The Hazards Analysis of Nickel-Rich Lithium-Ion Battery Thermal Runaway under Different States of Charge
Abstract
:1. Introduction
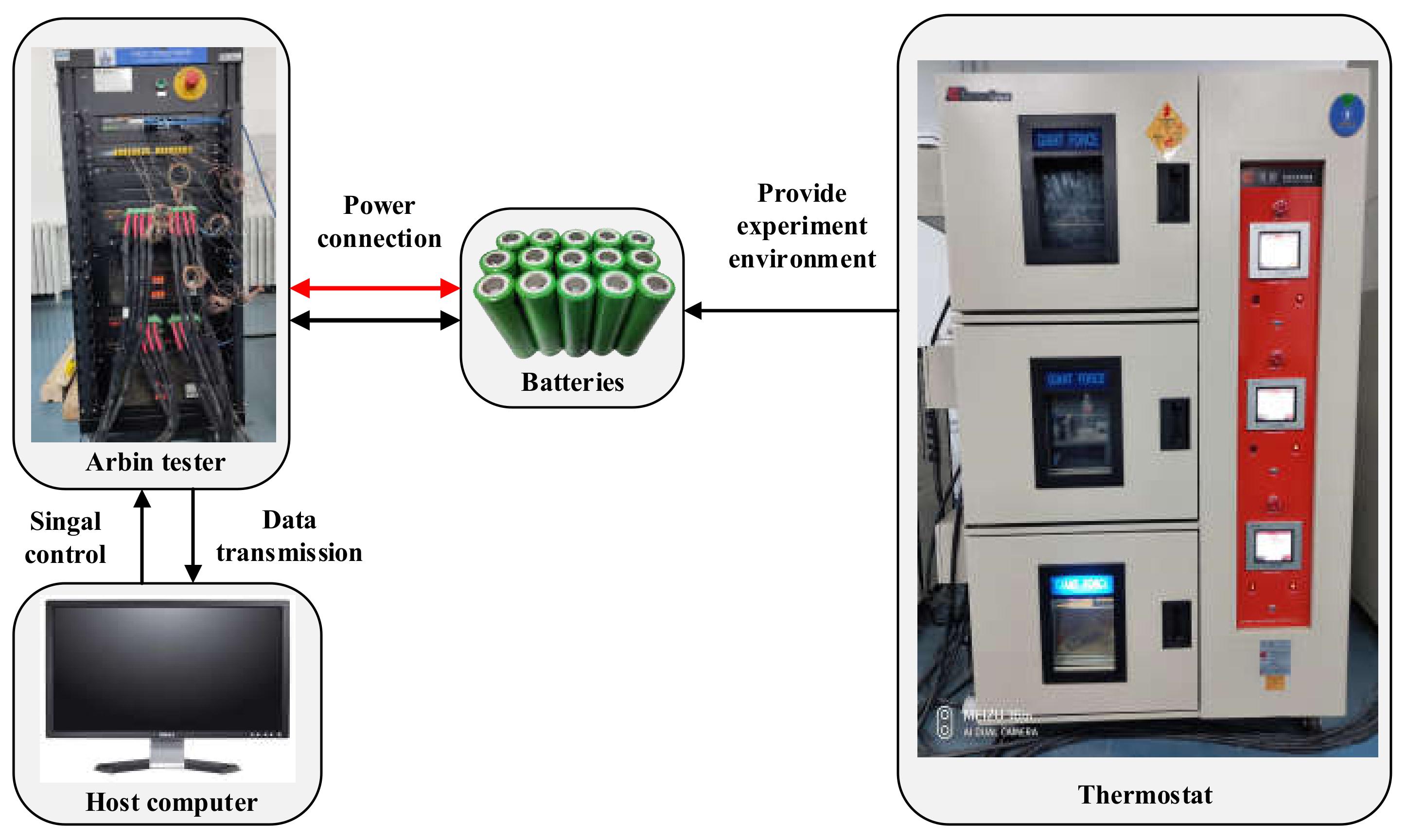
2. Experiment
2.1. Battery Samples and the SOC Method
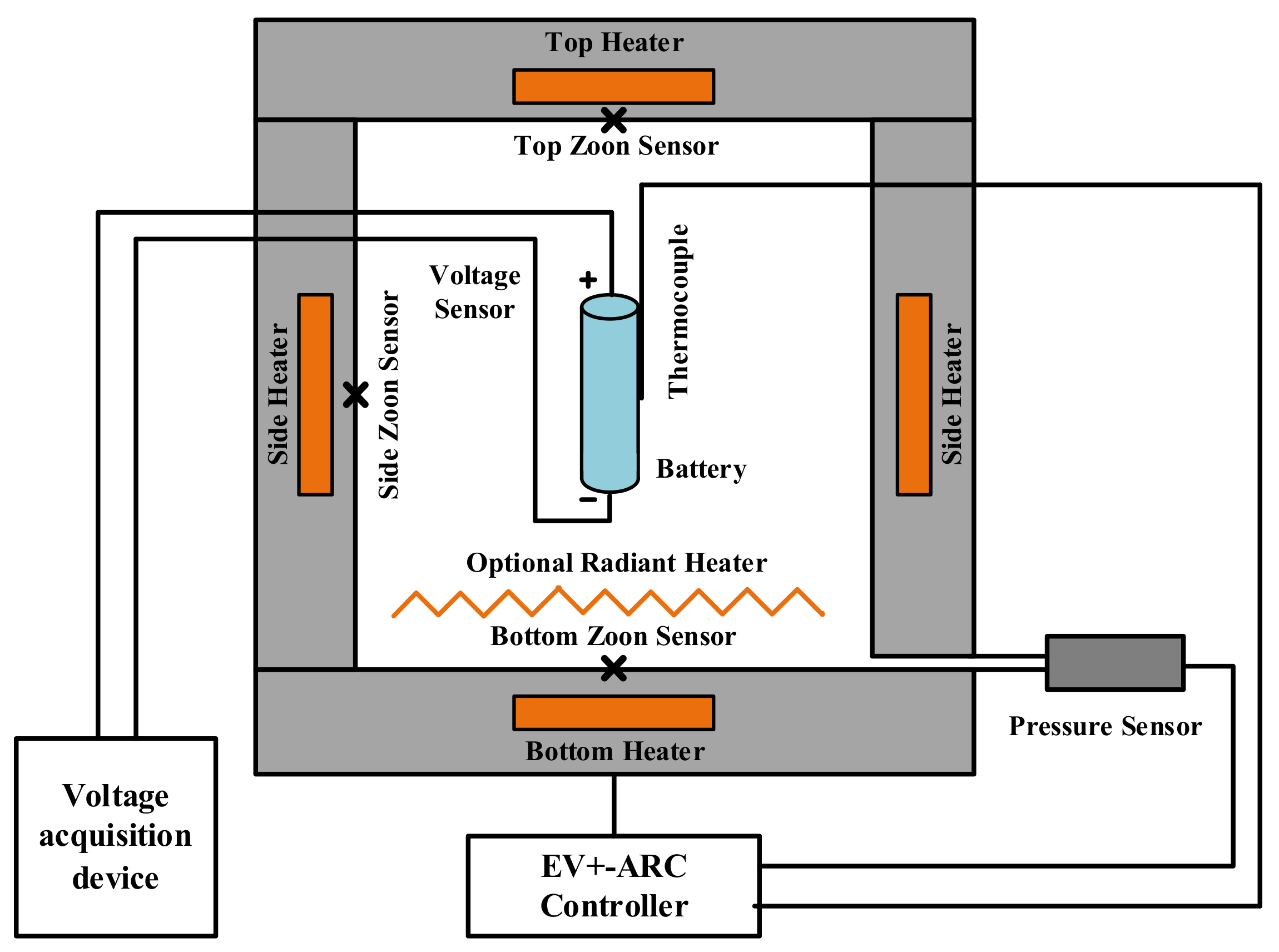
2.2. Thermal Runaway Experiment
3. Results and Discussion
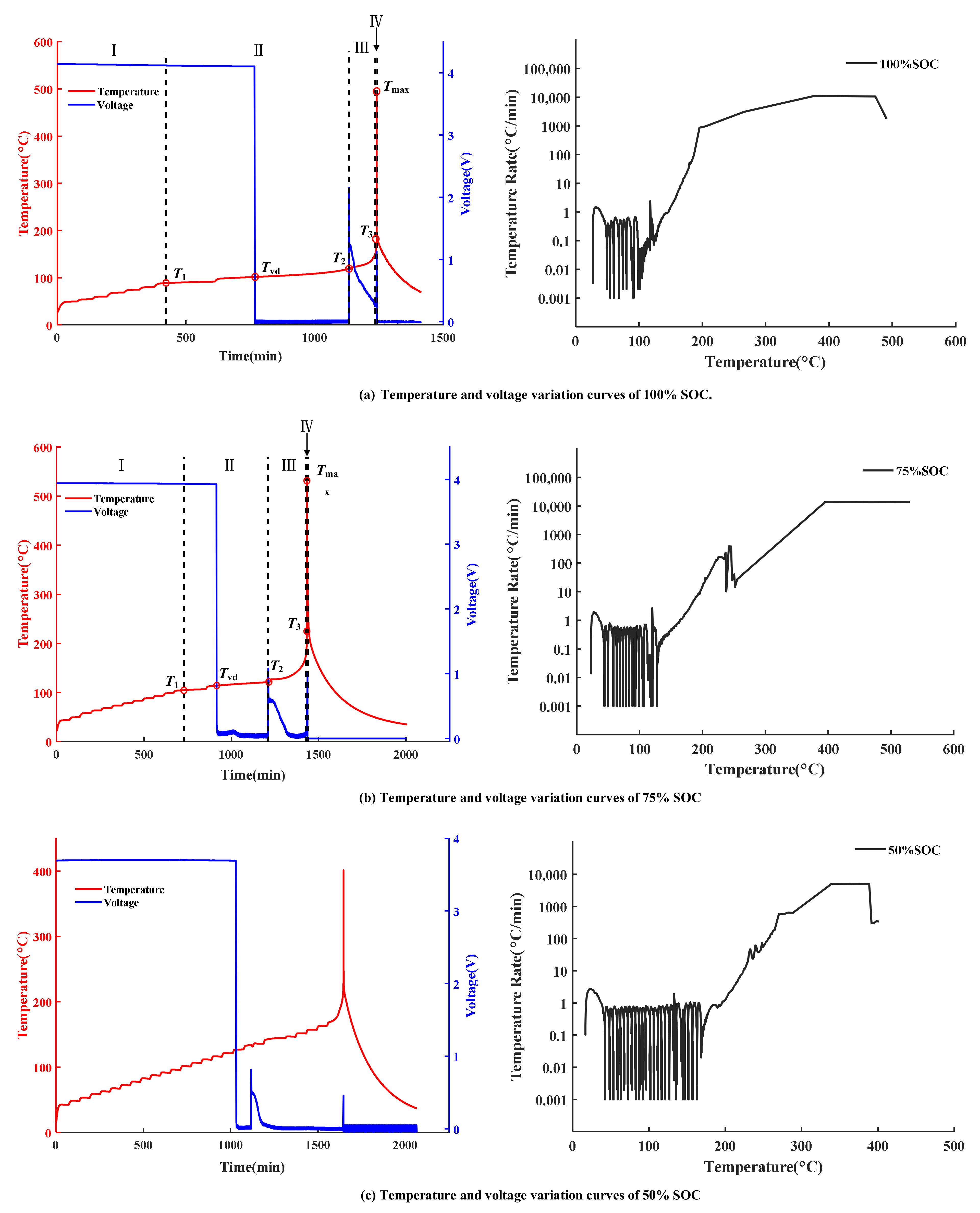
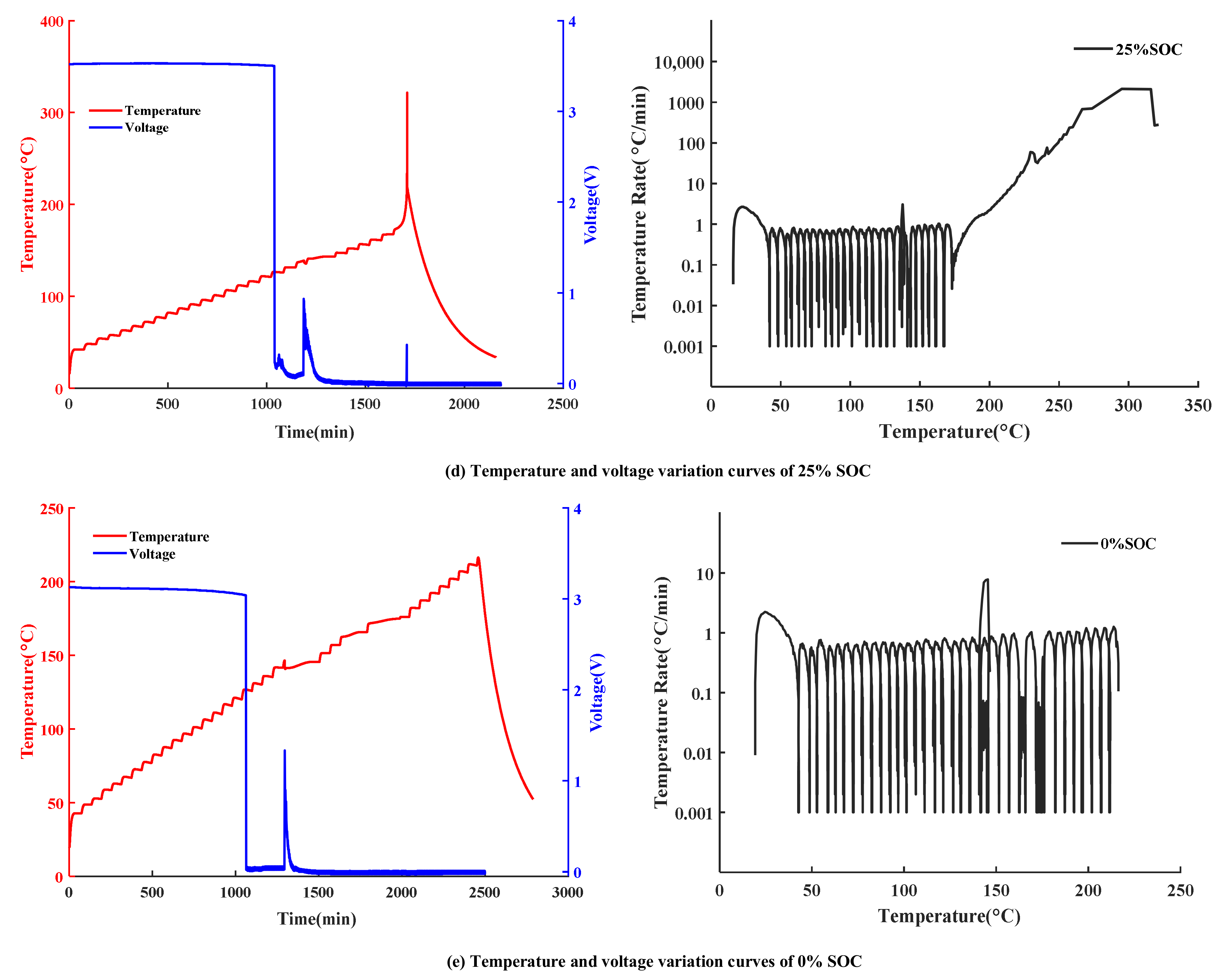
3.1. Temperature and Voltage Change in TR Experiments
3.2. Influence of Different SOC Conditions on the Harm of Battery Thermal Runaway
3.3. Energy Calculation and Conversion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TR | thermal runaway |
| SOC | state of charge |
| EV+-ARC | extended volume plus acceleration calorimeter |
| VSP2 | vent sizing package 2 |
| LCO | LiCoO2 |
| LMO | LiMnO2 |
| LFP | LiFePO4 |
| NCM111 | Li[Ni1/3Co1/3Mn1/3]O2 |
| NCM622 | Li[Ni0.6Co0.2Mn0.2]O2 |
| NCM811 | Li[Ni0.8Co0.1Mn0.1]O2 |
| EVs | electric vehicles |
| BMS | battery management system |
| EV-ARC | extended volume acceleration rate calorimeter |
| DSC | differential scanning calorimeter |
| DC | direct current |
| CC-CV | constant current-constant voltage |
| H-W-S | heat-wait-seek |
| SEI | solid electrolyte interface |
References
- Duan, B.; Zhang, Q.; Geng, F.; Zhang, C. Remaining useful life prediction of lithium-ion battery based on extended Kalman particle filter. Int. J. Energy Res. 2019, 44, 1724–1734. [Google Scholar] [CrossRef]
- Duan, B.; Li, Z.; Gu, P.; Zhou, Z.; Zhang, C. Evaluation of battery inconsistency based on information entropy. J. Energy Storage 2018, 16, 160–166. [Google Scholar] [CrossRef]
- Shan, X.-Y.; Li, F.; Wang, D.-W.; Cheng, H.-M. The smart era of electrochemical energy storage devices. Energy Storage Mater. 2016, 3, 66–68. [Google Scholar] [CrossRef] [Green Version]
- Cano, Z.P.; Banham, D.; Ye, S.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 2018, 3, 279–289. [Google Scholar] [CrossRef]
- Julien, C. Design Considerations for Lithium Batteries. In Materials for Lithium-ion Batteries; Julien, C., Stoynov, Z., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 1–20. [Google Scholar] [CrossRef]
- Yoshio, M.; Noguchi, H. A review of positive electrode materials for lithium-ion batteries. In Lithium-ion Batteries; Yoshio, M., Brodd, R.J., Kozawa, A., Eds.; Springer: New York, NY, USA, 2009; pp. 31–33. [Google Scholar]
- Ehrlich, G.M. Lithium-ion batteries. In Handbook of Batteries, 3rd ed.; Reddy, T.B., Linden, D., Eds.; McGraw-Hill: New York, NY, USA, 2002; pp. 35.1–35.94. [Google Scholar]
- Schipper, F.; Erickson, E.M.; Erk, C.; Shin, J.-Y.; Chesneau, F.F.; Aurbach, D. Review-Recent advances and remaining challenges for lithium ion battery cathodes I. Nickel-rich, LiNixCoyMnzO2. J. Electrochem. Soc. 2017, 164, A6220–A6228. [Google Scholar] [CrossRef]
- Jiang, K.; Wang, T.; Li, X.; Duan, B.; Zhang, C. Simulation of Thermal Runaway Prediction Model for Nickel-Rich Lithium-ion Batteries. In Proceedings of the 2020 Chinese Automation Congress, Shanghai, China, 6–8 November 2020. [Google Scholar]
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Lyon, R.E.; Walters, R.N. Energetics of lithium ion battery failure. J. Hazard. Mater. 2016, 318, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Yu, Y.; Chen, C. A Review on Lithium-Ion Batteries Safety Issues: Existing Problems and Possible Solutions. Mater. Express 2012, 2, 197–212. [Google Scholar] [CrossRef]
- Li, H.; Kong, X.; Liu, C.; Zhao, J. Study on thermal stability of nickel-rich/silicon-graphite large capacity lithium ion battery. Appl. Therm. Eng. 2019, 161, 114144. [Google Scholar] [CrossRef]
- Jhu, C.-Y.; Wang, Y.-W.; Shu, C.-M.; Chang, J.-C.; Wu, H.-C. Thermal explosion hazards on 18650 lithium ion batteries with a VSP2 adiabatic calorimeter. J. Hazard. Mater. 2011, 192, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2017, 10, 246–267. [Google Scholar] [CrossRef]
- Feng, X.; Fang, M.; He, X.; Ouyang, M.; Lu, L.; Wang, H.; Zhang, M. Thermal runaway features of large format prismatic lithium ion battery using extended volume accelerating rate calorimetry. J. Power Sources 2014, 255, 294–301. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Chen, X.; Chu, G.; Chen, C. Effects of solvents and salt on the thermal stability of charged LiCoO2. Mater. Res. Bull. 2009, 44, 543–548. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Q.; Sun, J. Electrochemical performance and thermal stability analysis of LiNixCoyMnzO2 cathode based on a composite safety electrolyte. J. Hazard. Mater. 2018, 351, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.; Chen, H.; Cui, Z.; Wu, T.; Wang, Q. Failure mechanism of the lithium ion battery during nail penetration. Int. J. Heat Mass Transf. 2018, 122, 1103–1115. [Google Scholar] [CrossRef]
- Ren, D.; Feng, X.; Lu, L.; Ouyang, M.; Zheng, S.; Li, J.; He, X. An electrochemical-thermal coupled over-charge-to-thermal-runaway model for lithium ion battery. J. Power Sources 2017, 364, 328–340. [Google Scholar] [CrossRef]
- Liu, C.; Li, H.; Kong, X.; Zhao, J. Modeling analysis of the effect of battery design on internal short circuit hazard in LiNi0.8Co0.1Mn0.1O2/SiOx-graphite lithium ion batteries. Int. J. Heat Mass Transf. 2020, 153, 119590. [Google Scholar] [CrossRef]
- Ryou, M.-H.; Lee, J.-N.; Lee, D.J.; Kim, W.-K.; Jeong, Y.K.; Choi, J.W.; Park, J.-K.; Lee, Y.M. Effects of lithium salts on thermal stabilities of lithium alkyl carbonates in SEI layer. Electrochim. Acta 2012, 83, 259–263. [Google Scholar] [CrossRef]
- Yang, H.; Shen, X.-D. Dynamic TGA–FTIR studies on the thermal stability of lithium/graphite with electrolyte in lithium-ion cell. J. Power Sources 2007, 167, 515–519. [Google Scholar] [CrossRef]
- Mao, B.; Huang, P.; Chen, H.; Wang, Q.; Sun, J. Self-heating reaction and thermal runaway criticality of the lithium ion battery. Int. J. Heat Mass Transf. 2019, 149, 119178. [Google Scholar] [CrossRef]
- Bak, S.-M.; Hu, E.; Zhou, Y.; Yu, X.; Senanayake, S.; Cho, S.-J.; Kim, K.-B.; Chung, K.Y.; Yang, X.-Q.; Nam, K.-W. Structural Changes and Thermal Stability of Charged LiNixMnyCozO2 Cathode Materials Studied by Combined In Situ Time-Resolved XRD and Mass Spectroscopy. ACS Appl. Mater. Interfaces 2014, 6, 22594–22601. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-M.; Li, X.; Wang, Z.; Xu, W.; Liu, J.; Gao, F.; Kovarik, L.; Zhang, J.-G.; Howe, J.; Burton, D.J.; et al. In situ TEM investigation of congruent phase transition and struc-tural evolution of nanostructured silicon/carbon anode for lithium ion batteries. Nano Lett. 2012, 12, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Wang, L.; Feng, X.; Ren, D.; Wu, Y.; Xu, G.; Lu, L.; Hou, J.; Zhang, W.; et al. Thermal runaway mechanism of lithium-ion battery with LiNi0.8Mn0.1Co0.1O2 cathode materials. Nano Energy 2021, 85, 105878. [Google Scholar] [CrossRef]
- Tsukasaki, H.; Fukuda, W.; Morimoto, H.; Arai, T.; Mori, S.; Hayashi, A.; Tatsumisago, M. Thermal behavior and microstruc-tures of cathodes for liquid electrolyte-based lithium batteries. Sci. Rep. 2018, 8, 15613. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.-Y.; Chiang, C.-C.; Wu, S.-H.; Chen, K.-C.; Lin, S.-J.; Wen, C.-Y.; Shu, C.-M. Thermal hazard evaluations of 18650 lithium-ion batteries by an adiabatic calorimeter. J. Therm. Anal. Calorim. 2013, 114, 1083–1088. [Google Scholar] [CrossRef]
- Chen, W.-C.; Wang, Y.-W.; Shu, C.-M. Adiabatic calorimetry test of the reaction kinetics and self-heating model for 18650 Li-ion cells in various states of charge. J. Power Sources 2016, 318, 200–209. [Google Scholar] [CrossRef]





| Parameter | Value |
|---|---|
| Length (mm) | 65.1 |
| Diameter (mm) | 18.5 |
| Surface area (m2) | 0.0043 |
| Volume (m3) | 1.75 × 10−5 |
| Mass (g) | 47 |
| Nominal capacity (mAh) | 3350 |
| Nominal voltage (V) | 3.635 |
| Charge and discharge cut-off voltage (V) | 2.5–4.2 |
| SOC (%) | Mass (g) | Voltage (V) | T1 (°C) | Tvd (°C) | T2 (°C) | T3 (°C) | Tmax (°C) |
|---|---|---|---|---|---|---|---|
| 0 | 47 | 3.13 | 141.8 | 126.7 | 146.6 | - | - |
| 25 | 47 | 3.52 | 142 | 126.5 | 138.8 | 243.6 | 321.5 |
| 50 | 47 | 3.69 | 143 | 126.7 | 134.2 | 239.2 | 401.4 |
| 75 | 47 | 3.94 | 104.8 | 114.1 | 121.4 | 215.3 | 530.9 |
| 100 | 47 | 4.14 | 88.9 | 101.5 | 118.7 | 183.3 | 490.9 |
| SOC (%) | T3 (°C) | Tmax (°C) | ΔH (kJ) | TNT-Equivalent (g) |
|---|---|---|---|---|
| 100 | 183.3 | 490.9 | 14.75 | 3.28 |
| 75 | 215.3 | 530.9 | 15.13 | 3.36 |
| 50 | 239.2 | 401.4 | 7.78 | 1.73 |
| 25 | 243.6 | 321.5 | 3.73 | 0.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, K.; Gu, P.; Huang, P.; Zhang, Y.; Duan, B.; Zhang, C. The Hazards Analysis of Nickel-Rich Lithium-Ion Battery Thermal Runaway under Different States of Charge. Electronics 2021, 10, 2376. https://doi.org/10.3390/electronics10192376
Jiang K, Gu P, Huang P, Zhang Y, Duan B, Zhang C. The Hazards Analysis of Nickel-Rich Lithium-Ion Battery Thermal Runaway under Different States of Charge. Electronics. 2021; 10(19):2376. https://doi.org/10.3390/electronics10192376
Chicago/Turabian StyleJiang, Kun, Pingwei Gu, Peng Huang, Ying Zhang, Bin Duan, and Chenghui Zhang. 2021. "The Hazards Analysis of Nickel-Rich Lithium-Ion Battery Thermal Runaway under Different States of Charge" Electronics 10, no. 19: 2376. https://doi.org/10.3390/electronics10192376
APA StyleJiang, K., Gu, P., Huang, P., Zhang, Y., Duan, B., & Zhang, C. (2021). The Hazards Analysis of Nickel-Rich Lithium-Ion Battery Thermal Runaway under Different States of Charge. Electronics, 10(19), 2376. https://doi.org/10.3390/electronics10192376







