Efficacy of Smart EEG Monitoring Amidst the COVID-19 Pandemic
Abstract
1. Introduction
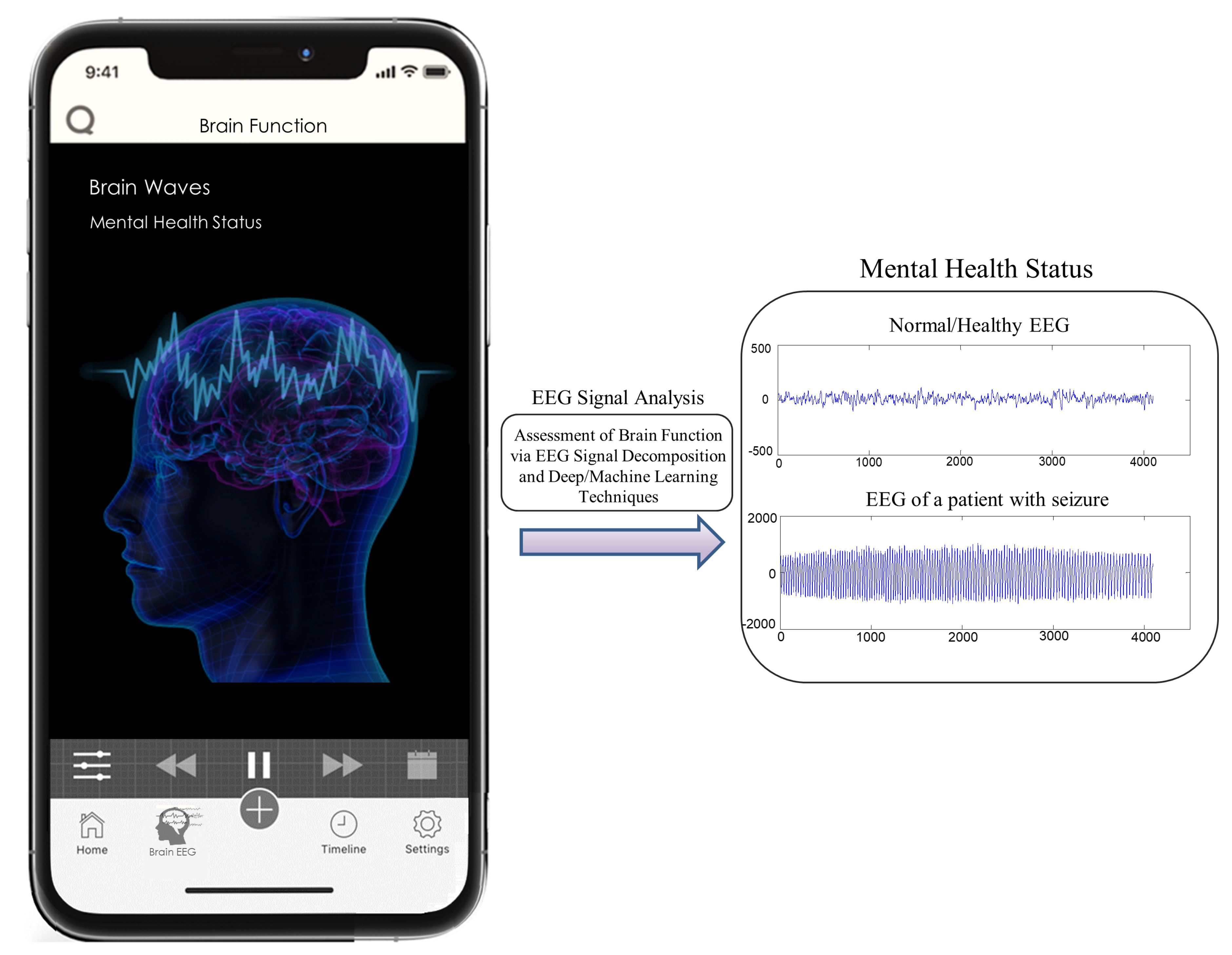
2. Proposed Ideas and Perspectives
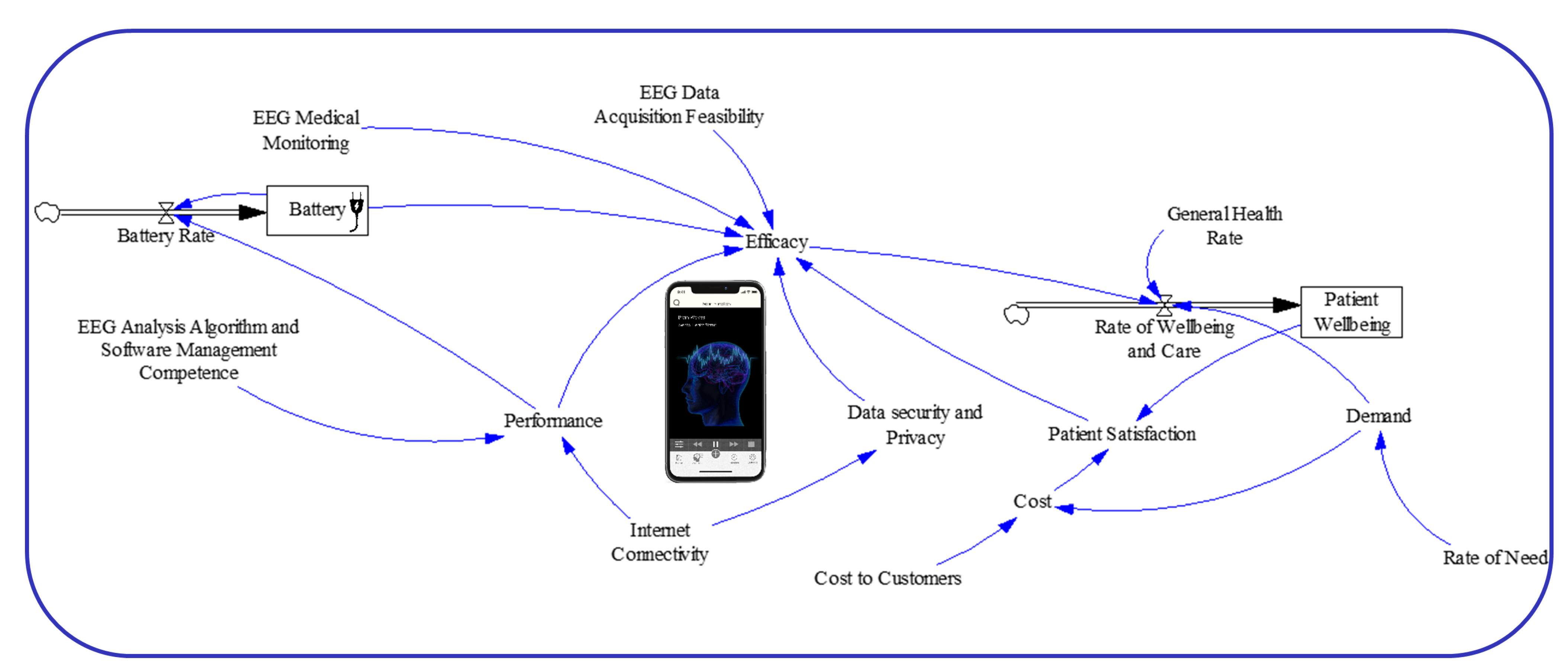
2.1. Causal Model
2.2. System Dynamics Model
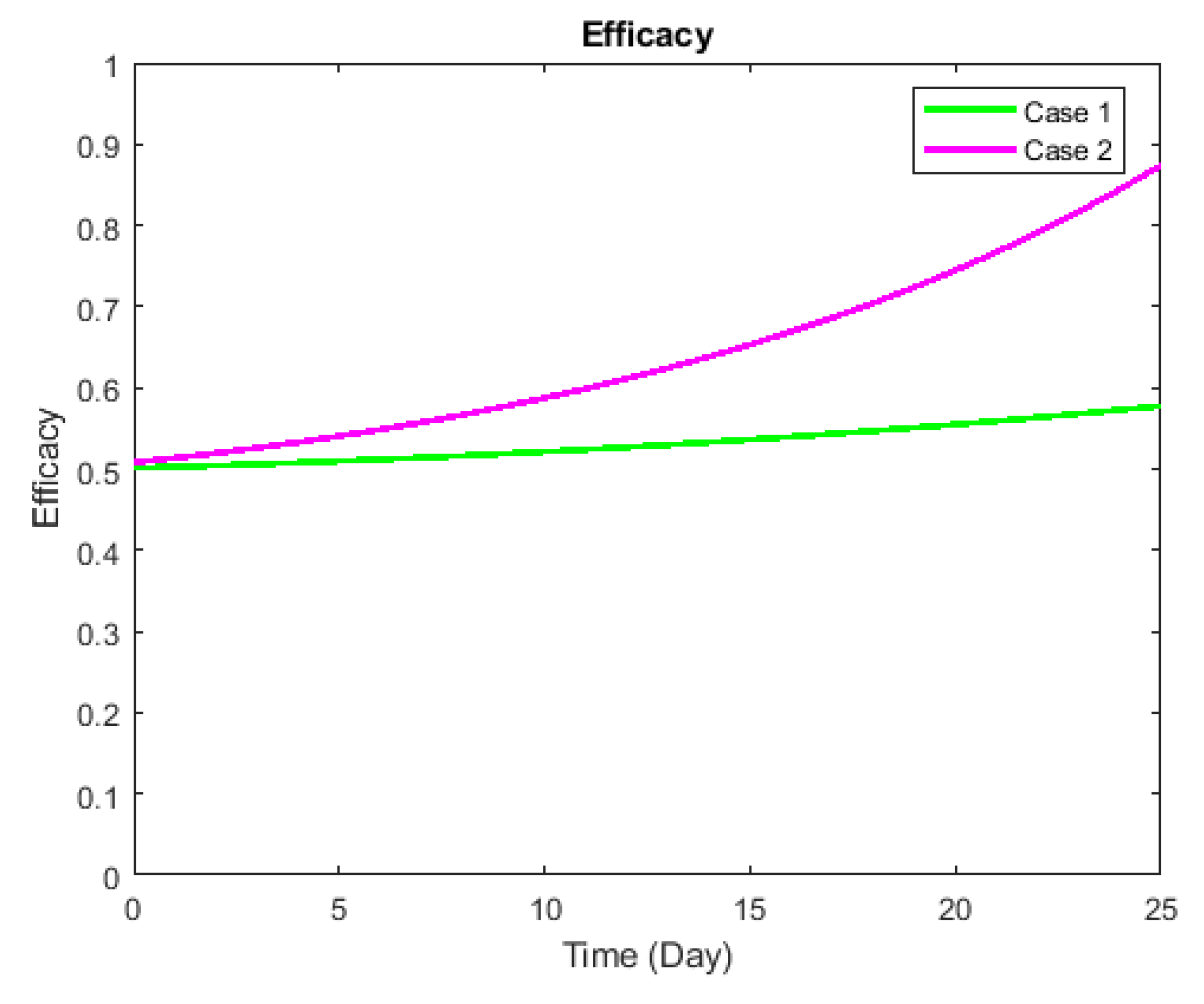
3. Validation
Preliminary Simulations
4. Discussion and Expected Outcome
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khera, A.; Baum, S.J.; Gluckman, T.J.; Gulati, M.; Martin, S.S.; Michos, E.D.; Navar, A.M.; Taub, P.R.; Toth, P.P.; Virani, S.S.; et al. Continuity of care and outpatient management for patients with and at high risk for cardiovascular disease during the COVID-19 pandemic: A scientific statement from the American Society for Preventive Cardiology. Am. J. Prev. Cardiol. 2020, 1, 100009. [Google Scholar] [CrossRef]
- Amaral, D.G.; de Vries, P.J. COVID-19 and Autism Research: Perspectives from Around the Globe. Autism Res. 2020, 13, 844. [Google Scholar] [CrossRef]
- Xiong, J.; Lipsitz, O.; Nasri, F.; Lui, L.M.; Gill, H.; Phan, L.; Chen-Li, D.; Iacobucci, M.; Ho, R.; Majeed, A.; et al. Impact of COVID-19 pandemic on mental health in the general population: A systematic review. J. Affect. Disord. 2020, 277, 55–64. [Google Scholar] [CrossRef]
- What EEGs Tell Us about COVID-19 and The Brain. Available online: https://www.bcm.edu/news/what-eegs-tell-us-about-covid-19-and-the-brain (accessed on 19 March 2021).
- Antony, A.; Haneef, Z. Systematic review of EEG findings in 617 patients diagnosed with COVID-19. Seizure 2020, 83, 234–241. [Google Scholar] [CrossRef]
- de Aguiar Neto, F.S.; Rosa, J.L.G. Depression biomarkers using non-invasive EEG: A review. Neurosci. Biobehav. Rev. 2019, 105, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Aboalayon, K.A.I.; Faezipour, M.; Almuhammadi, W.S.; Moslehpour, S. Sleep stage classification using EEG signal analysis: A comprehensive survey and new investigation. Entropy 2016, 18, 272. [Google Scholar] [CrossRef]
- Aboalayon, K.; Faezipour, M. Single Channel EEG for Near Real-Time Sleep Stage Detection. In Proceedings of the 2019 International Conference on Computational Science and Computational Intelligence (CSCI), Las Vegas, NV, USA, 5–7 December 2019; pp. 641–645. [Google Scholar]
- Bradway, M.; Carrion, C.; Vallespin, B.; Saadatfard, O.; Puigdomènech, E.; Espallargues, M.; Kotzeva, A. mHealth assessment: Conceptualization of a global framework. JMIR mHealth uHealth 2017, 5, e60. [Google Scholar] [CrossRef] [PubMed]
- Kratzke, C.; Cox, C. Smartphone technology and apps: Rapidly changing health promotion. Glob. J. Health Educ. Promot. 2012, 15, 72–82. [Google Scholar]
- Baniasadi, T.; Niakan Kalhori, S.R.; Ayyoubzadeh, S.M.; Zakerabasali, S.; Pourmohamadkhan, M. Study of challenges to utilise mobile-based health care monitoring systems: A descriptive literature review. J. Telemed. Telecare 2018, 24, 661–668. [Google Scholar] [CrossRef]
- EEG App-Brain Wave App, NeuroSky Store. Available online: https://store.neurosky.com/collections/developer-tools (accessed on 19 March 2021).
- Mosadeghi-Nik, M.; Askari, M.S.; Fatehi, F. Mobile health (mHealth) for headache disorders: A review of the evidence base. J. Telemed. Telecare 2016, 22, 472–477. [Google Scholar] [CrossRef]
- Wasimuddin, M.; Elleithy, K.; Abuzneid, A.; Faezipour, M.; Abuzaghleh, O. Multiclass ECG Signal Analysis Using Global Average-Based 2-D Convolutional Neural Network Modeling. Electron. Spec. Issue Biomed. Signal Process. 2021, 10, 170. [Google Scholar] [CrossRef]
- Khan, M.A.; Kim, Y. Cardiac arrhythmia disease classification using LSTM deep learning approach. Comput. Mater. Contin. 2021, 67, 427–443. [Google Scholar] [CrossRef]
- Alsos, O.A.; Das, A.; Svanæs, D. Mobile health IT: The effect of user interface and form factor on doctor–patient communication. Int. J. Med. Inform. 2012, 81, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Faezipour, M.; Saeed, A.; Bulusu, S.C.; Nourani, M.; Minn, H.; Tamil, L. A patient-adaptive profiling scheme for ECG beat classification. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 1153–1165. [Google Scholar] [CrossRef]
- Abushakra, A.; Faezipour, M. Augmenting breath regulation using a mobile driven virtual reality therapy framework. IEEE J. Biomed. Health Inform. 2014, 18, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Abushakra, A.; Faezipour, M. Acoustic signal classification of breathing movements to virtually aid breath regulation. IEEE J. Biomed. Health Inform. 2013, 17, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Faezipour, M.; Abuzneid, A. Smartphone-Based Self-Testing of COVID-19 Using Breathing Sounds. Telemed. e-Health 2020, 26, 1202–1205. [Google Scholar] [CrossRef]
- Abuzaghleh, O.; Barkana, B.D.; Faezipour, M. Noninvasive real-time automated skin lesion analysis system for melanoma early detection and prevention. IEEE J. Transl. Eng. Health Med. 2015, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Aloudat, M.; Faezipour, M.; El-Sayed, A. Automated Vision-Based High Intraocular Pressure Detection Using Frontal Eye Images. IEEE J. Transl. Eng. Health Med. 2019, 7, 1–13. [Google Scholar] [CrossRef]
- Amlani, A.M.; Taylor, B.; Levy, C.; Robbins, R. Utility of smartphone-based hearing aid applications as a substitute to traditional hearing aids. Hear. Rev. 2013, 20, 16–18. [Google Scholar]
- Sterman, J. System Dynamics: Systems Thinking and Modeling for a Complex World; ESD Intern. Symp. ESD-WP-2003; Massachusetts Institute of Technology: Cambridge, MA, USA, 2002; Volume 1, pp. 1–31. [Google Scholar]
- Forrester, J.W. System dynamics, systems thinking, and soft OR. Syst. Dyn. Rev. 1994, 10, 245–256. [Google Scholar] [CrossRef]
- Faezipour, M.; Ferreira, S. Applying systems thinking to assess sustainability in healthcare system of systems. Int. J. Syst. Syst. Eng. 2011, 2, 290–308. [Google Scholar] [CrossRef]
- Faezipour, M.; Ferreira, S. A System Dynamics Perspective of Patient Satisfaction in Healthcare. Procedia Comput. Sci. 2013, 16, 148–156. [Google Scholar] [CrossRef]
- Faezipour, M.; Ferreira, S. A system dynamics approach for sustainable water management in hospitals. IEEE Syst. J. 2018, 12, 1278–1285. [Google Scholar] [CrossRef]
- de Andrade, L.; Lynch, C.; Carvalho, E.; Rodrigues, C.G.; Vissoci, J.R.N.; Passos, G.F.; Pietrobon, R.; Nihei, O.K.; de Barros Carvalho, M.D. System dynamics modeling in the evaluation of delays of care in ST-segment elevation myocardial infarction patients within a tiered health system. PLoS ONE 2014, 9, e103577. [Google Scholar] [CrossRef] [PubMed]
- Faezipour, M.; Faezipour, M. System Dynamics Modeling for Smartphone-Based Healthcare Tools: Case Study on ECG Monitoring. IEEE Syst. J. 2020. [Google Scholar] [CrossRef]
- Faezipour, M.; Faezipour, M. Sustainable smartphone-based healthcare systems: A systems engineering approach to assess the efficacy of respiratory monitoring apps. Sustainability 2020, 12, 5061. [Google Scholar] [CrossRef]
- Mohamed, S.; Chinda, T. System dynamics modelling of construction safety culture. Eng. Constr. Archit. Manag. 2011, 18, 266–281. [Google Scholar] [CrossRef]
- Darabi, N.; Hosseinichimeh, N. System dynamics modeling in health and medicine: A systematic literature review. Syst. Dyn. Rev. 2020, 36, 29–73. [Google Scholar] [CrossRef]
- Davahli, M.R.; Karwowski, W.; Taiar, R. A System Dynamics Simulation Applied to Healthcare: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 5741. [Google Scholar] [CrossRef]
- Gu, X.; Cao, Z.; Jolfaei, A.; Xu, P.; Wu, D.; Jung, T.P.; Lin, C.T. EEG-based brain-computer interfaces (BCIS): A survey of recent studies on signal sensing technologies and computational intelligence approaches and their applications. arXiv 2020, arXiv:2001.11337. [Google Scholar]
- McKenzie, E.D.; Lim, A.S.; Leung, E.C.; Cole, A.J.; Lam, A.D.; Eloyan, A.; Nirola, D.K.; Tshering, L.; Thibert, R.; Garcia, R.Z.; et al. Validation of a smartphone-based EEG among people with epilepsy: A prospective study. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.; Kosinski, M.; Gandek, B. SF-36 health survey: Manual and interpretation guide Lincoln. RI Qual. Inc. 2000, 6.1–6.22. [Google Scholar]
- Faezipour, M. The Empowered Patient Wants Shared Decision Making–How can System Dynamics Modeling Help? In Proceedings of the International System Dynamics Conference, Reykjavik, Iceland, 7–9 August 2018.
- Vensim Software. Available online: https://vensim.com/vensim-software/#professional-amp-dss. (accessed on 11 April 2021).
- Forrester, J.W.; Senge, P.M. Tests for building confidence in system dynamics models. Syst. Dyn. Tims Stud. Manag. Sci. 1980, 14, 209–228. [Google Scholar]
- Richardson, G.P.; Pugh III, A.I. Introduction to System Dynamics Modeling with DYNAMO; Productivity Press Inc.: New York, NY, USA, 1981. [Google Scholar]
- Barlas, Y. Formal aspects of model validity and validation in system dynamics. Syst. Dyn. Rev. J. Syst. Dyn. Soc. 1996, 12, 183–210. [Google Scholar] [CrossRef]
- Currie, D.J.; Smith, C.; Jagals, P. The application of system dynamics modelling to environmental health decision-making and policy-a scoping review. BMC Public Health 2018, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faezipour, M.; Faezipour, M. Efficacy of Smart EEG Monitoring Amidst the COVID-19 Pandemic. Electronics 2021, 10, 1001. https://doi.org/10.3390/electronics10091001
Faezipour M, Faezipour M. Efficacy of Smart EEG Monitoring Amidst the COVID-19 Pandemic. Electronics. 2021; 10(9):1001. https://doi.org/10.3390/electronics10091001
Chicago/Turabian StyleFaezipour, Misagh, and Miad Faezipour. 2021. "Efficacy of Smart EEG Monitoring Amidst the COVID-19 Pandemic" Electronics 10, no. 9: 1001. https://doi.org/10.3390/electronics10091001
APA StyleFaezipour, M., & Faezipour, M. (2021). Efficacy of Smart EEG Monitoring Amidst the COVID-19 Pandemic. Electronics, 10(9), 1001. https://doi.org/10.3390/electronics10091001






