Curcuma aromatica and Curcuma comosa Extracts and Isolated Constituents Provide Protection against UVB-Induced Damage and Attenuate Matrix Metalloproteinase-1 Expression in HaCaT Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Plant Materials
2.3. Preparation of Plant Extracts
2.4. Total Phenolic Content
2.5. Antioxidant Assays
2.5.1. DPPH Free Radical Scavenging Assay
2.5.2. FRAP Radical Scavenging Assay
2.5.3. ABTS Radical Scavenging Assay
2.5.4. NO Radical Scavenging Assay
2.6. Isolation of Marker Compounds from Crude Extracts
2.7. Quantification of Marker Compounds in Crude Extracts using High-Performance Liquid Chromatography-Diode Array (HPLC-DAD)
2.8. Validation of HPLC Analysis
2.9. Cell Line and Culture Conditions
2.10. UV Irradiation
2.11. Cell Viability
2.12. Cell Proliferation
2.13. Enzyme-Linked Immunosorbent Assay (ELISA)
2.14. Cell-cycle by Flow Cytometry
2.15. Statistical Analysis
3. Results and Discussion
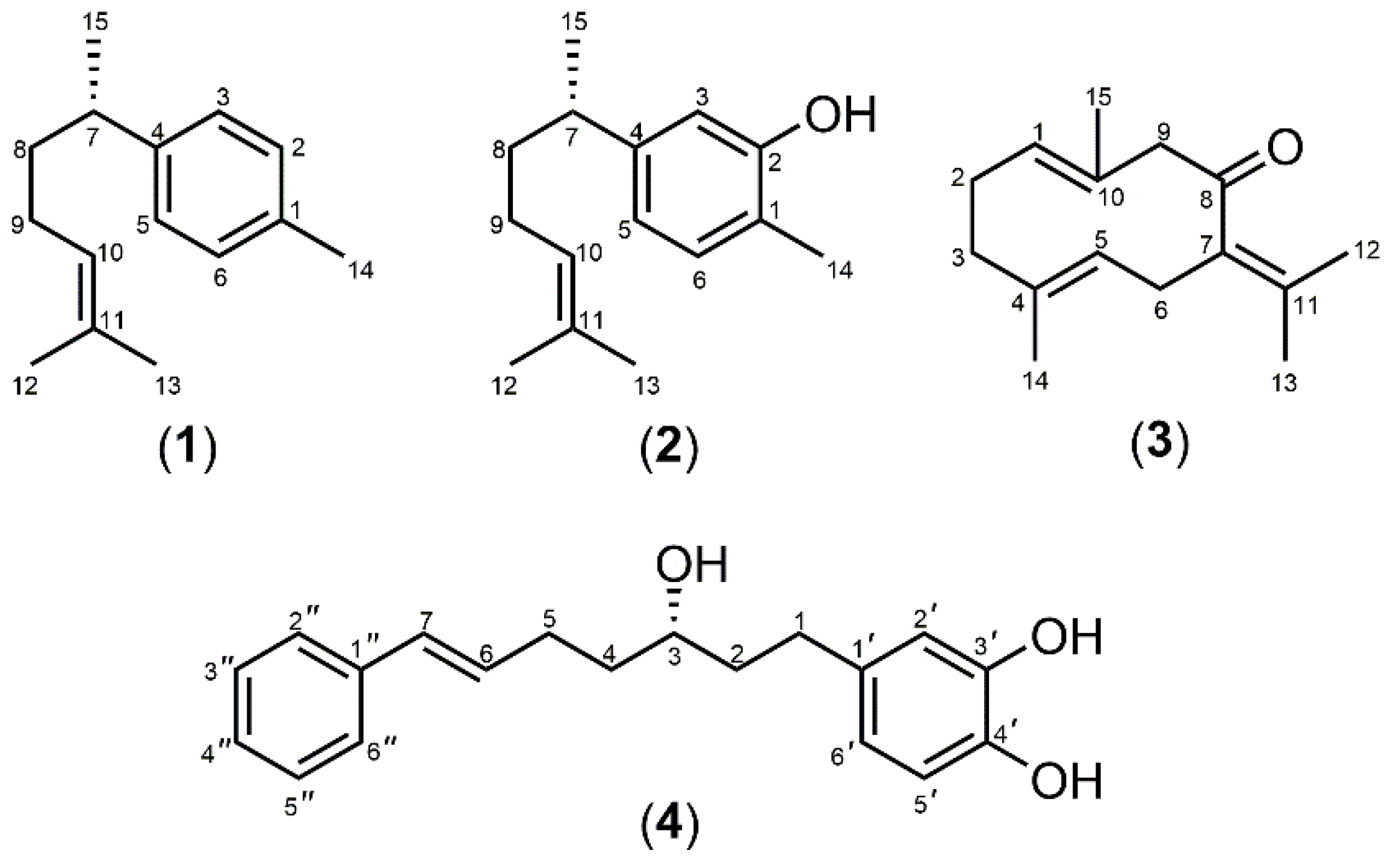
3.1. Extraction and Isolation
3.2. Antioxidant Activities of CA and CC Extracts
3.3. Validation and Quantitative Analysis of Marker Compounds by using HPLC
3.4. Selection of Optimal UVB Dose
3.5. Cytotoxicity of the Extracts to Human HaCat Keratinocytes
3.6. Evaluation of the Extracts and Isolated Constituents on Cell Proliferation and MMP-1 after UVB Irradiation in Human HaCaT Keratinocyte Cells
3.7. Cell Cycle Arrest of UVB-Exposed Cells to the Extracts and Isolated Constituents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Dermatoendocrinology 2012, 4, 308–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cevenini, E.; Invidia, L.; Lescai, F.; Salvioli, S.; Tieri, P.; Castellani, G.; Franceschi, C. Human models of aging and longevity. Expert Opin. Biol. Ther. 2008, 8, 1393–1405. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Piao, M.J.; Han, X.; Kang, K.A.; Kang, H.K.; Yoon, W.J.; Ko, M.H.; Lee, N.H.; Lee, M.Y.; Chae, S.; et al. Anti-wrinkle effects of Sargassum muticum ethyl acetate fraction on ultraviolet B-irradiated hairless mouse skin and mechanistic evaluation in the human HaCaT keratinocyte cell line. Mol. Med. Rep. 2016, 14, 2937–2944. [Google Scholar] [CrossRef] [Green Version]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The skin aging exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef] [Green Version]
- Pambianchi, E.; Hagenberg, Z.; Pecorelli, A.; Grace, M.; Therrien, J.-P.; Lila, M.A.; Valacchi, G. Alaskan bog blueberry (Vaccinium uliginosum) extract as an innovative topical approach to prevent UV-induced skin damage. Cosmetics 2021, 8, 112. [Google Scholar] [CrossRef]
- Morocho-Jácome, A.L.; Freire, T.B.; de Oliveira, A.C.; de Almeida, T.S.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. In vivo SPF from multifunctional sunscreen systems developed with natural compounds–A review. J. Cosmet. Dermatol. 2021, 20, 729–737. [Google Scholar] [CrossRef]
- Kwon, K.-R.; Alam, M.B.; Park, J.-H.; Kim, T.-H.; Lee, S.-H. Attenuation of UVB-induced photo-aging by polyphenolic-rich Spatholobus suberectus stem extract via modulation of MAPK/AP-1/MMPs signaling in human keratinocytes. Nutrients 2019, 11, 1341. [Google Scholar] [CrossRef] [Green Version]
- Verma, R.P.; Hansch, C. Matrix metalloproteinases (MMPs): Chemical-biological functions and (Q)SARs. Bioorg. Med. Chem. 2007, 15, 2223–2268. [Google Scholar] [CrossRef]
- Jabłońska-Trypuć, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzyme Inhib. Med. Chem. 2016, 31, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Dong, K.K.; Damaghi, N.; Picart, S.D.; Markova, N.G.; Obayashi, K.; Okano, Y.; Masaki, H.; Grether-Beck, S.; Krutmann, J.; Smiles, K.A.; et al. UV-induced DNA damage initiates release of MMP-1 in human skin. Exp. Dermatol. 2008, 17, 1037–1044. [Google Scholar] [CrossRef]
- Kim, C.; Ryu, H.C.; Kim, J.H. Low-dose UVB irradiation stimulates matrix metalloproteinase-1 expression via a BLT2-linked pathway in HaCaT cells. Exp. Mol. Med. 2010, 42, 833–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, J.Y.; Na, E.J. MMP expression alteration and MMP-1 production control by syringic acid via AP-1 mechanism. Biomed. Dermatol. 2018, 2, 15. [Google Scholar] [CrossRef] [Green Version]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuroyanagi, M.; Ueno, A.; Ujiie, K.; Sato, S. Structures of sesquiterpenes from Curcuma aromatica Salisb. Chem. Pharm. Bull. 1987, 35, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Al-Reza, S.M.; Rahman, A.; Sattar, M.A.; Rahman, M.O.; Fida, H.M. Essential oil composition and antioxidant activities of Curcuma aromatica Salisb. Food Chem. Toxicol. 2010, 48, 1757–1760. [Google Scholar] [CrossRef]
- Ahmed, S.; Ansari, S.H.; Ali, M.; Bhatt, D.; Ansari, F. Phytochemical and biological investigations on Curcuma aromatica. Pharmacog. Rev. 2008, 2, 151–156. [Google Scholar]
- Pintatum, A.; Maneerat, W.; Logie, E.; Tuenter, E.; Sakavitsi, M.E.; Pieters, L.; Berghe, W.V.; Sripisut, T.; Deachathai, S.; Laphookhieo, S. In vitro anti-inflammatory, antioxidant, and cytotoxic activities of four Curcuma species and the isolation of compounds from Curcuma aromatica rhizome. Biomolecules 2020, 10, 799. [Google Scholar] [CrossRef]
- Sun, W.; Wang, S.; Zhao, W.; Wu, C.; Guo, S.; Gao, H.; Tao, H.; Lu, J.; Wang, Y.; Chen, X. Chemical constituents and biological research on plants in the genus Curcuma. Crit. Rev. Food Sci. Nutr. 2016, 57, 1451–1523. [Google Scholar] [CrossRef]
- Soontornchainaksaeng, P.; Jenjittikul, T. Chromosome number variation of phytoestrogen-producing Curcuma (Zingiberaceae) from Thailand. J. Nat. Med. 2010, 64, 370–377. [Google Scholar] [CrossRef]
- Piyachaturawat, P.; Ercharuporn, S.; Suksamrarn, A. Uterotrophic effect of Curcuma comosa in rats. Int. J. Pharmacogn. 1995, 33, 334–338. [Google Scholar] [CrossRef]
- Piyachaturawat, P.; Ercharuporn, S.; Suksamrarn, A. Estrogenic activity of Curcuma comosa extract in rats. Asia Pac. J. Pharmacol. 1995, 10, 121–126. [Google Scholar]
- Winuthayanon, W.; Piyachaturawat, P.; Suksamrarn, A.; Ponglikitmongkol, M.; Arao, Y.; Hewitt, S.C.; Korach, K.S. Diarylheptanoid phytoestrogens isolated from the medicinal plant Curcuma comosa: Biological actions in vitro and in vivo indicate estrogen receptor-dependent mechanisms. Environ. Health Persp. 2009, 117, 1155–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suksamrarn, A.; Ponglikitmongkol, M.; Wongkrajang, K.; Chindaduang, A.; Kittidanairak, S.; Jankam, A.; Yingyongnarongkul, B.; Kittipanumat, N.; Chokchaisiri, R.; Khetkam, P.; et al. Diarylheptanoids, new phytoestrogens from the rhizomes of Curcuma comosa: Isolation, chemical modification and estrogenic activity evaluation. Bioorg. Med. Chem. 2008, 16, 6891–6902. [Google Scholar] [CrossRef]
- Piyachaturawat, P.; Charoenpiboonsin, J.; Toskulkao, C.; Suksamrarn, A. Reduction of plasma cholesterol by Curcuma comosa extract in hypercholesterolaemic hamsters. J. Ethnopharmacol. 1999, 66, 199–204. [Google Scholar] [CrossRef]
- Sutjarit, N.; Sueajai, J.; Boonmuen, N.; Sornkaew, N.; Suksamrarn, A.; Tuchinda, P.; Zhu, W.; Weerachayaphorn, J.; Piyachaturawat, P. Curcuma comosa reduces visceral adipose tissue and improves dyslipidemia in ovariectomized rats. J. Ethnopharmacol. 2017, 215, 167–175. [Google Scholar] [CrossRef]
- Tantikanlayaporn, D.; Wichit, P.; Weerachayaphorn, J.; Chairoungdua, A.; Chuncharunee, A.; Suksamrarn, A.; Piyachaturawat, P. Bone sparing effect of a novel phytoestrogen diarylheptanoid from Curcuma comosa Roxb. in ovariectomized rats. PLoS ONE 2013, 8, e78739. [Google Scholar] [CrossRef]
- Tanhuad, N.; Thongsa-Ad, U.; Sutjarit, N.; Yoosabai, P.; Panvongsa, W.; Wongniam, S.; Suksamrarn, A.; Piyachaturawat, P.; Anurath-apan, U.; Borwornpinyo, S.; et al. Ex vivo expansion and functional activity preservation of adult hematopoietic stem cells by a diarylheptanoid from Curcuma comosa. Biomed. Pharm. 2021, 143, 112102. [Google Scholar] [CrossRef]
- Chuaicharoen, P.; Charaslertrangsi, T.; Chuncharunee, A.; Suksamrarn, A.; Piyachaturawat, P. Non-phenolic diarylheptanoid from Curcuma comosa protects against thioacetamide-induced acute hepatotoxicity in mice. Pharm. Sci. Asia 2020, 47, 74–85. [Google Scholar] [CrossRef]
- Jantaratnotai, N.; Utaisincharoen, P.; Piyachaturawat, P.; Chongthammakun, S.; Sanvarinda, Y. Inhibitory effect of Curcuma comosa on NO production and cytokine expression in LPS-activated microglia. Life Sci. 2006, 78, 571–577. [Google Scholar] [CrossRef]
- Sodsai, A.; Piyachaturawat, P.; Sophasan, A.; Suksamrarn, A.; Vongsakul, M. Suppression by Curcuma comosa Roxb. of pro-inflammatory cytokine secretion in phorbol-12-myristate-13-acetate stimulated human mononuclear cells. Int. Immunopharmacol. 2007, 7, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Nakyai, W.; Pabuprapap, W.; Sroimee, W.; Ajavakom, V.; Yingyongnarongkul, B.; Suksamrarn, A. Anti-acne vulgaris potential of the ethanolic extract of Mesua ferrea L. flowers. Cosmetics 2021, 8, 107. [Google Scholar] [CrossRef]
- Ehara, T.; Tanikawa, S.; Ono, M.; Akita, H. Synthesis of (R)-curcumene and (R)-xanthorrizol based on 1,2-aryl migration via phenonium ion. Chem. Pharm. Bull. 2007, 55, 1361–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chavan, S.P.; Thakkar, M.; Kalkote, U.R. An enantiospecific synthesis of (+)-isoparvifolinone and (−)-parvifoline. Tetrahedron Lett. 2007, 48, 535–537. [Google Scholar] [CrossRef]
- Barrero, A.F.; Herrador, M.M.; López-Pérez, J.-L.; Arteaga, J.F.; Catalán, J. New pathways in transannular cyclization of germacrone [germacra-1(10),4,7(11)-trien-8-one]: Evidence regarding a concerted mechanism. Org. Lett. 2009, 11, 4782–4785. [Google Scholar] [CrossRef]
- Demirci-Çekiça, S.; Özkan, G.; Avan, A.N.; Uzunboy, S.; Çapanoğlu, E.; Apak, R. Biomarkers of oxidative stress and antioxidant defense. J. Pharm. Biomed. Anal. 2022, 209, 114477. [Google Scholar] [CrossRef]
- Tiraravesit, N.; Yakaew, S.; Rukchay, R.; Luangbudnark, W.; Viennet, C.; Humbert, P.; Viyoch, J. Artocarpus altilis heartwood extract protects skin against UVB in vitro and in vivo. J. Ethnopharmacol. 2015, 175, 153–162. [Google Scholar] [CrossRef]
- Kwon, S.H.; Wang, Z.; Hwang, S.H.; Kang, Y.-H.; Lee, J.-Y.; Lim, S.S. Comprehensive evaluation of the antioxidant capacity of Perilla frutescens leaves extract and isolation of free radical scavengers using step-wise HSCCC guided by DPPH-HPLC. Int. J. Food Prop. 2017, 20, 921–934. [Google Scholar] [CrossRef] [Green Version]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant activity/capacity measurement. 1. classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Kuntasal, O.O.; Karman, D.; Wang, D.; Tuncel, S.G.; Tuncel, G. Determination of volatile organic compounds in different microenvironments by multibed adsorption and short-path thermal desorption followed by gas chromatographic-mass spectrometric analysis. J. Chromatogr. A 2005, 1099, 43–54. [Google Scholar] [CrossRef]
- Huber, L. Validation and Qualification in Analytical Laboratories, 2nd ed.; Informa Healthcare USA, Inc.: New York, NY, USA, 2007; pp. 125–154. [Google Scholar]
- Behzadi, M.A.; Choi, A.; Duehr, J.; Feyznezhad, R.; Upadhyay, C.; Schotsaert, M.; Palese, P.; Nachbagauer, R. A cross-reactive mouse monoclonal antibody against rhinovirus mediates phagocytosis in vitro. Sci. Rep. 2020, 10, 9750. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.-I.; Shim, J.-S.; Gwon, S.-H.; Kwon, H.-J.; Hwang, J.-K. The effect of xanthorrhizol on the expression of matrix metalloproteinase-1 and type-I procollagen in ultraviolet-irradiated human skin fibroblasts. Phytother. Res. 2009, 23, 1299–1302. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Mohamed, M.A.A.; Jung, Y.-J.; Shrestha, S.; Lee, T.H.; Lee, C.-H.; Han, D.; Kim, J.; Baek, N.-I. Germacrane sesquiterpenes isolated from the rhizome of Curcuma xanthorrhiza Roxb. inhibit UVB-induced upregulation of MMP-1, -2, and -3 expression in human keratinocytes. Arch. Pharm. Res. 2014, 38, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, J.; Li, X.; Sun, N.; Yu, R.; Zhao, B.; Yu, D.; Cheng, Y.; Liu, Y. Huaier aqueous extract induces hepatocellular carcinoma cells arrest in S phase via JNK signaling pathway. Evid. Based Complement. Altern. Med. 2015, 171356, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, N.; Chen, X.; Rui, J.; Yu, Y.; Gu, D.; Ruan, J.J.; Ruan, B.H. Cell growth measurement. In Cell Growth; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef] [Green Version]
- Tiraravesit, N.; Humbert, P.; Robin, S.; Tissot, M.; Viennet, C.; Viyoch, J. Artocarpin-enriched (Artocarpus altilis) heartwood extract provides protection against UVB-induced mechanical damage in dermal fibroblasts. Photochem. Photobiol. 2017, 93, 1232–1239. [Google Scholar] [CrossRef]
- Nakyai, W.; Saraphanchotiwitthaya, A.; Viennet, C.; Humbert, P.; Viyoch, J. An in vitro model for fibroblast photoaging comparing single and repeated UVA irradiations. Photochem. Photobiol. 2017, 93, 1462–1471. [Google Scholar] [CrossRef]
- Deng, M.; Li, D.; Zhang, Y.; Zhou, G.; Liu, W.; Cao, Y.; Zhang, W. Protective effect of crocin on ultraviolet B-induced dermal fibroblast photoaging. Mol. Med. Rep. 2018, 18, 1439–1446. [Google Scholar] [CrossRef] [Green Version]









| Sample | Total Phenolic Contents (µg GAE/mg) | Antioxidant Activities (IC50, µg/mL) | |||
|---|---|---|---|---|---|
| DPPH | ABTS | FRAP | NO | ||
| CA extract | 90.89 ± 0.14 | 78.77 ± 1.02 | 19.17 ± 0.07 | 86.85 ± 1.11 | 159.90 ± 1.04 |
| CC extract | 186.99 ± 0.01 | 21.22 ± 1.01 | 2.67 ± 1.02 | 32.47 ± 1.04 | 0.26 ± 1.04 |
| L-Ascorbic acid 1 | - | 5.15 ± 1.04 | 0.86 ± 1.03 | 2.06 ± 1.56 | 0.04 ± 1.04 |
| Compounds | Linearity | Correlation Coefficient (R²) | LOD (µg/mL) | LOQ (µg/mL) | % RSD | % Recovery | |
|---|---|---|---|---|---|---|---|
| Inter-Day (n = 9) | Intra-Day (n = 9) | ||||||
| 1 | y = 91.53x + 427.86 | 0.9990 | 1.48 | 4.72 | 0.45 ± 0.09 | 0.63 ± 0.30 | 98.93 ± 1.06 |
| 2 | y = 89.04x + 272.38 | 0.9992 | 0.89 | 2.85 | 1.17 ± 0.16 | 0.58 ± 0.39 | 101.79 ± 1.48 |
| 3 | y = 98.72x + 560.04 | 0.9992 | 0.42 | 1.34 | 1.30 ± 0.35 | 0.65 ± 0.10 | 100.43 ± 0.35 |
| 4 | y = 174.91x + 165.03 | 0.9997 | 0.05 | 0.15 | 0.98 ± 0.18 | 1.53 ± 0.59 | 99.13 ± 0.60 |
| Sample | % of Total Cell Cycle Population in: | ||
|---|---|---|---|
| G0/G1 | S | G2/M | |
| Non-UVB-treated cells | 59.64 ± 2.01 | 7.96 ± 0.05 | 32.05 ± 2.09 |
| UVB-treated cells | 64.47 ± 0.79 | 13.73 ± 0.11 | 21.17 ± 0.81 |
| CA extract | 61.73 ± 1.24 | 10.93 ± 1.28 | 26.77 ± 2.52 |
| Compound 1 | 62.28 ± 0.42 | 10.03 ± 0.54 | 27.15 ± 0.81 |
| Compound 2 | 61.25 ± 0.54 | 11.34 ± 1.17 | 26.83 ± 1.29 |
| Compound 3 | 62.69 ± 0.86 | 9.76 ± 0.10 | 26.95 ± 0.96 |
| CC extract | 63.31 ± 0.37 | 11.15 ± 0.56 | 24.98 ± 0.22 |
| Compound 4 | 63.35 ± 0.19 | 10.40 ± 1.29 | 25.74 ± 2.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pabuprapap, W.; Nakyai, W.; Chaichompoo, W.; Pheedee, N.; Phetkeereerat, S.; Viyoch, J.; Yingyongnarongkul, B.-e.; Ajavakom, V.; Chompoosor, A.; Piyachaturawat, P.; et al. Curcuma aromatica and Curcuma comosa Extracts and Isolated Constituents Provide Protection against UVB-Induced Damage and Attenuate Matrix Metalloproteinase-1 Expression in HaCaT Cells. Cosmetics 2022, 9, 23. https://doi.org/10.3390/cosmetics9010023
Pabuprapap W, Nakyai W, Chaichompoo W, Pheedee N, Phetkeereerat S, Viyoch J, Yingyongnarongkul B-e, Ajavakom V, Chompoosor A, Piyachaturawat P, et al. Curcuma aromatica and Curcuma comosa Extracts and Isolated Constituents Provide Protection against UVB-Induced Damage and Attenuate Matrix Metalloproteinase-1 Expression in HaCaT Cells. Cosmetics. 2022; 9(1):23. https://doi.org/10.3390/cosmetics9010023
Chicago/Turabian StylePabuprapap, Wachirachai, Wongnapa Nakyai, Waraluck Chaichompoo, Nattharika Pheedee, Saowanee Phetkeereerat, Jarupa Viyoch, Boon-ek Yingyongnarongkul, Vachiraporn Ajavakom, Apiwat Chompoosor, Pawinee Piyachaturawat, and et al. 2022. "Curcuma aromatica and Curcuma comosa Extracts and Isolated Constituents Provide Protection against UVB-Induced Damage and Attenuate Matrix Metalloproteinase-1 Expression in HaCaT Cells" Cosmetics 9, no. 1: 23. https://doi.org/10.3390/cosmetics9010023
APA StylePabuprapap, W., Nakyai, W., Chaichompoo, W., Pheedee, N., Phetkeereerat, S., Viyoch, J., Yingyongnarongkul, B.-e., Ajavakom, V., Chompoosor, A., Piyachaturawat, P., & Suksamrarn, A. (2022). Curcuma aromatica and Curcuma comosa Extracts and Isolated Constituents Provide Protection against UVB-Induced Damage and Attenuate Matrix Metalloproteinase-1 Expression in HaCaT Cells. Cosmetics, 9(1), 23. https://doi.org/10.3390/cosmetics9010023






