An Overview of Wearable Piezoresistive and Inertial Sensors for Respiration Rate Monitoring
Abstract
:1. Introduction
- A comprehensive overview of the methodologies, materials, and techniques applied to piezoresistive breathing sensors. Specifically, novel IoT-based wearable devices for monitoring respiration activity are discussed and analyzed [19,39]. Also, innovative piezoresistive materials are introduced, analyzing their manufacturing processes and improvements enhancing their performances or reduce production costs or, last but not least, improve user’s experience by making the sensor more comfortable. Furthermore, we report a comparative analysis of discussed piezoresistive devices to define the features and functionalities of the next generation of RR sensors.
- An accurate survey of IoT-based wearable devices using inertial sensors (accelerometers, gyroscope, magnetometer, etc.) are analyzed for detecting the breathing movements and thus extracting the respiration rate [40,41,42]. Several embedded systems are proposed in the scientific literature, including one or more inertial sensors, a processing unit, and a communication module for wirelessly transmits the acquired data toward a host device or cloud platform, allowing remote monitoring of user’s conditions [43,44]. Furthermore, an overview of the main algorithms for extracting the respiratory rate from the raw inertial data is reported. Finally, a comparison of discussed devices based on inertial sensors is reported.
2. Review of Innovative Piezoresistive System and Materials for Detecting the Respiration Rate
2.1. A Survey of Innovative Piezoresistive Sensing Systems for Monitoring the Respiratory Activity
2.2. Overview of Smart Piezoresistive Textiles and Materials Used to Monitor Respiration Rate
3. State of the Art on Systems for Respiration Monitoring Based on Inertial Sensors
3.1. Overview of Innovative Wearable Systems Based on Inertial Sensors to Monitor the Respiratory Activity
3.2. A Survey of Algorithms to Measure Respiration Rate Using Inertial Sensors
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iqbal, M.H.; Aydin, A.; Brunckhorst, O.; Dasgupta, P.; Ahmed, K. A review of wearable technology in medicine. J. R. Soc. Med. 2016, 109, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, B.; Velázquez, R.; Del-Valle-Soto, C.; de Fazio, R.; Giannoccaro, N.I.; Visconti, P. Solar-powered deep learning-based recognition system of daily used objects and human faces for assistance of the visually impaired. Energies 2020, 13, 6104. [Google Scholar] [CrossRef]
- Ennafiri, M.; Mazri, T. Internet of things for smart healthcare: A review on a potential IOT based system and technologies to control covid-19 pandemic. Innov. Smart Cities Appl. Vol. 4 2020, 183, 1256–1269. [Google Scholar]
- Kadhim, K.T.; Alsahlany, A.M.; Wadi, S.M.; Kadhum, H.T. An overview of patient’s health status monitoring system based on internet of things (IoT). Wirel. Pers. Commumun. 2020, 114, 2235–2262. [Google Scholar] [CrossRef]
- De Fazio, R.; Sponziello, A.; Cafagna, D.; Velazquez, R.; Visconti, P. An overview on technologies and devices against covid-19 pandemic diffusion: Virus detection and monitoring solutions. Int. J. Smart Sens. Intell. Syst. 2021, 14, 1–28. [Google Scholar] [CrossRef]
- Molinaro, N.; Massaroni, C.; Lo Presti, D.; Saccomandi, P.; di Tomaso, G.; Zollo, L.; Perego, P.; Andreoni, G.; Schena, E. Wearable textile based on silver plated knitted sensor for respiratory rate monitoring. In Proceedings of the 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 2865–2868. [Google Scholar]
- Reinvuo, T.; Hannula, M.; Sorvoja, H.; Alasaarela, E.; Myllyla, R. Measurement of respiratory rate with high-resolution accelerometer and emfit pressure sensor. In Proceedings of the IEEE Sensors Applications Symposium, Houston, TX, USA, 7–9 February 2006; pp. 192–195. [Google Scholar]
- Fang, Y.; Jiang, Z.; Wang, H. A novel sleep respiratory rate detection method for obstructive sleep apnea based on characteristic moment waveform. J. Healthc. Eng. 2018, 2018, 1902176. [Google Scholar] [CrossRef]
- Dieffenderfer, J.; Goodell, H.; Mills, S.; McKnight, M.; Yao, S.; Lin, F.; Beppler, E.; Bent, B.; Lee, B.; Misra, V.; et al. Low-power wearable systems for continuous monitoring of environment and health for chronic respiratory disease. IEEE J. Biomed. Health Inf. 2016, 20, 1251–1264. [Google Scholar] [CrossRef]
- Li, S.-H.; Lin, B.-S.; Tsai, C.-H.; Yang, C.-T.; Lin, B.-S. Design of wearable breathing sound monitoring system for real-time wheeze detection. Sensors 2017, 17, 171. [Google Scholar] [CrossRef] [Green Version]
- Ionescu, C.-M.; Copot, D. Monitoring respiratory impedance by wearable sensor device: Protocol and methodology. Biomed. Signal Process. Control 2017, 36, 57–62. [Google Scholar] [CrossRef]
- Singh, O.P.; Howe, T.A.; Malarvili, M.B. Real-Time human respiration carbon dioxide measurement device for cardiorespiratory assessment. J. Breath Res. 2018, 12, 026003. [Google Scholar] [CrossRef] [Green Version]
- Vincent, T.A.; Urasinska-Wojcik, B.; Gardner, J.W. Development of a low-cost NDIR System for ppm detection of carbon dioxide in exhaled breath analysis. Procedia Eng. 2015, 120, 388–391. [Google Scholar] [CrossRef] [Green Version]
- Krehel, M.; Schmid, M.; Rossi, R.M.; Boesel, L.F.; Bona, G.-L.; Scherer, L.J. An optical fibre-based sensor for respiratory monitoring. Sensors 2014, 14, 13088–13101. [Google Scholar] [CrossRef] [Green Version]
- Yatani, K.; Truong, K.N. BodyScope: A wearable acoustic sensor for activity recognition. In Proceedings of the 2012 ACM Conference on Ubiquitous Computing, Pittsburgh, PA, USA, 5–8 September 2012; Association for Computing Machinery: New York, NY, USA, 2012; pp. 341–350. [Google Scholar]
- Yuasa, Y.; Takahashi, K.; Suzuki, K. Wearable flexible device for respiratory phase measurement based on sound and chest movement. In Proceedings of the 2017 IEEE International Conference on Systems, Man, and Cybernetics (SMC), Banff, AB, Canada, 5–8 October 2017; pp. 2378–2383. [Google Scholar]
- Esfahani, M.I.M.; Narimani, R.; Ramezanzadehkoldeh, M. A wearable respiratory plethysmography using flexible sensor. Int. J. Biomed. Eng. Technol. 2013, 11, 364–380. [Google Scholar] [CrossRef]
- Jubran, A. Pulse oximetry. Crit. Care 2015, 19, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Aliverti, A. Wearable technology: Role in respiratory health and disease. Breathe 2017, 13, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Charlton, P.H.; Birrenkott, D.A.; Bonnici, T.; Pimentel, M.A.F.; Johnson, A.E.W.; Alastruey, J.; Tarassenko, L.; Watkinson, P.J.; Beale, R.; Clifton, D.A. Breathing rate estimation from the electrocardiogram and photoplethysmogram: A review. IEEE Rev. Biomed. Eng. 2018, 11, 2–20. [Google Scholar] [CrossRef] [Green Version]
- Jortberg, E.; Silva, I.; Bhatkar, V.; McGinnis, R.S.; Sen-Gupta, E.; Morey, B.; Wright, J.A.; Pindado, J.; Bianchi, M.T. A novel adhesive biosensor system for detecting respiration, cardiac, and limb movement signals during sleep: Validation with polysomnography. Nat. Sci. Sleep 2018, 10, 397–408. [Google Scholar] [CrossRef] [Green Version]
- Moussavi, Z.K.; Leopando, M.T.; Pasterkamp, H.; Rempel, G. Computerised acoustical respiratory phase detection without airflow measurement. Med. Biol. Eng. Comput. 2000, 38, 198–203. [Google Scholar] [CrossRef]
- Vahdatpour, A.; Amini, N.; Xu, W.; Sarrafzadeh, M. Accelerometer-based on-body sensor localization for health and medical monitoring applications. Pervasive Mob. Comput. 2011, 7, 746–760. [Google Scholar] [CrossRef] [Green Version]
- Hou, C.-L.; Wu, Y.; Zeng, X.; Zhao, S.; Zhou, Q.; Yang, G. Novel high sensitivity accelerometer based on a microfiber loop resonator. Opt. Eng. 2010, 49, 014402. [Google Scholar] [CrossRef]
- Gomathi, T.; Shaby, S.M. Capacitive accelerometers for microelectromechanical applications: A review. In Proceedings of the 2016 International Conference on Control, Instrumentation, Communication and Computational Technologies (ICCICCT), Kumaracoil, India, 16–17 December 2016; IEEE: Kumaracoil, India, 2016; pp. 486–490. [Google Scholar]
- Rendon, D.B.; Ojeda, J.L.R.; Foix, L.F.C.; Morillo, D.S.; Fernandez, M.A. Mapping the human body for vibrations using an accelerometer. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 23–26 August 2007; IEEE: Lyon, France, 2007; pp. 1671–1674. [Google Scholar]
- Ejupi, A.; Menon, C. Detection of talking in respiratory signals: A feasibility study using machine learning and wearable textile-based sensors. Sensors 2018, 18, 2474. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, E.; Coyle, S.; O’Connor, N.E.; Diamond, D.; Ward, T. Breathing feedback system with wearable textile sensors. In Proceedings of the 2010 International Conference on Body Sensor Networks, Singapore, 7–9 June 2010; pp. 56–61. [Google Scholar]
- Zięba, J.; Frydrysiak, M. Textronics—Electrical and electronic textiles. Sensors for breathing frequency measurement. Fibres Text. East. Eur. 2006, 14, 7. [Google Scholar]
- AL-Khalidi, F.Q.; Saatchi, R.; Burke, D.; Elphick, H.; Tan, S. Respiration rate monitoring methods: A review. Pediatric Pulmonol. 2011, 46, 523–529. [Google Scholar] [CrossRef] [Green Version]
- Janssen, R.; Wang, W.; Moço, A.; de Haan, G. Video-based respiration monitoring with automatic region of interest detection. Physiol. Meas. 2016, 37, 100–114. [Google Scholar] [CrossRef] [Green Version]
- Siam, A.I.; El-Bahnasawy, N.A.; Banby, G.M.E.; Elazm, A.A.; El-Samie, F.E.A.; El-Samie, F.E.A. Efficient video-based breathing pattern and respiration rate monitoring for remote health monitoring. J. Opt. Soc. Am. A 2020, 37, 118–124. [Google Scholar] [CrossRef]
- Capineri, L. Resistive sensors with smart textiles for wearable technology: From fabrication processes to integration with electronics. Procedia Eng. 2014, 87, 724–727. [Google Scholar] [CrossRef] [Green Version]
- Calvert, P.; Patra, P.; Lo, T.-C.; Chen, C.H.; Sawhney, A.; Agrawal, A. Piezoresistive sensors for smart textiles. In Proceedings of the Electroactive Polymer Actuators and Devices, Portland, OR, USA, 17 April 2007; Volume 6524, pp. 1–5. [Google Scholar]
- Tan, Y.; Ivanov, Z.M.; Li, H.; Lubich, L.; Wang, C.; Wang, C. A soft wearable and full textile piezoresistive sensor for plantar pressure capturing. Res. Sq. Nano Express 2020, 1, 110. [Google Scholar] [CrossRef]
- Kos, A.; Umek, A. Wearable sensor devices for prevention and rehabilitation in healthcare: Swimming exercise with real-time therapist feedback. IEEE Internet Things J. 2019, 6, 1331–1341. [Google Scholar] [CrossRef]
- Tarannum, S.; Farheen, S. Wireless sensor networks for healthcare monitoring: A review. In Inventive Computation Technologies; Smys, S., Bestak, R., Rocha, Á., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 669–676. [Google Scholar]
- Villeneuve, E.; Harwin, W.; Holderbaum, W.; Janko, B.; Sherratt, R.S. Reconstruction of angular kinematics from wrist-worn inertial sensor data for smart home healthcare. IEEE Access 2017, 5, 2351–2363. [Google Scholar] [CrossRef]
- Liu, H.; Allen, J.; Zheng, D.; Chen, F. Recent development of respiratory rate measurement technologies. Physiol. Meas. 2019, 40, 07TR01. [Google Scholar] [CrossRef] [Green Version]
- Buke, A.; Gaoli, F.; Yongcai, W.; Lei, S.; Zhiqi, Y. Healthcare algorithms by wearable inertial sensors: A survey. China Commun. 2015, 12, 1–12. [Google Scholar] [CrossRef]
- Liu, G.-Z.; Guo, Y.-W.; Zhu, Q.-S.; Huang, B.-Y.; Wang, L. Estimation of respiration rate from three-dimensional acceleration data based on body sensor network. Telemed. E-Health 2011, 17, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Ponciano, V.; Pires, I.M.; Ribeiro, F.R.; Marques, G.; Villasana, M.V.; Garcia, N.M.; Zdravevski, E.; Spinsante, S. Identification of diseases based on the use of inertial sensors: A systematic review. Electronics 2020, 9, 778. [Google Scholar] [CrossRef]
- Malasinghe, L.P.; Ramzan, N.; Dahal, K. Remote patient monitoring: A comprehensive study. J. Ambient Intell. Hum. Comput. 2019, 10, 57–76. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Elsayed, A.; Mendez, A.; Savaria, Y.; Sawan, M. Contact and remote breathing rate monitoring techniques: A review. IEEE Sens. J. 2021, 21, 14569–14586. [Google Scholar] [CrossRef]
- Fiorillo, A.S.; Critello, C.D.; Pullano, S.A. Theory, technology and applications of piezoresistive sensors: A review. Sens. Actuators A Phys. 2018, 281, 156–175. [Google Scholar] [CrossRef]
- Atalay, O.; Kennon, W.R.; Demirok, E. Weft-knitted strain sensor for monitoring respiratory rate and its electro-mechanical modeling. IEEE Sens. J. 2015, 15, 110–122. [Google Scholar] [CrossRef]
- Bergmann, J.H.M.; Chandaria, V.; McGregor, A. Wearable and implantable sensors: The patient’s perspective. Sensors 2012, 12, 16695–16709. [Google Scholar] [CrossRef] [Green Version]
- Bergmann, J.H.M.; McGregor, A.H. Body-worn sensor design: What do patients and clinicians want? Annu. Biomed. Eng. 2011, 39, 2299–2312. [Google Scholar] [CrossRef]
- Mokhlespour Esfahani, M.I.; Nussbaum, M.A. Preferred Placement and usability of a smart textile system vs. inertial measurement units for activity monitoring. Sensors 2018, 18, 2501. [Google Scholar] [CrossRef] [Green Version]
- Vanegas, E.; Igual, R.; Plaza, I. Piezoresistive breathing sensing system with 3D printed wearable casing. J. Sens. 2019, 2019, 2431731. [Google Scholar] [CrossRef] [Green Version]
- Saha, U.; Kamat, A.; Sengupta, D.; Jayawardhana, B.; Kottapalli, A.G.P. A low-cost lung monitoring point-of-care device based on a flexible piezoresistive flow sensor. In Proceedings of the 2020 IEEE Sensors, Rotterdam, The Netherlands, 25–28 October 2020; pp. 1–4. [Google Scholar]
- Nguyen, T.-V.; Ichiki, M. MEMS-based sensor for simultaneous measurement of pulse wave and respiration rate. Sensors 2019, 19, 4942. [Google Scholar] [CrossRef] [Green Version]
- Raji, R.K.; Miao, X.; Wan, A.; Niu, L.; Li, Y.; Boakye, A. Knitted Piezoresistive smart chest band and its application for respiration patterns assessment. J. Eng. Fibers Fabr. 2019, 14. [Google Scholar] [CrossRef] [Green Version]
- Raji, R.K.; Adjeisah, M.; Miao, X.; Wan, A. A novel respiration pattern biometric prediction system based on artificial neural network. Sens. Rev. 2020, 40, 8–16. [Google Scholar] [CrossRef]
- Abbasnejad, B.; Thorby, W.; Razmjou, A.; Jin, D.; Asadnia, M.; Ebrahimi Warkiani, M. MEMS piezoresistive flow sensors for sleep apnea therapy. Sens. Actuators A Phys. 2018, 279, 577–585. [Google Scholar] [CrossRef]
- Thaysen, J.; Yalçinkaya, A.D.; Vettiger, P.; Menon, A. Polymer-based stress sensor with integrated readout. J. Phys. D Appl. Phys. 2002, 35, 2698–2703. [Google Scholar] [CrossRef]
- Kottapalli, A.G.P.; Tan, C.W.; Olfatnia, M.; Miao, J.M.; Barbastathis, G.; Triantafyllou, M. A liquid crystal polymer membrane mems sensor for flow rate and flow direction sensing applications. J. Micromech. Microeng. 2011, 21, 085006. [Google Scholar] [CrossRef]
- Zheng, Q.; Liu, X.; Xu, H.; Cheung, M.-S.; Choi, Y.-W.; Huang, H.-C.; Lei, H.-Y.; Shen, X.; Wang, Z.; Wu, Y.; et al. Sliced graphene foam films for dual-functional wearable strain sensors and switches. Nanoscale Horiz. 2018, 3, 35–44. [Google Scholar] [CrossRef]
- De Fazio, R.; Perrone, E.; Velázquez, R.; de Vittorio, M.; Visconti, P. Development of a self-powered piezo-resistive smart insole equipped with low-power ble connectivity for remote gait monitoring. Sensors 2021, 21, 4539. [Google Scholar] [CrossRef]
- Raiano, L.; Di Tocco, J.; Massaroni, C.; Di Pino, G.; Schena, E.; Formica, D. Clean-breathing: A novel sensor fusion algorithm based on ica to remove motion artifacts from breathing signal. In Proceedings of the 2020 IEEE International Workshop on Metrology for Industry 4.0 IoT, Rome, Italy, 3–5 June 2020; IEEE: Rome, Italy, 2020; pp. 734–739. [Google Scholar]
- Chu, M.; Nguyen, T.; Pandey, V.; Zhou, Y.; Pham, H.N.; Bar-Yoseph, R.; Radom-Aizik, S.; Jain, R.; Cooper, D.M.; Khine, M. Respiration rate and volume measurements using wearable strain sensors. NPJ Digit. Med. 2019, 2, 8. [Google Scholar] [CrossRef]
- Pegan, J.D.; Zhang, J.; Chu, M.; Nguyen, T.; Park, S.-J.; Paul, A.; Kim, J.; Bachman, M.; Khine, M. Skin-mountable stretch sensor for wearable health monitoring. Nanoscale 2016, 8, 17295–17303. [Google Scholar] [CrossRef]
- Konno, K.; Mead, J. Measurement of the separate volume changes of rib cage and abdomen during breathing. J. Appl. Physiol. 1967, 22, 407–422. [Google Scholar] [CrossRef]
- Marani, R.; Gelao, G.; Perri, A.G. A new system for continuous monitoring of breathing and kinetic activity. J. Sens. 2010, 2010, 434863. [Google Scholar] [CrossRef]
- Massaroni, C.; Di Tocco, J.; Lo Presti, D.; Longo, U.G.; Miccinilli, S.; Sterzi, S.; Formica, D.; Saccomandi, P.; Schena, E. Smart Textile based on piezoresistive sensing elements for respiratory monitoring. IEEE Sens. J. 2019, 19, 7718–7725. [Google Scholar] [CrossRef]
- Bauer, S.; Bauer-Gogonea, S.; Graz, I.; Kaltenbrunner, M.; Keplinger, C.; Schwödiauer, R. 25th anniversary article: A soft future: From robots and sensor skin to energy harvesters. Adv. Mater. 2014, 26, 149–162. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Zhang, J.; Chen, D.; Meng, X.; Liu, L.; Wang, K.; Jiao, Z.; Sun, T.; Wang, D.; Niu, S.; et al. Crack-based and hair-like sensors inspired from arthropods: A review. J. Bionic Eng. 2020, 17, 867–898. [Google Scholar] [CrossRef]
- De Fazio, R.; Cafagna, D.; Marcuccio, G.; Minerba, A.; Visconti, P. A multi-source harvesting system applied to sensor-based smart garments for monitoring workers’ bio-physical parameters in harsh environments. Energies 2020, 13, 2161. [Google Scholar] [CrossRef]
- Gaetani, F.; Primiceri, P.; Antonio Zappatore, G.; Visconti, P. Hardware design and software development of a motion control and driving system for transradial prosthesis based on a wireless myoelectric armband. IET Sci. Meas. Technol. 2019, 13, 354–362. [Google Scholar] [CrossRef]
- Visconti, P.; de Fazio, R.; Costantini, P.; Miccoli, S.; Cafagna, D. Innovative complete solution for health safety of children unintentionally forgotten in a car: A smart arduino-based system with user app for remote control. IET Sci. Meas. Technol. 2020, 14, 665–675. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, Z.; Hu, H.; Liu, Y.; Wang, X.; Zhou, S.; Qiu, J. Highly stretchable and ultrasensitive strain sensor based on reduced graphene oxide microtubes–elastomer composite. ACS Appl. Mater. Interfaces 2015, 7, 27432–27439. [Google Scholar] [CrossRef]
- Pang, C.; Lee, G.-Y.; Kim, T.; Kim, S.M.; Kim, H.N.; Ahn, S.-H.; Suh, K.-Y. A flexible and highly sensitive strain-gauge sensor using reversible interlocking of nanofibres. Nat. Mater. 2012, 11, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, X.; Gao, E.; Jian, M.; Xia, K.; Wang, Q.; Xu, Z.; Ren, T.; Zhang, Y. Carbonized silk fabric for ultrastretchable, highly sensitive, and wearable strain sensors. Adv. Mater. 2016, 28, 6640–6648. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, M.I.M. Chapter 6—Smart textiles in healthcare: A summary of history, types, applications, challenges, and future trends. In Nanosensors and Nanodevices for Smart Multifunctional Textiles; Ehrmann, A., Nguyen, T.A., Nguyen Tri, P., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2021; pp. 93–107. ISBN 978-0-12-820777-2. [Google Scholar]
- Angelucci, A.; Cavicchioli, M.; Cintorrino, I.A.; Lauricella, G.; Rossi, C.; Strati, S.; Aliverti, A. Smart textiles and sensorized garments for physiological monitoring: A review of available solutions and techniques. Sensors 2021, 21, 814. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, M.; Pichitpajongkit, A.; Lee, S.; Ryu, S.; Park, I. Highly stretchable and sensitive strain sensor based on silver nanowire–elastomer nanocomposite. ACS Nano 2014, 8, 5154–5163. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.-U.; Lee, J.-H.; Trung, T.Q.; Roh, E.; Kim, D.-I.; Kim, S.-W.; Lee, N.-E. Transparent stretchable self-powered patchable sensor platform with ultrasensitive recognition of human activities. ACS Nano 2015, 9, 8801–8810. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, M.; Shim, H.J.; Ghaffari, R.; Cho, H.R.; Son, D.; Jung, Y.H.; Soh, M.; Choi, C.; Jung, S.; et al. Stretchable silicon nanoribbon electronics for skin prosthesis. Nat. Commun. 2014, 5, 5747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, N.; Lu, C.; Yang, S.; Rogers, J. Highly sensitive skin-mountable strain gauges based entirely on elastomers. Adv. Funct. Mater. 2012, 22, 4044–4050. [Google Scholar] [CrossRef]
- Park, J.; Lee, Y.; Hong, J.; Lee, Y.; Ha, M.; Jung, Y.; Lim, H.; Kim, S.Y.; Ko, H. Tactile-direction-sensitive and stretchable electronic skins based on human-skin-inspired interlocked microstructures. ACS Nano 2014, 8, 12020–12029. [Google Scholar] [CrossRef]
- Daňová, R.; Olejnik, R.; Slobodian, P.; Matyas, J. The piezoresistive highly elastic sensor based on carbon nanotubes for the detection of breath. Polymers 2020, 12, 713. [Google Scholar] [CrossRef] [Green Version]
- Tadakaluru, S.; Thongsuwan, W.; Singjai, P. Stretchable and flexible high-strain sensors made using carbon nanotubes and graphite films on natural rubber. Sensors 2014, 14, 868–876. [Google Scholar] [CrossRef] [Green Version]
- Hajjaj, A.Z.; Chappanda, K.N.; Batra, N.M.; Hafiz, M.A.A.; Costa, P.M.F.J.; Younis, M.I. Miniature pressure sensor based on suspended MWCNT. Sens. Actuators A Phys. 2019, 292, 11–16. [Google Scholar] [CrossRef]
- Slobodian, P.; Riha, P.; Olejnik, R.; Cvelbar, U.; Saha, P. Enhancing effect of KMnO4 oxidation of carbon nanotubes network embedded in elastic polyurethane on overall electro-mechanical properties of composite. Compos. Sci. Technol. 2013, 81, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhang, F.; Lu, K.; Abdulaziz, M.; Li, C.; Zhang, C.; Chen, J.; Li, Y. Nano-copper enhanced flexible device for simultaneous measurement of human respiratory and electro-cardiac activities. J. Nanobiotechnol. 2020, 18, 1–15. [Google Scholar] [CrossRef]
- Mao, L.; Meng, Q.; Ahmad, A.; Wei, Z. Mechanical analyses and structural design requirements for flexible energy storage devices. Adv. Energy Mater. 2017, 7, 1700535. [Google Scholar] [CrossRef] [Green Version]
- Martinsson, E. Nanoplasmonic Sensing Using Metal Nanoparticles; Department of Physics, Chemistry and Biology, Molecular Physics, Linköping University; Linköping University Electronic Press: Linköping, Sweden, 2014; ISBN 978-91-7519-223-9. [Google Scholar]
- Zahn, M.; Pao, S.-C. Effects of step changes in excitation from a steady state on the transient electric field and space charge behavior for unipolar ion conduction: I. Step changes in current. J. Electrost. 1975, 1, 235–248. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, S.; Lin, Y.; Yuan, X.; Liu, L. Silver nanowires coated on cotton for flexible pressure sensors. J. Mater. Chem. C 2016, 4, 935–943. [Google Scholar] [CrossRef]
- Zhou, R.; Li, P.; Fan, Z.; Du, D.; Ouyang, J. Stretchable heaters with composites of an intrinsically conductive polymer, reduced graphene oxide and an elastomer for wearable thermotherapy. J. Mater. Chem. C 2017, 5, 1544–1551. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Lu, H.; Zhang, Q.; Yao, Y.; Chen, M.; Li, Q. Flexible carbon nanotube/polyurethane electrothermal films. Carbon 2016, 110, 343–349. [Google Scholar] [CrossRef] [Green Version]
- Pang, Y.; Tian, H.; Tao, L.; Li, Y.; Wang, X.; Deng, N.; Yang, Y.; Ren, T.-L. Flexible, highly sensitive, and wearable pressure and strain sensors with graphene porous network structure. ACS Appl. Mater. Interfaces 2016, 8, 26458–26462. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, N.; Li, L.; Hu, X.; Zou, Z.; Wang, J.; Luo, S.; Gao, Y. A highly flexible and sensitive piezoresistive sensor based on mxene with greatly changed interlayer distances. Nat. Commun. 2017, 8, 1207. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Tian, M.; Sun, X.; Pan, N.; Chen, F.; Zhu, S.; Zhang, X.; Chen, S. Highly sensitive wearable 3D piezoresistive pressure sensors based on graphene coated isotropic non-woven substrate. Compos. Part A Appl. Sci. Manuf. 2019, 117, 202–210. [Google Scholar] [CrossRef]
- Tannarana, M.; Solanki, G.K.; Bhakhar, S.A.; Patel, K.D.; Pathak, V.M.; Pataniya, P.M. 2D-SnSe 2 nanosheet functionalized piezo-resistive flexible sensor for pressure and human breath monitoring. ACS Sustain. Chem. Eng. 2020, 8, 7741–7749. [Google Scholar] [CrossRef]
- Lian, Y.; Yu, H.; Wang, M.; Yang, X.; Zhang, H. Ultrasensitive wearable pressure sensors based on silver nanowire-coated fabrics. Nanoscale Res. Lett. 2020, 15, 70. [Google Scholar] [CrossRef]
- Pacelli, M.; Caldani, L.; Paradiso, R. Textile piezoresistive sensors for biomechanical variables monitoring. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; IEEE: New York, NY, USA, 2006; pp. 5358–5361. [Google Scholar]
- Jang, S.; Choi, J.Y.; Yoo, E.S.; Lim, D.Y.; Lee, J.Y.; Kim, J.K.; Pang, C. Printable wet-resistive textile strain sensors using bead-blended composite ink for robustly integrative wearable electronics. Compos. Part B Eng. 2021, 210, 108674. [Google Scholar] [CrossRef]
- Tamura, T. Wearable inertial sensors and their applications. In Wearable Sensors; Sazonov, E., Neuman, M.R., Eds.; Academic Press: Oxford, UK, 2014; pp. 85–104. ISBN 978-0-12-418662-0. [Google Scholar]
- Rahmani, M.H.; Berkvens, R.; Weyn, M. Chest-worn inertial sensors: A survey of applications and methods. Sensors 2021, 21, 2875. [Google Scholar] [CrossRef]
- Gaidhani, A.; Moon, K.S.; Ozturk, Y.; Lee, S.Q.; Youm, W. Extraction and analysis of respiratory motion using wearable inertial sensor system during trunk motion. Sensors 2017, 17, 2932. [Google Scholar] [CrossRef] [Green Version]
- Gjoreski, H.; Lustrek, M.; Gams, M. Accelerometer placement for posture recognition and fall detection. In Proceedings of the 2011 Seventh International Conference on Intelligent Environments, Nottingham, UK, 25–28 July 2011; IEEE: Nottingham, UK, 2011; pp. 47–54. [Google Scholar]
- Da Costa, T.D.; Vara, M.D.F.F.; Cristino, C.S.; Zanella, T.Z.; Neto, G.N.N.; Nohama, P. Breathing Monitoring and Pattern Recognition with Wearable Sensors; IntechOpen: London, UK, 2019; ISBN 978-1-78984-497-9. [Google Scholar]
- Phan, D.H.; Bonnet, S.; Guillemaud, R.; Castelli, E.; Pham Thi, N.Y. Estimation of respiratory waveform and heart rate using an accelerometer. In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008; pp. 4916–4919. [Google Scholar]
- Chan, A.M.; Ferdosi, N.; Narasimhan, R. Ambulatory respiratory rate detection using ecg and a triaxial accelerometer. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; Volume 2013, pp. 4058–4061. [Google Scholar] [CrossRef]
- Elfaramawy, T.; Fall, C.L.; Arab, S.; Morissette, M.; Lellouche, F.; Gosselin, B. A wireless respiratory monitoring system using a wearable patch sensor network. IEEE Sens. J. 2019, 19, 650–657. [Google Scholar] [CrossRef]
- Cleland, I.; Kikhia, B.; Nugent, C.; Boytsov, A.; Hallberg, J.; Synnes, K.; McClean, S.; Finlay, D. Optimal placement of accelerometers for the detection of everyday activities. Sensors 2013, 13, 9183–9200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preejith, S.P.; Jeelani, A.; Maniyar, P.; Joseph, J.; Sivaprakasam, M. Accelerometer based system for continuous respiratory rate monitoring. In Proceedings of the 2017 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Rochester, MN, USA, 7–10 May 2017; pp. 171–176. [Google Scholar]
- Shabeeb, A.G.; Al-Askery, A.J.; Humadi, A.F. Design and implementation of breathing rate measurement systembased on accelerometer sensor. IOP Conf. Ser. Mater. Sci. Eng. 2020, 745, 012100. [Google Scholar] [CrossRef]
- Beck, S.; Laufer, B.; Krueger-Ziolek, S.; Moeller, K. Measurement of respiratory rate with inertial measurement units. Curr. Dir. Biomed. Eng. 2020, 6, 237–240. [Google Scholar] [CrossRef]
- Cesareo, A.; Nido, S.A.; Biffi, E.; Gandossini, S.; D’Angelo, M.G.; Aliverti, A. A wearable device for breathing frequency monitoring: A pilot study on patients with muscular dystrophy. Sensors 2020, 20, 5346. [Google Scholar] [CrossRef]
- Fekr, A.R.; Janidarmian, M.; Radecka, K.; Zilic, Z. A medical cloud-based platform for respiration rate measurement and hierarchical classification of breath disorders. Sensors 2014, 14, 11204–11224. [Google Scholar] [CrossRef]
- Ruminski, J.; Bujnowski, A.; Czuszynski, K.; Kocejko, T. Estimation of respiration rate using an accelerometer and thermal camera in EGlasses. In Proceedings of the 2016 Federated Conference on Computer Science and Information Systems (FedCSIS), Gdansk, Poland, 11–14 September 2016; pp. 1431–1434. [Google Scholar]
- Kano, S.; Mekaru, H. Preliminary comparison of respiratory signals using acceleration on neck and humidity in exhaled air. Microsyst. Technol. 2021, 27, 1–9. [Google Scholar] [CrossRef]
- Manoni, A.; Loreti, F.; Radicioni, V.; Pellegrino, D.; Della Torre, L.; Gumiero, A.; Halicki, D.; Palange, P.; Irrera, F. A new wearable system for home sleep apnea testing, screening, and classification. Sensors 2020, 20, 7014. [Google Scholar] [CrossRef]
- Röddiger, T.; Wolffram, D.; Laubenstein, D.; Budde, M.; Beigl, M. Towards respiration rate monitoring using an in-ear headphone inertial measurement unit. In Proceedings of the 1st International Workshop on Earable Computing; Association for Computing Machinery, New York, NY, USA, 21–26 September 2019; pp. 48–53. [Google Scholar]
- Jayarathna, T.; Gargiulo, G.D.; Breen, P.P. Continuous vital monitoring during sleep and light activity using carbon-black elastomer sensors. Sensors 2020, 20, 1583. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Wang, K.; Xu, H.; Li, T.; Jin, Q.; Cui, D. Recent developments in sensors for wearable device applications. Anal. Bioanal. Chem. 2021. [Google Scholar] [CrossRef]
- Shen, C.-L.; Huang, T.-H.; Hsu, P.-C.; Ko, Y.-C.; Chen, F.-L.; Wang, W.-C.; Kao, T.; Chan, C.-T. Respiratory rate estimation by using ecg, impedance, and motion sensing in smart clothing. J. Med. Biol. Eng. 2017, 37, 826–842. [Google Scholar] [CrossRef]
- Fedotov, A.A.; Akulov, S.A.; Akulova, A.S. Motion artifacts reduction in wearable respiratory monitoring device. In EMBEC & NBC 2017; Eskola, H., Väisänen, O., Viik, J., Hyttinen, J., Eds.; Springer: Singapore, 2018; pp. 1121–1124. [Google Scholar]
- Nabavi, S.; Bhadra, S. A robust fusion method for motion artifacts reduction in photoplethysmography signal. IEEE Trans. Instrum. Meas. 2020, 69, 9599–9608. [Google Scholar] [CrossRef]
- Lepine, N.N.; Tajima, T.; Ogasawara, T.; Kasahara, R.; Koizumi, H. Robust respiration rate estimation using adaptive kalman filtering with textile ecg sensor and accelerometer. In Proceedings of the 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; Volume 2016, pp. 3797–3800. [Google Scholar] [CrossRef]
- Yoon, J.-W.; Noh, Y.-S.; Kwon, Y.-S.; Kim, W.-K.; Yoon, H.-R. Improvement of dynamic respiration monitoring through sensor fusion of accelerometer and gyro-sensor. J. Electr. Eng. Technol. 2014, 9, 334–343. [Google Scholar] [CrossRef] [Green Version]
- Vanegas, E.; Igual, R.; Plaza, I. Sensing systems for respiration monitoring: A technical systematic review. Sensors 2020, 20, 5446. [Google Scholar] [CrossRef]
- Huang, C.-C.; Lin, W.-Y.; Lee, M.-Y. Development and verification of an accelerometer-based respiratory detection algorithm with wearable instrumented smart clothes. In Proceedings of the 2017 IEEE International Conference on Systems, Man, and Cybernetics (SMC), Banff, AB, Canada, 5–8 October 2017; IEEE: Banff, AB, Canada, 2017; pp. 578–581. [Google Scholar]
- Antony Raj, A.; Preejith, S.P.; Raja, V.S.; Joseph, J.; Sivaprakasam, M. Clinical validation of a wearable respiratory rate device for neonatal monitoring. In Proceedings of the 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; IEEE: Honolulu, HI, USA, 2018; pp. 1628–1631. [Google Scholar]
- Doheny, E.P.; Lowery, M.M.; Russell, A.; Ryan, S. Estimation of respiration rate and sleeping position using a wearable accelerometer. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine Biology Society (EMBC), Montral, QC, Canada, 20–24 July 2020; IEEE: Montral, QC, Canada, 2020; pp. 4668–4671. [Google Scholar]
- Fekr, A.R.; Radecka, K.; Zilic, Z. Tidal volume variability and respiration rate estimation using a wearable accelerometer sensor. In Proceedings of the 2014 4th International Conference on Wireless Mobile Communication and Healthcare—Transforming Healthcare Through Innovations in Mobile and Wireless Technologies (MOBIHEALTH), Athens, Greece, 3–5 November 2014; pp. 1–6. [Google Scholar]
- Dan, G.; Zhao, J.; Chen, Z.; Yang, H.; Zhu, Z. A novel signal acquisition system for wearable respiratory monitoring. IEEE Access 2018, 6, 34365–34371. [Google Scholar] [CrossRef]
- Sun, X.; Qiu, L.; Wu, Y.; Tang, Y.; Cao, G. SleepMonitor: Monitoring respiratory rate and body position during sleep using smartwatch. In Proceedings of the ACM on Interactive, Mobile, Wearable and Ubiquitous Technologies, New York, NY, USA, 11 September 2017; Volume 1, pp. 1–22. [Google Scholar] [CrossRef]
- Jafari Tadi, M.; Koivisto, T.; Pänkäälä, M.; Paasio, A. Accelerometer-based method for extracting respiratory and cardiac gating information for dual gating during nuclear medicine imaging. Int. J. Biomed. Imaging 2014, 2014, 690124. [Google Scholar] [CrossRef]
- Madhav, K.V.; Ram, M.R.; Krishna, E.H.; Reddy, K.N.; Reddy, K.A. Estimation of respiratory rate from principal components of photoplethysmographic signals. In Proceedings of the 2010 IEEE EMBS Conference on Biomedical Engineering and Sciences (IECBES), Kuala Lumpur, Malaysia, 30 November–2 December 2010; IEEE: Kuala Lumpur, Malaysia, 2010; pp. 311–314. [Google Scholar]
- Jin, A.; Yin, B.; Morren, G.; Duric, H.; Aarts, R.M. Performance evaluation of a tri-axial accelerometry-based respiration monitoring for ambient assisted living. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MI, USA, 3–6 September 2009; pp. 5677–5680. [Google Scholar]
- Liu, G.-Z.; Wu, D.; Mei, Z.; Zhu, Q.; Wang, L. Automatic detection of respiratory rate from electrocardiogram, respiration induced plethysmography and 3d acceleration signals. J. Cent. South Univ. 2013, 20, 2423–2431. [Google Scholar] [CrossRef]
- Jarchi, D.; Rodgers, S.J.; Tarassenko, L.; Clifton, D. Accelerometry-based estimation of respiratory rate for post-intensive care patient monitoring. IEEE Sens. J. 2018, 18, 4981–4989. [Google Scholar] [CrossRef] [Green Version]
- Warnecke, J.M.; Wang, J.; Deserno, T.M. Noise reduction for efficient in-vehicle respiration monitoring with accelerometers. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; IEEE: Berlin, Germany, 2019; pp. 3257–3261. [Google Scholar]
- Alafeef, M.; Fraiwan, M. Smartphone-based respiratory rate estimation using photoplethysmographic imaging and discrete wavelet transform. J Ambient Intell. Hum. Comput. 2020, 11, 693–703. [Google Scholar] [CrossRef]
- Jiang, P.; Zhu, R. Dual tri-axis accelerometers for monitoring physiological parameters of human body in sleep. In Proceedings of the 2016 IEEE Sensors, Orlando, FL, USA, 30 October–3 November 2016; IEEE: Orlando, FL, USA, 2016; pp. 1–3. [Google Scholar]
- Cesareo, A.; Previtali, Y.; Biffi, E.; Aliverti, A. Assessment of breathing parameters using an inertial measurement unit (imu)-based system. Sensors 2019, 19, 88. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Yoo, S.K. Respiration rate estimation based on independent component analysis of accelerometer data: Pilot single-arm intervention study. JMIR Mhealth Uhealth 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, M.D.; Shrivastav, R.; Hunter, E.J. Cepstral peak sensitivity: A theoretic analysis and comparison of several implementations. J. Voice 2015, 29, 670–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, A.E.W.; Cholleti, S.R.; Buchman, T.G.; Clifford, G.D. Improved respiration rate estimation using a kalman filter and wavelet cross-coherence. In Proceedings of the Computing in Cardiology 2013, Zaragoza, Spain, 22–25 September 2013; IEEE: Zaragoza, Spain, 2013; pp. 791–794. [Google Scholar]
- Wang, S.; Liu, M.; Pang, B.; Li, P.; Yao, Z.; Zhang, X.; Chen, H. A new physiological signal acquisition patch designed with advanced respiration monitoring algorithm based on 3-axis accelerator and gyroscope. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; IEEE: Honolulu, HI, USA, 2018; pp. 441–444. [Google Scholar]
- Hadiyoso, S.; Dewi, E.M.; Mengko, T.L.E.R.; Zakaria, H. Respiratory rate extraction based on plethysmographic wave analysis. IOP Conf. Ser. Mater. Sci. Eng. 2020, 830, 032050. [Google Scholar] [CrossRef]
- Mabrouk, M.; Rajan, S.; Bolic, M.; Forouzanfar, M.; Dajani, H.R.; Batkin, I. Human breathing rate estimation from radar returns using harmonically related filters. J. Sens. 2016, 2016, 9891852. [Google Scholar] [CrossRef]
- Gomes, N.; Iranfar, S.; Maksymenko, K.; Aridhi, S.; Guyon, A. Physiological effects (Heart Rate, Respiration Rate and EEG) of rapid relaxation devices with sensorial immersion: A pilot study. J. Interdiscip. Methodol. Issues Sci. 2020, 9. [Google Scholar] [CrossRef]
- El-Sheimy, N.; Youssef, A. Inertial sensors technologies for navigation applications: State of the art and future trends. Satell. Navig. 2020, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- Rast, F.M.; Labruyère, R. Systematic review on the application of wearable inertial sensors to quantify everyday life motor activity in people with mobility impairments. J. Neuroeng. Rehabil. 2020, 17, 148. [Google Scholar] [CrossRef]
- Karacocuk, G.; Höflinger, F.; Zhang, R.; Reindl, L.M.; Laufer, B.; Möller, K.; Röell, M.; Zdzieblik, D. Inertial sensor-based respiration analysis. IEEE Trans. Instrum. Meas. 2019, 68, 4268–4275. [Google Scholar] [CrossRef]
- Balestrieri, E.; Boldi, F.; Colavita, A.R.; de Vito, L.; Laudato, G.; Oliveto, R.; Picariello, F.; Rivaldi, S.; Scalabrino, S.; Torchitti, P.; et al. The architecture of an innovative smart t-shirt based on the internet of medical things paradigm. In Proceedings of the 2019 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Istanbul, Turkey, 26–28 June 2019; pp. 1–6. [Google Scholar]




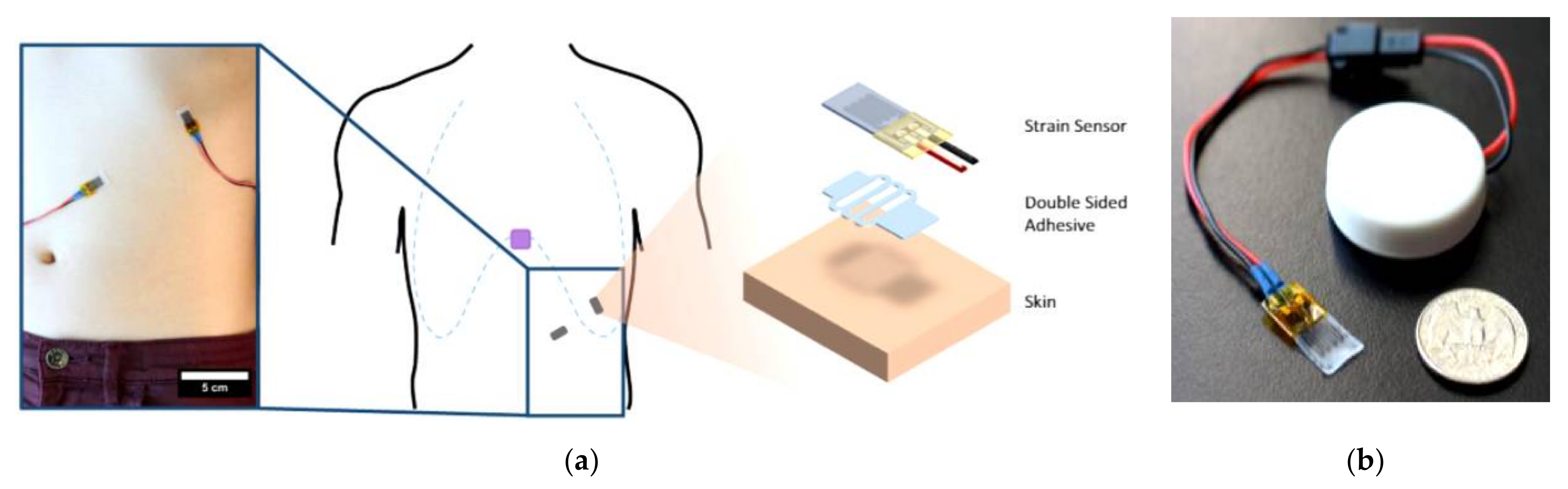

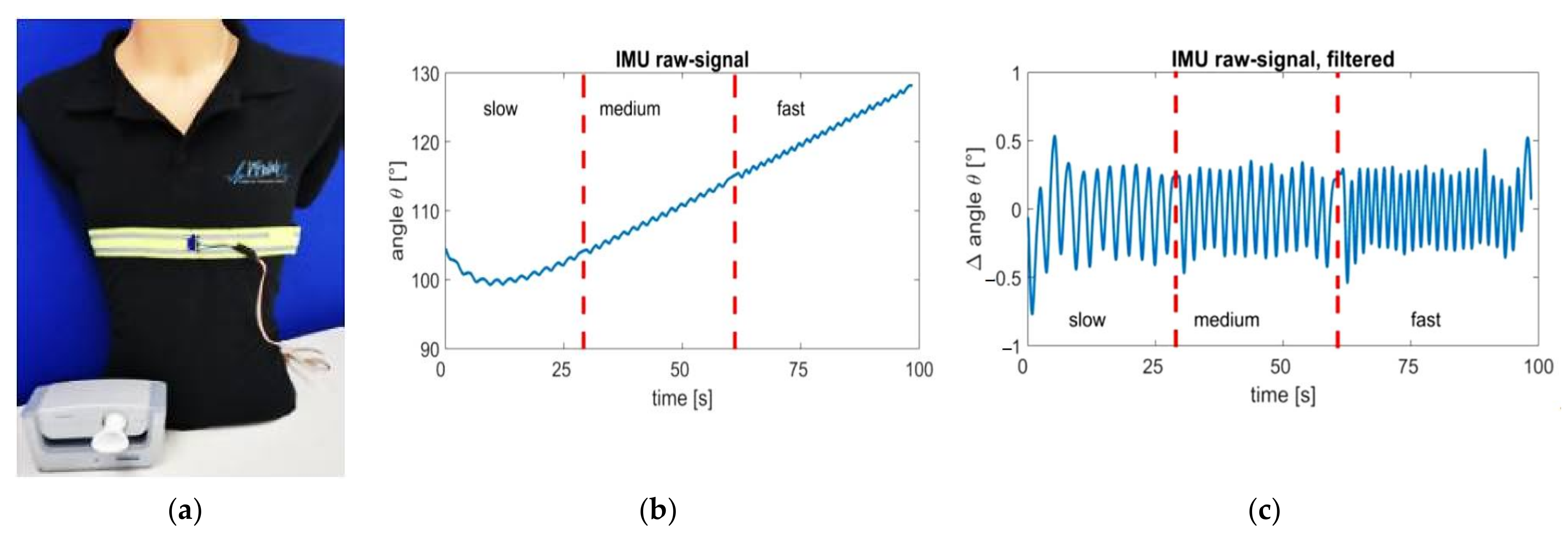
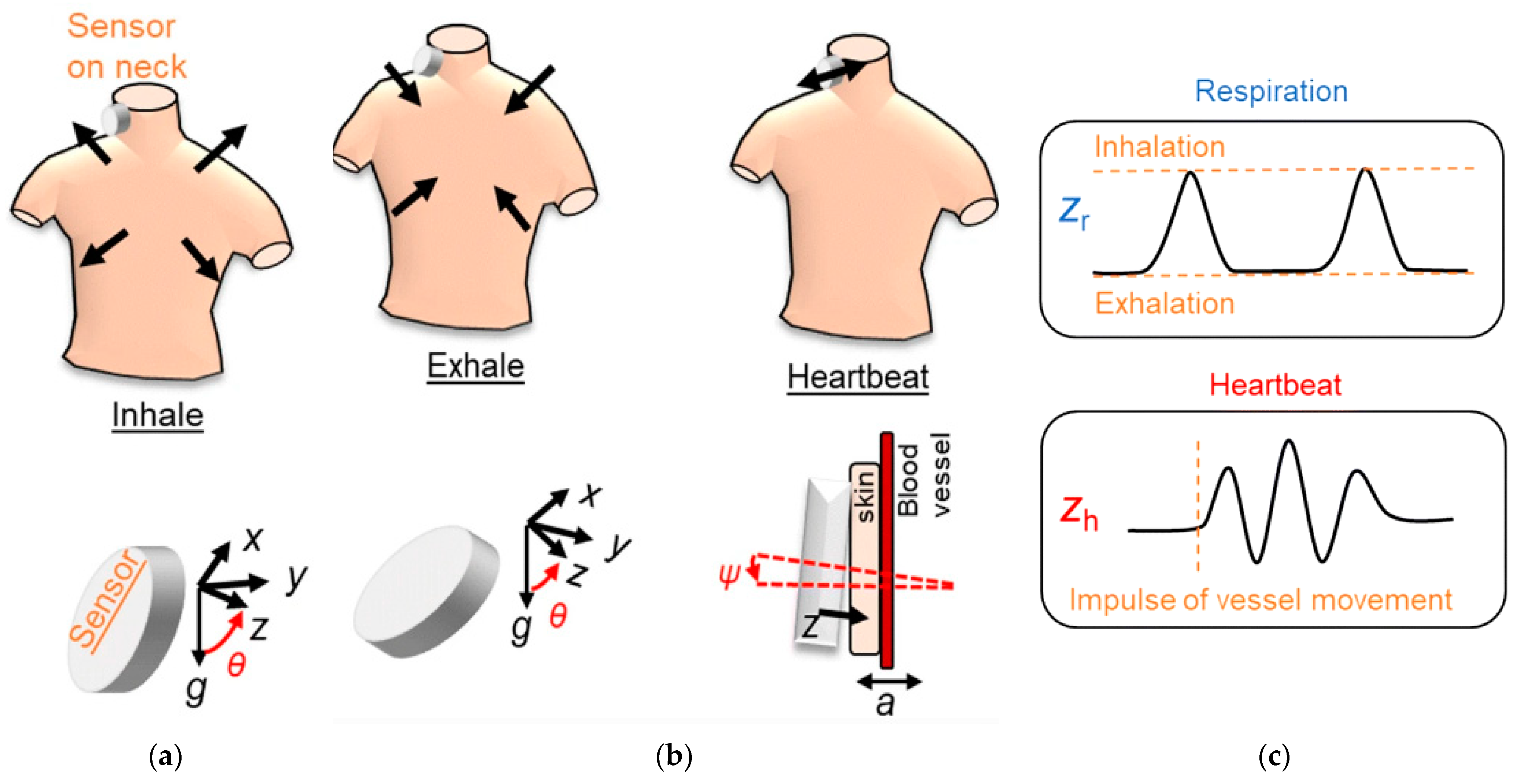
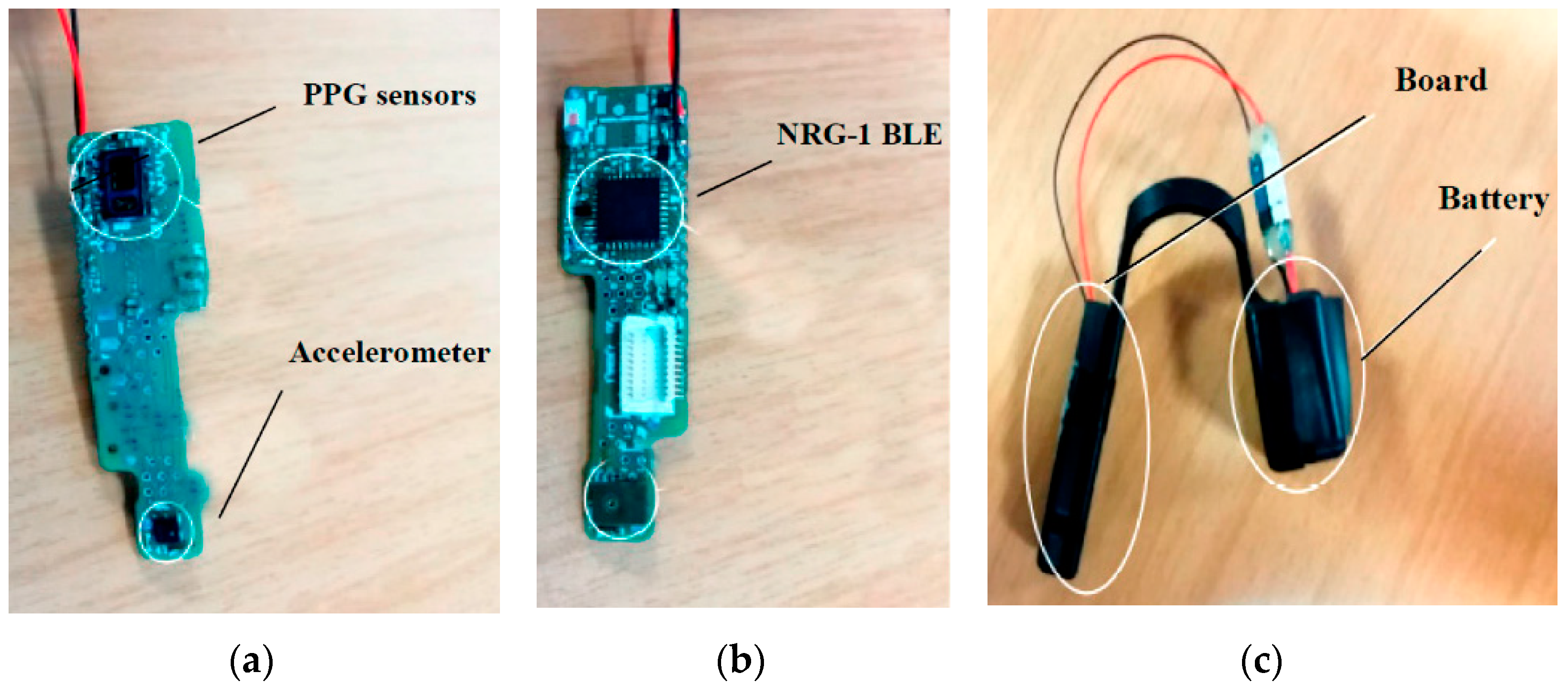
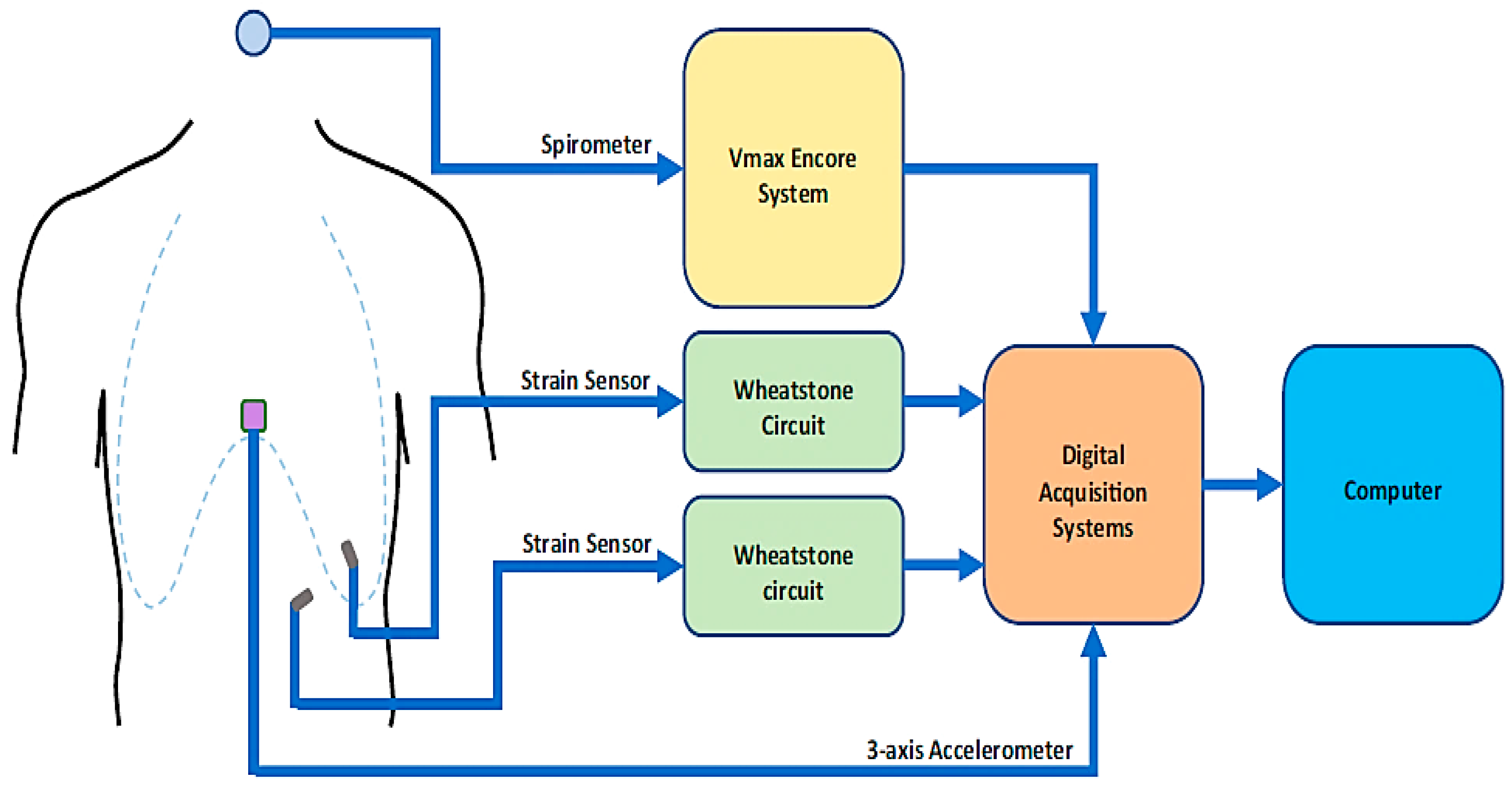


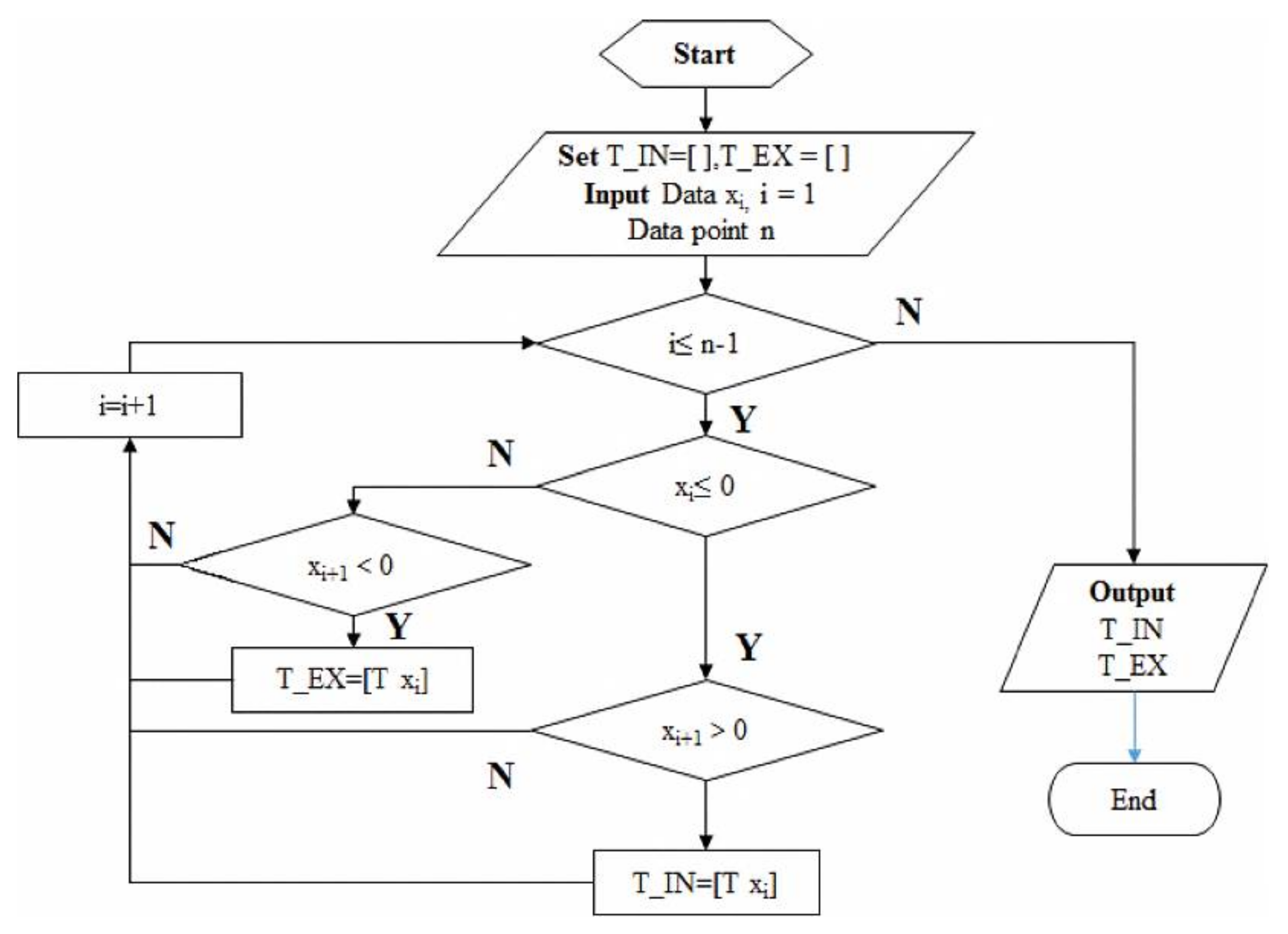

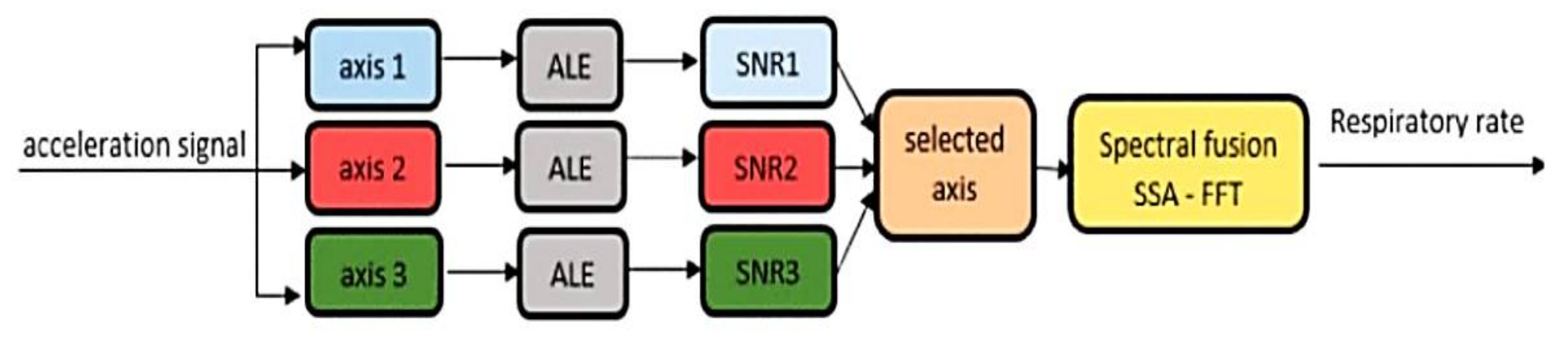
| Sensor Type | Advantages | Limitations |
|---|---|---|
| Acoustic Sensor | Portable, cheap, and easy-to-use. | Optimal sensor position should be determined. Background acoustic noise and movement artefacts must be deleted. In some settings, they are not usable. |
| Humidity Sensor | Less affected by the environmental conditions than pressure, flow, temperature sensors | Not suitable for long-time usage as it is uncomfortable. It needs some improvements to be commercialized. |
| Oximeter Sensor | Versatile, Simple, Noninvasive | There are no particular disadvantages with this kind of sensor. |
| CO2 Sensor | High Sensitivity, Wide Linearity range High Accuracy | Body movements seriously impact their performance. Affected by the environmental conditions |
| ECG Sensor | Versatile, Simple | Require the application of electrodes |
| Accelerometer Sensor | Wide spectral range Small dimensions The sensitivity can be adjusted to detect from gross movements to small artery pulsation. | The sensor’s position is crucial. Body movements seriously impact their performance. Unwanted artefacts can affect the detected signal. |
| Textile Sensor | Simple integration into smart clothes or wearable devices. | This kind of sensor is affected by movement artefacts. |
| Capacitive Sensor | High resolution, Low cost, Vibration-immunity and Low power consumption | This kind of sensor is affected by movement artefacts. |
| Work | Sensing Device | Processing Unit | Sampling Frequency [Hz] | Availability of Wireless Communication | Wearability |
|---|---|---|---|---|---|
| E. Venegas et al. [50] | A201 FlexiForce sensor | Arduino Pro Mini (ATMega32U) | 50 | Bluetooth (HC-05) | Medium |
| U. Saha et al. [51] | PDMS/Graphene sensor | Arduino Uno (ATMega328p) | 1000 | WiFi (ESP8266) | Low |
| T.V. Nguyen et al. [52] | MEMS pressure sensor | DL850 Acquisition board/PC | 1000 | No | High |
| R. K. Raji et al. [53] | piezoresistive fabric | STM32F401RCT6 | 1000 | BLE (HC-08) | Medium |
| B. Abbasnejad et al. [55] | MEMS LCP pressure sensor | NI-USB6003 board/PC | 2000 | No | Low |
| M. Chu et al. [61] | Strain sensor | NI-USB6003 board/PC | 1000 | Bluetooth | High |
| C. Massironi et al. [65] | Silver conductive yarns | PIC18F46J50 | 60 | Bluetooth (SPBT2632C2A) | Medium |
| Work | Substrate | Sensitive Material | Operative Range | Sensitivity | Cost |
|---|---|---|---|---|---|
| R. Danovà et al. [81] | TPU | MWCNTs oxidated by KMnO4 | 0.167 ÷ 1.066 MPa | 1.197 MPa−1 2–5 (GF) | Low |
| L. Wang et al. [85] | PDMS | carbon fiber enriched with nano-copper NPs | 0.1 ÷ 0.6 kPa | 0.053 ± 0.00079 kPa−1 0.98 (GF) | Medium |
| Y. Lu et al. [94] | 3D non-woven PET fabric | rGO | 0 ÷ 30 kPa | 35.37 kPa−1 | Low |
| M. Tannarana et al. [95] | Paper | 2D-SnSe2 nanosheets | 2 ÷ 25 kPa | 1.79 kPa−1 | Low |
| Y. Lian et al. [96] | cotton fabrics | Ag nanowire | 0 ÷ 10 kPa | 3.24 × 105 kPa−1 | Medium |
| S. Jang et al. [98] | PDMS | carbon-based ink | 0 ÷ 2 MPa | 57 (GF) | Medium |
| Work | Number of Used IMUs | Processing Unit | Application Area | Availability of Wireless Communication | Wearability |
|---|---|---|---|---|---|
| T. Elfaramawy et al. [78] | 2 | MSP-EXP430F5529LP | Chest Abdomen | RF 2.4 GHz | High |
| S. P. Preejith et al. [108] | 1 (MMA8451) | ARM Cortex M0 microcontroller | Abdomen | BLE | Medium |
| A. Shabeeb et al. [109] | 1 (ADXL345) | Arduino UNO (ATmega 328p) | Chest | No | Medium |
| S. Beck et al. [110] | 2 (MPU-6050) | Arduino MKR1010 (SAMD21) | Chest | No | Medium |
| J. Ruminski et al. [113] | 1 (MPU-6500) | OMAP 4460 | Nasal septum | Bluetooth WiFi | High |
| S. Kano et al. [114] | 2 (BM1160) | ARM Cortex M4 microcontroller | Neck | Bluetooth | High |
| A. Cesareo et al. [111] | 3 | PC | Chest Abdomen | BLE | Medium |
| A. Manoni et al. [115] | 1 (LSM6DSM) | BlueNRG-1 | Nasal septum | BLE | High |
| A. R. Fekr et al. [112] | 1 (KXTJ9) | PC | Chest | WiFi | Medium |
| M. Chu et al. [61] | 1 (ADXL326) | PC | Ribcage Abdomen | BLE | Medium |
| T. Röddiger et al. [116] | 1 | Smartphone/Tablet | Auricle | BLE | High |
| T. Jayarathna et al. [117] | 1 (LIS2DH) | CC2640R2F | Chest | BLE | High |
| Work | Number of Used of IMUs | Processing Methods | Additional Information | Performances | Complexity |
|---|---|---|---|---|---|
| C.-C. Huang et al. [125] | 1 (LSM6DS3) | Peak detection | No | 95% | Low |
| A. Raj [126] | 1 (LIS2HH12) | Peak detection | No | 97.4% | Low |
| E.P. Doheny et al. [127] | 1 (MC10) | Peak detection | PSG signal | 1.58 ± 0.54 BrPM 1 | Low |
| G. Dan et al. [129] | 1 (MPU6050) | Peak detection | CO2 analysis | 99.8% | Low |
| A. Manoni et al. [115] | 1 (LSM6DSM) | PSD PWA | PPG signal | 93% | Medium |
| M. Jafari Tadi [131] | 1 (MMA8451Q) | FFT Peak detection | SCG signal | 99.41 ÷ 99.81% | Medium |
| G.-Z. Liu et al. [134] | 1 | Kalman filter PCA FFT | EDR signal RIP signal CO2 analysis | 95.63% | Medium |
| D. Jarchi et al. [135] | 1 | SSA FFT | PPG signal | 2.56 BrPM 1 | High |
| J. Warnecke et al. [136] | 3 (Shimmer3) | PCA FFT | ECG signal | 3.04 BrPM 1 | Medium |
| A. Cesareo et al. [139] | 1 | PCA FFT | - | 2 BrPM 1 | Medium |
| J. Lee et al. [140] | 1 | ICA | - | 0.47 BrPM 1 | Medium |
| S. Wang et al. [143] | 1 | Kalman filter VCS | RIP signal | 1.58 ± 0.54 BrPM 1 (MAE) | High |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Fazio, R.; Stabile, M.; De Vittorio, M.; Velázquez, R.; Visconti, P. An Overview of Wearable Piezoresistive and Inertial Sensors for Respiration Rate Monitoring. Electronics 2021, 10, 2178. https://doi.org/10.3390/electronics10172178
De Fazio R, Stabile M, De Vittorio M, Velázquez R, Visconti P. An Overview of Wearable Piezoresistive and Inertial Sensors for Respiration Rate Monitoring. Electronics. 2021; 10(17):2178. https://doi.org/10.3390/electronics10172178
Chicago/Turabian StyleDe Fazio, Roberto, Marco Stabile, Massimo De Vittorio, Ramiro Velázquez, and Paolo Visconti. 2021. "An Overview of Wearable Piezoresistive and Inertial Sensors for Respiration Rate Monitoring" Electronics 10, no. 17: 2178. https://doi.org/10.3390/electronics10172178
APA StyleDe Fazio, R., Stabile, M., De Vittorio, M., Velázquez, R., & Visconti, P. (2021). An Overview of Wearable Piezoresistive and Inertial Sensors for Respiration Rate Monitoring. Electronics, 10(17), 2178. https://doi.org/10.3390/electronics10172178









