism of action and indications in dermatology. Indian J. Dermatol. Venereol. Leprol. 2018, 84, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Seemal Desai, M.D.; Eloisa Ayres, M.D.; Hana Bak, M.D.; Green, B.S.; PhDg, Q.Z. Effect of a tranexamic acid, kojic acid, and niacin-amide containing serum on facial dyschromia: A clinical evaluation. J. Drugs Dermatol. 2019, 18, 454–459. [Google Scholar]
- Cosmetic Ingredient Review Expert Panel. Final report of the safety assessment of niacinamide and niacin. Int. J. Toxicol. 2005, 24, 1–31. [Google Scholar]
- Caritá, A.C.; Fonseca-Santos, B.; Shultz, J.D.; Michniak-Kohn, B.; Chorilli, M.; Leonardi, G.R. Vitamin C: One compound, several uses. Advances for delivery, efficiency and stability. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102117. [Google Scholar] [CrossRef] [PubMed]
- Sunil Kumar, B.V.; Singh, S.; Verma, R. Anticancer potential of dietary vitamin D and ascorbic acid: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 2623–2635. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-Y.; Selim, M.; Shea, C.R.; Grichnik, J.M.; Omar, M.M.; Monteiro-Riviere, N.; Pinnell, S.R. UV photoprotection by combination topical antioxidants vitamin C and vitamin E. J. Am. Acad. Dermatol. 2003, 48, 866–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastrangelo, D.; Pelosi, E.; Castelli, G.; Lo-Coco, F.; Testa, U. Mechanisms of anti-cancer effects of ascorbate: Cytotoxic activity and epigenetic modulation. Blood CellsMol. Dis. 2018, 69, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marionnet, C.; Pierrard, C.; Sok, J.; Asselineau, D.; Bernerd, F.; Vioux-Chagnoleau, C. Morphogenesis of dermal-epidermal junction in a model of reconstructed skin: Beneficial effects of vitamin C. Exp. Dermatol. 2006, 15, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Blaschke, K.; Ebata, K.; Karimi, M.M.; Zepeda-Martínez, J.A.; Goyal, P.; Mahapatra, S.; Tam, A.; Laird, D.J.; Hirst, M.; Rao, A.; et al. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature 2013, 500, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Mao, S.-Q.; Zhao, B.; Chong, Z.; Yang, Y.; Zhao, C.; Zhang, D.; Huang, H.; Gao, J.; Li, Z.; et al. Ascorbic Acid Enhances Tet-Mediated 5-Methylcytosine Oxidation and Promotes DNA Demethylation in Mammals. J. Am. Chem. Soc. 2013, 135, 10396–10403. [Google Scholar] [CrossRef] [PubMed]
- Keen, M.A.; Hassan, I. Vitamin E in dermatology. Indian Dermatol. Online J. 2016, 7, 311. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, E.; Faintuch, J.; Moreira, E.A.M.; Da Silva, V.R.G.; Pereima, M.J.L.; Fagundes, R.L.M.; Filho, D.W. Supplementation of Vitamin E, Vitamin C, and Zinc Attenuates Oxidative Stress in Burned Children: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. J. Burn. Care Res. 2009, 30, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Kondo, R.; Sakai, K.; Takeda, N.; Nagahata, T.; Oniki, T. Novel vitamin E derivative with 4-substituted resorcinol moiety has both antioxidant and tyrosinase inhibitory properties. Lipids 2001, 36, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Kelly, F.J.; Salonen, J.T.; Neuzil, J.; Zingg, J.-M.; Azzi, A. The European perspective on vitamin E: Current knowledge and future research. Am. J. Clin. Nutr. 2002, 76, 703–716. [Google Scholar] [CrossRef]
- Ellinger, S.; Stehle, P. Efficacy of vitamin supplementation in situations with wound healing disorders: Results from clinical in-tervention studies. Curr. Opin. Clin. Nutr. Metabol. Care 2009, 12, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Shearer, M.J.; Bach, A.; Kohlmeier, M. Chemistry, nutritional sources, tissue distribution and metabolism of vitamin K with special reference to bone health. J. Nutr. 1996, 126, 1181S–1186S. [Google Scholar] [CrossRef] [PubMed]
- Fitzmaurice, S.; Sivamani, R.; Isseroff, R. Antioxidant Therapies for Wound Healing: A Clinical Guide to Currently Commercially Available Products. Ski. Pharmacol. Physiol. 2011, 24, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanzadeh, B.; Nemati, M.; Behmanesh, M.A.; Hemmati, A.A.; Houshmand, G. Topical vitamin K1promotes repair of full thickness wound in rat. Indian J. Pharmacol. 2014, 46, 409–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunaway, S.; Odin, R.; Zhou, L.; Ji, L.; Zhang, Y.; Kadekaro, A.L. Natural Antioxidants: Multiple Mechanisms to Protect Skin from Solar Radiation. Front. Pharmacol. 2018, 9, 392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saewan, N.; Jimtaisong, A. Natural products as photoprotection. J. Cosmet. Dermatol. 2015, 14, 47–63. [Google Scholar] [CrossRef]
- F’Guyer, S.; Afaq, F.; Mukhtar, H. Photochemoprevention of skin cancer by botanical agents. Photodermatol. Photoimmunol. Photomed. 2003, 19, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Wijeratne, S.S.; Abou-Zaid, M.M.; Shahidi, F. Antioxidant Polyphenols in Almond and Its Coproducts. J. Agric. Food Chem. 2005, 54, 312–318. [Google Scholar] [CrossRef]
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2009, 302, 71–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katiyar, S.K.; Afaq, F.; Azizuddin, K.; Mukhtar, H. Inhibition of UVB-induced oxidative stress-mediated phosphorylation of mi-togen-activated protein kinase signaling pathways in cultured human epidermal keratinocytes by green tea polyphenol (−)-epigallocatechin-3-gallate. Toxicol. Appl. Pharmacol. 2001, 176, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Adhami, V.M.; Ahmad, N.; Mukhtar, H. Inhibition of ultraviolet B-mediated activation of nuclear factor κB in normal human epidermal keratinocytes by green tea Constituent (−)-epigallocatechin-3-gallate. Oncogene 2003, 22, 1035–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michna, L.; Lu, Y.-P.; Lou, Y.-R.; Wagner, G.C.; Conney, A.H. Stimulatory effect of oral administration of green tea and caffeine on locomotor activity in SKH-1 mice. Life Sci. 2003, 73, 1383–1392. [Google Scholar] [CrossRef]
- Laine, A.-L. Resistance variation within and among host populations in a plant-pathogen metapopulation: Implications for regional pathogen dynamics. J. Ecol. 2004, 92, 990–1000. [Google Scholar] [CrossRef]
- Afaq, F.; Adhami, V.M.; Ahmad, N. Prevention of short-term ultraviolet B radiation-mediated damages by resveratrol in SKH-1 hairless mice. Toxicol. Appl. Pharmacol. 2003, 186, 28–37. [Google Scholar] [CrossRef]
- Cao, C.; Lu, S.; Kivlin, R.; Wallin, B.; Card, E.; Bagdasarian, A.; Tamakloe, T.; Wang, W.J.; Song, X.; Chu, W.M.; et al. SIRT1 confers protection against UVB-and H2O2-induced cell death via modulation of p53 and JNK in cultured skin keratinocytes. J. Cell. Mol. Med. 2009, 13, 3632–3643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thimmulappa, R.K.; Mai, K.H.; Srisuma, S.; Kensler, T.W.; Yamamoto, M.; Biswal, S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002, 62, 5196–5203. [Google Scholar] [PubMed]
- Suh, J.H.; Shenvi, S.V.; Dixon, B.M.; Liu, H.; Jaiswal, A.K.; Liu, R.M.; Hagen, T.M. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc. Natl. Acad. Sci. USA 2004, 101, 3381–3386. [Google Scholar] [CrossRef] [Green Version]
- Kubo, E.; Chhunchha, B.; Singh, P.; Sasaki, H.; Singh, D.P. Sulforaphane reactivates cellular antioxidant defense by inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress. Sci. Rep. 2017, 7, 14130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandur, S.K.; Pandey, M.K.; Sung, B.; Ahn, K.S.; Murakami, A.; Sethi, G.; Limtrakul, P.; Badmaev, V.; Aggarwal, B.B. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis 2007, 28, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.Y.; Cai, X.F.; Lee, J.J.; Kang, S.S.; Shin, E.M.; Zhou, H.Y.; Jung, J.W.; Kim, Y.S. Comparison of suppressive effects of demethoxycur-cumin and bisdemethoxycurcumin on expressions of inflammatory mediators in vitro and in vivo. Arch. Pharm. Res. 2008, 31, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Xie, C.; Zhu, J.; Meng, Y.; Chen, Y.; Li, Y.; Jiang, Y.; Yang, X.; Wang, S.; et al. Sonic hedgehog and Wnt/β-catenin pathways mediate curcumin inhibition of breast cancer stem cells. Anti-Cancer Drugs 2018, 29, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Holzinger, A.; Ganzera, M.; Karsten, U. Prasiolin, a new UV-sunscreen compound in the terrestrial green macroalga Prasiola calophylla (Carmichael ex Greville) Kützing (Trebouxiophyceae, Chlorophyta). Planta 2016, 243, 161–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.; Cozad, M.A.; Holland, D.A.; Zhang, Y.; Luesch, H.; Ding, Y. Photosynthetic Production of Sunscreen Shinorine Using an Engineered Cyanobacterium. ACS Synth. Biol. 2018, 7, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-J.; Ko, S.-C.; Cha, S.-H.; Kang, D.-H.; Park, H.-S.; Choi, Y.-U.; Kim, D.; Jung, W.-K.; Jeon, Y.-J. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol. Vitr. 2009, 23, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-J.; Jeon, Y.-J. Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J. Photochem. Photobiol. B Biol. 2009, 95, 101–107. [Google Scholar] [CrossRef]
- Hur, S.; Lee, H.; Kim, Y.; Lee, B.-H.; Shin, J.; Kim, T.-Y. Sargaquinoic acid and sargachromenol, extracts of Sargassum sagamianum, induce apoptosis in HaCaT cells and mice skin: Its potentiation of UVB-induced apoptosis. Eur. J. Pharmacol. 2008, 582, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bałan, B.J.; Niemcewicz, M.; Kocik, J.; Jung, L.; Skopińska-Różewska, E.; Skopiński, P. Oral administration of Aloe vera gel, an-ti-microbial and anti-inflammatory herbal remedy, stimulates cell-mediated immunity and antibody production in a mouse model. Cent. Eur. J. Immunol. 2014, 39, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hęś, M.; Dziedzic, K.; Górecka, D.; Jędrusek-Golińska, A.; Gujska, E. Aloe vera (L.) Webb.: Natural sources of antioxidants—A review. Plant. Foods Hum. Nutr. 2019, 74, 255–265. [Google Scholar] [CrossRef] [Green Version]
- Hajheidari, Z.; Saeedi, M.; Morteza-Semnani, K.; Soltani, A. Effect of Aloe vera topical gel combined with tretinoin in treatment of mild and moderate acne vulgaris: A randomized, double-blind, prospective trial. J. Dermatol. Treat. 2013, 25, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Kitture, R.; Ghosh, S.; More, P.A.; Date, K.; Gaware, S.; Datar, S.; Chopade, B.A.; Kale, S.N. Curcumin-Loaded, Self-Assembled Aloevera Template for Superior Antioxidant Activity and Trans-Membrane Drug Release. J. Nanosci. Nanotechnol. 2015, 15, 4039–4045. [Google Scholar] [CrossRef] [PubMed]
- Arct, J.; Pytkowska, K. Flavonoids as components of biologically active cosmeceuticals. Clin. Dermatol. 2008, 26, 347–357. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.M.; Ruzindana-Umunyana, A.; Imbeault, L.; Sircar, S. Inhibition of adenovirus infection and adenain by green tea catechins. Antivir. Res. 2002, 58, 167–173. [Google Scholar] [CrossRef]
- Alvesalo, J.; Vuorela, H.; Tammela, P.; Leinonen, M.; Saikku, P. Inhibitory effect of dietary phenolic compounds on Chlamydia pneumoniae in cell cultures. Biochem. Pharmacol. 2006, 71, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Subarnas, A.; Wagner, H. Analgesic and anti-inflammatory activity of the proanthocyanidin shellegueain A from Polypodium feei METT. Phytomedicine 2000, 7, 401–405. [Google Scholar] [CrossRef]
- Mladěnka, P.; Zatloukalová, L.; Filipský, T.; Hrdina, R. Cardiovascular effects of flavonoids are not caused only by direct antiox-idant activity. Free Radic. Biol. Med. 2010, 49, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Kostelac, D.; Rechkemmer, G.; Briviba, K. Phytoestrogens Modulate Binding Response of Estrogen Receptors α and β to the Estrogen Response Element. J. Agric. Food Chem. 2003, 51, 7632–7635. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.J.M.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; Van Der Saag, P.T.; Van Der Burg, B.; Gustafsson, J. Interaction of Estrogenic Chemicals and Phytoestrogens with Estrogen Receptor β. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef] [PubMed]
- Guardia, T.; Rotelli, A.E.; Juarez, A.O.; Pelzer, L.E. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco 2001, 56, 683–687. [Google Scholar] [CrossRef]
- Virgili, F.; Kobuchi, H.; Packer, L. Nitrogen monoxide (NO) metabolism antioxidant properties and modulation of inducible NO synthase activity in activated macrophages by procyanidins extracted from Pinus maritima (Pycnogenol). Free Radic. Biol. Med. 1998, 24, 1120–1129. [Google Scholar] [CrossRef]
- Kempuraj, D.; Madhappan, B.; Christodoulou, S.; Boucher, W.; Cao, J.; Papadopoulou, N.; Cetrulo, C.L.; Theoharides, T.C. Flavonols inhibit proinflammatory mediator release, intracellular calcium ion levels and protein kinase C theta phosphorylation in human mast cells. Br. J. Pharmacol. 2005, 145, 934–944. [Google Scholar] [CrossRef] [Green Version]
- Marzocchella, L.; Fantini, M.; Benvenuto, M.; Masuelli, L.; Tresoldi, I.; Modesti, A.; Bei, R. Dietary Flavonoids: Molecular Mechanisms of Action as Anti- Inflammatory Agents. Recent Pat. Inflamm. Allergy Drug Discov. 2011, 5, 200–220. [Google Scholar] [CrossRef] [PubMed]
- Kumazawa, Y.; Takimoto, H.; Matsumoto, T.; Kawaguchi, K. Potential Use of Dietary Natural Products, Especially Polyphenols, for Improving Type-1 Allergic Symptoms. Curr. Pharm. Des. 2014, 20, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Takahashi, R. Flavonoids and Asthma. Nutrients 2013, 5, 2128–2143. [Google Scholar] [CrossRef]
- Finn, D.F.; Walsh, J.J. Twenty-first century mast cell stabilizers. Br. J. Pharmacol. 2013, 170, 23–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saklani, A.; Kutty, S. Plant-derived compounds in clinical trials. Drug Discov. Today 2008, 13, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Vasir, J.; Reddy, M.; Labhasetwar, V. Nanosystems in Drug Targeting: Opportunities and Challenges. Curr. Nanosci. 2005, 1, 47–64. [Google Scholar] [CrossRef]
- Musthaba, S.M.; Ahmad, S.; Ahuja, A.; Ali, J.; Baboota, S. Nano approaches to enhance pharmacokinetic and pharmacodynamic activity of plant origin drugs. Curr. Nanosci. 2009, 5, 344–352. [Google Scholar] [CrossRef]
- Raj, S.; Sumod, U.S.; Jose, S.; Sabitha, M. Nanotechnology in cosmetics: Opportunities and challenges. J. Pharm. Bioallied Sci. 2012, 4, 186–193. [Google Scholar] [CrossRef]
- Sanna, V.; Lubinu, G.; Madau, P.; Pala, N.; Nurra, S.; Mariani, A.; Sechi, M. Polymeric Nanoparticles Encapsulating White Tea Extract for Nutraceutical Application. J. Agric. Food Chem. 2015, 63, 2026–2032. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.C.; Choi, W.Y.; Seo, Y.C.; Kim, J.S.; Yoon, C.S.; Lim, H.W.; Kim, H.S.; Ahn, J.H.; Lee, H.Y. Enhancement of the Skin-Protective Activities of Centella asiatica L. Urban by a Nano-encapsulation Process. J. Biotechnol. 2012, 157, 100–106. [Google Scholar] [CrossRef]
- Gadkari, P.V.; Balaraman, M. Extraction of catechins from decaffeinated green tea for development of nanoemulsion using palm oil and sunflower oil based lipid carrier systems. J. Food Eng. 2015, 147, 14–23. [Google Scholar] [CrossRef]
- Gibis, M.; Zeeb, B.; Weiss, J. Formation, characterization, and stability of encapsulated hibiscus extract in multilayered liposomes. Food Hydrocoll. 2013, 38, 28–39. [Google Scholar] [CrossRef]
- Kaur, C.D.; Saraf, S. Topical vesicular formulations of Curcuma longa extract on recuperating the ultraviolet radiation-damaged skin. J. Cosmet. Dermatol. 2011, 10, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Moulaoui, K.; Caddeo, C.; Manca, M.L.; Castangia, I.; Valenti, D.; Escribano, E.; Atmani, D.; Fadda, A.M.; Manconi, M. Identification and nanoentrapment of polyphenolic phytocomplex from Fraxinus angustifolia: In vitro and in vivo wound healing potential. Eur. J. Med. Chem. 2015, 89, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Kitamoto, D.; Asikin, Y.; Takara, K.; Wada, K. Liposomes encapsulating Aloe vera leaf gel extract significantly en-hance proliferation and collagen synthesis in human skin cell lines. J. Oleo Sci. 2009, 58, 643–650. [Google Scholar] [CrossRef] [Green Version]
- Aisha, A.F.; Majid, A.M.; Ismail, Z. Preparation and characterization of nano liposomes of Orthosiphon stamineus ethanolic extract in soybean phospholipids. BMC Biotechnol. 2014, 14, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spigno, G.; Donsì, F.; Amendola, D.; Sessa, M.; Ferrari, G.; De Faveri, D.M. Nanoencapsulation systems to improve solubility and antioxidant efficiency of a grape marc extract into hazelnut paste. J. Food Eng. 2013, 114, 207–214. [Google Scholar] [CrossRef]
- Tsai, W.C.; Li, W.C.; Yin, H.Y.; Yu, M.C.; Wen, H.W. Constructing liposomal nanovesicles of ginseng extract against hydrogen per-oxide-induced oxidative damage to L929 cells. Food Chem. 2012, 132, 744–751. [Google Scholar] [CrossRef]
- Kwon, S.S.; Kim, S.Y.; Kong, B.J.; Kim, K.J.; Noh, G.Y.; Im, N.R.; Lim, J.W.; Ha, J.H.; Kim, J.; Park, S.N. Cell penetrating peptide conjugated liposomes as transdermal delivery system of Polygonum aviculare L. extract. Int. J. Pharm. 2015, 483, 26–37. [Google Scholar] [CrossRef]
- Mahdi, E.S.; Sakeena, M.H.; Abdulkarim, M.F.; Sattar, M.A.; Noor, A.M.; Abdullah, G.Z. Formulation and in vitro release evaluation of newly synthesized palm kernel oil esters-based nanoemulsion delivery system for 30% ethanolic dried extract derived from local Phyllanthus urinaria for skin antiaging. Int. J. Nanomed. 2011, 6, 2499–2512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorzi, G.K.; Caregnato, F.F.; Moreira, J.C.F.; Teixeira, H.F.; Carvalho, E.L.S. Antioxidant Effect of Nanoemulsions Containing Extract of Achyrocline satureioides (Lam) D.C.—Asteraceae. AAPS PharmSciTech 2015, 17, 844–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| S. No | Source | Antioxidant | Potential Activity | Reference |
|---|---|---|---|---|
| 1. | Apple | Phenolic compounds | Inhibitors of sulfotransferases, influence epigenetic processes and heritable changes not encoded in the DNA sequence, DNA protection against UV radiation | [50,51] |
| 2. | Baccharis species | Phenolic compounds | Inhibit reactive oxygen and nitrogen species (RONS), inhibit carrageenan induced edema | [52] |
| 3. | Basil leaves | Phenolic compounds | Antiacne, antiaging, remove dead skin cells | [53,54] |
| 4. | Blueberry pomace | Phenolic compounds | Enhance polyphenol oxidase activity, potent antioxidant | [55,56] |
| 5. | Cape gooseberry | Phenolic compounds and carotenoids | Anticoagulant, antispasmodic | [57,58] |
| 6. | Carrot | Carotenoids, anthocyanins | Protection from UV-induced lipid peroxidation, in treatment of erythropoietic protoporphyria | [59,60] |
| 7. | Chest nut | Polyphenols | Moisturizer, in treatment of oxidative stress-mediated diseases and photoaging | [61,62] |
| 8. | Coffee leaves | Chlorophylls and carotenoids | Antioxidant, antimicrobial, antiaging | [63,64] |
| 9. | Feijoa | Phenolic compounds | Antioxidant, antimicrobial | [65,66] |
| 10. | Ginkgo biloba leaves | Flavonoids | Prevent UVB-induced photoaging, anti-inflammatory, antioxidant, blood microcirculation | [67,68] |
| 11. | Goji berry | Phenolic compounds | Antioxidant, prevent skin aging, immunomodulatory | [69,70] |
| 12. | Goldenberry | Polyphenols | Anti-inflammatory, antiallergic | [71] |
| 13. | Grape | Anthocyanins and phenolic compounds | Protection from UV radiation, antioxidant and antiaging, depigmenting, anti-inflammatory, wound healing | [72,73] |
| 14. | Green algae | Carotenoids and phenolic compounds | Prevention of skin aging, protection from UVR, inhibition of melanogenesis, anti-inflammatory, antioxidant | [74,75] |
| 15. | Green propolis | Phenolic compounds | Anti-inflammatory, antimicrobial, wound healing | [76,77] |
| 16. | Jussara fruit | Phenolic compounds | Antioxidant, natural coolant | [78,79] |
| 17. | Kumquat peel | Phenols and flavonoids | Antioxidant, anti-inflammatory, skin lightening, suppression of lipid accumulation | [80,81] |
| 18. | Mango | Carotenoids | Wound healing, prevent skin aging, antioxidant | [82,83] |
| 19. | Myrtle | Phenolic compounds, flavonoids, and anthocyanins | Treatment of burn injury, anti-inflammatory, antifungal | [84,85] |
| 20. | Olive | Phenolic compounds | Antioxidant, anticancer, antiallergic, antiatherogenic, antimutagenic effects | [86,87] |
| 21. | Papaya seeds | Phenolic compounds | Antioxidant, insecticidal and repellent, antibacterial, wound healing, anti-inflammatory and immunomodulatory | [88,89] |
| 22. | Peach fruit | Flavonoids and phenolic compounds | Anticancer, antioxidant | [90,91] |
| 23. | Peel of egg plant | Phenolic compounds, flavonoids, tannins, and anthocyanins | Antioxidant, anti-inflammatory, antiviral and antimicrobial | [92] |
| 24. | Peppermint | Phenolic compound and essential oils | Antioxidant, antiaging | [93] |
| 25. | Pineapple | Polyphenols | Antimalarial, antinociceptive, and anti-inflammatory activities, improve skin barrier function | [94,95] |
| 26. | Pomegranate | Phenolic compounds | Anti-inflammatory, antioxidant, antimicrobial, promote hair follicles | [96,97] |
| 27. | Propolis | Phenolic compounds | Wound healing, immunomodulatory, anti-inflammatory | [98,99] |
| 28. | Red Macroalgae | Proteins, polyphenols and polysaccharides | Prevent skin-aging processes, promote transepidermal water loss, simulate sebum content, and increase erythema and melanin production | [100,101] |
| 29. | Bananas | Phenolic compounds and flavonoids | Provide UV protection, antimicrobial, wound healing | [102,103] |
| 30. | Spent grain | Phenolic compounds | Antioxidant, skin lightening, anti-inflammatory | [104,105] |
| 31. | Turmeric | Phenolic compounds | Anti-inflammatory, antioxidant, treatment of psoriasis | [106,107] |
| 32. | Strawberry | Anthocyanins and phenolic compounds | Antimicrobial, antioxidant, antiaging | [108,109] |
| 33. | Sweet potato | Polyphenols and anthocyanins | Antioxidant, wound healing, serve as natural, safe and effective colorants, antimicrobial, antifungal | [110,111] |
| 34. | Tomato | Flavonoids and lycopene | Antioxidant, protection from cell damage, provide protection against UV rays, wound repair | [112,113] |
| 35. | Horse radish | Phenolic compounds and flavonoids | Antimicrobial, antioxidants | [114] |
| 36. | Withania somnifera | Phenolic compounds | Antioxidant, skin whitening | [115,116] |
| S. No. | Vitamin A and Its Derivatives | Functions | Application | References |
|---|---|---|---|---|
| 1. | Retinol | Inhibits collagenase and the expression of MMP, stimulates GAGS synthesis and collagen type 1 | Used in dyspigmentation, dryness, anti-wrinkle treatment | [137] |
| 2. | Retinoic acid | Reduces inflammation in sebaceous glands, inhibits keratosis, stimulates epidermal cell proliferation | Used in treatment of psoriasis, chronic inflammation of hair | [138] |
| 3. | Retinyl acetate and palmitate | Stimulates epidermal cell proliferation, regulation of sebum, coverts into retinoid acid | Stabilizes properties in wrinkle treatment, acts as antioxidant | [139] |
| 4. | Retinaldehyde | Stimulates epidermal cell proliferation, oxidizes into retinoic acid | Works as stabilizer in treatment of wrinkle | [140] |
| 5. | Naphthalenecarboxylic acid | Acts as a strong modulator for keratinization in hair follicles, increases proliferation, changes expression of genes and synthesis of mRNAt | Reduces inflammation, acne, excessive keratosis | [141] |
| 6. | Tazarotene | Regulates keratinocyte differentiation, proliferation, and inflammation | Used in treatment of psoriasis and acne, works as photoprotection from sunlight | [142] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoang, H.T.; Moon, J.-Y.; Lee, Y.-C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics 2021, 8, 106. https://doi.org/10.3390/cosmetics8040106
Hoang HT, Moon J-Y, Lee Y-C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics. 2021; 8(4):106. https://doi.org/10.3390/cosmetics8040106
Chicago/Turabian StyleHoang, Hien Thi, Ju-Young Moon, and Young-Chul Lee. 2021. "Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives" Cosmetics 8, no. 4: 106. https://doi.org/10.3390/cosmetics8040106
APA StyleHoang, H. T., Moon, J.-Y., & Lee, Y.-C. (2021). Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics, 8(4), 106. https://doi.org/10.3390/cosmetics8040106







