Performance Improvement of ZnSnO Thin-Film Transistors with Low-Temperature Self-Combustion Reaction
Abstract
1. Introduction
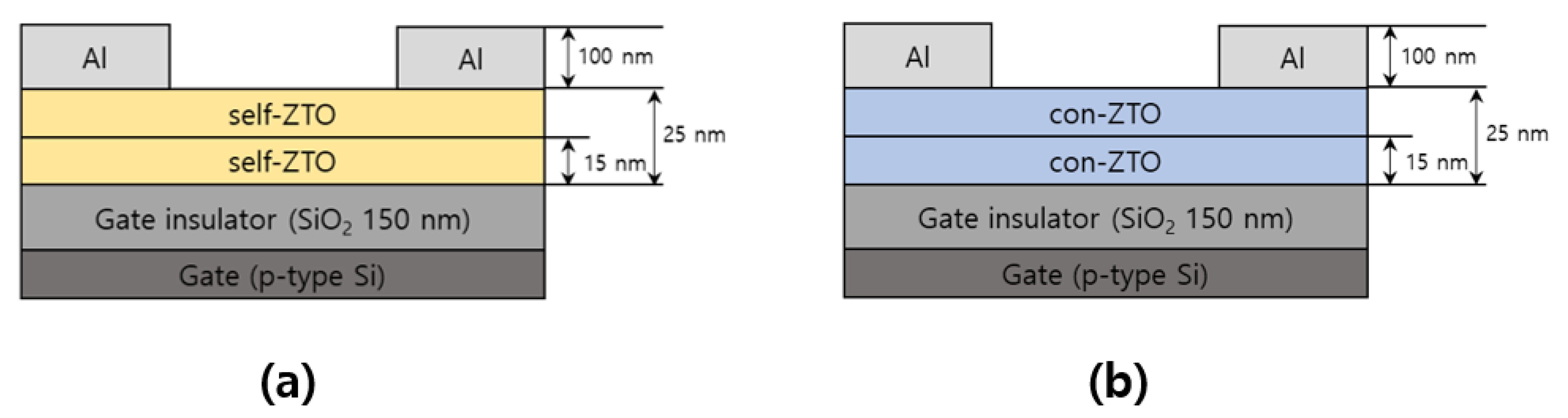
2. Materials and Methods
3. Results
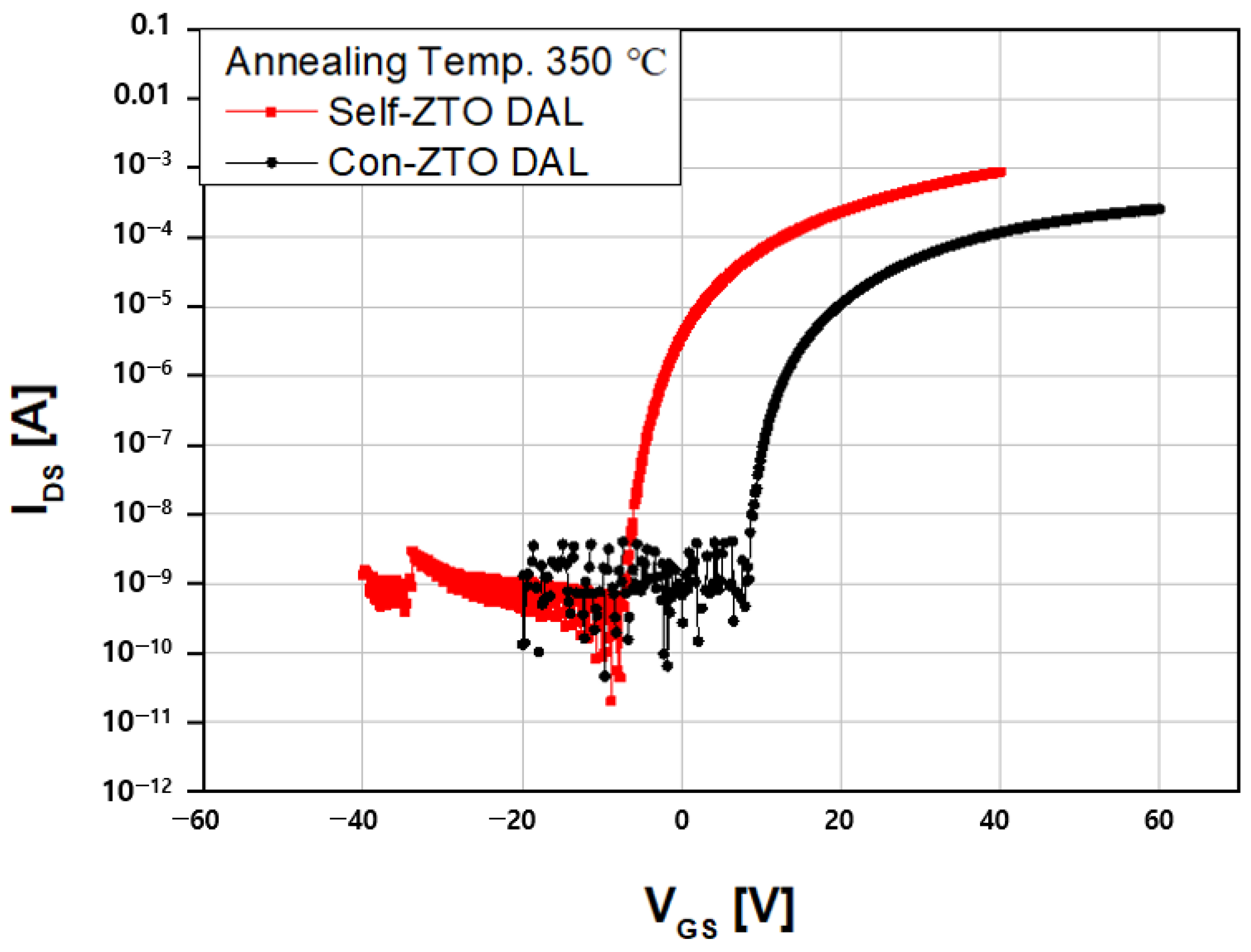
3.1. Electronical Performance
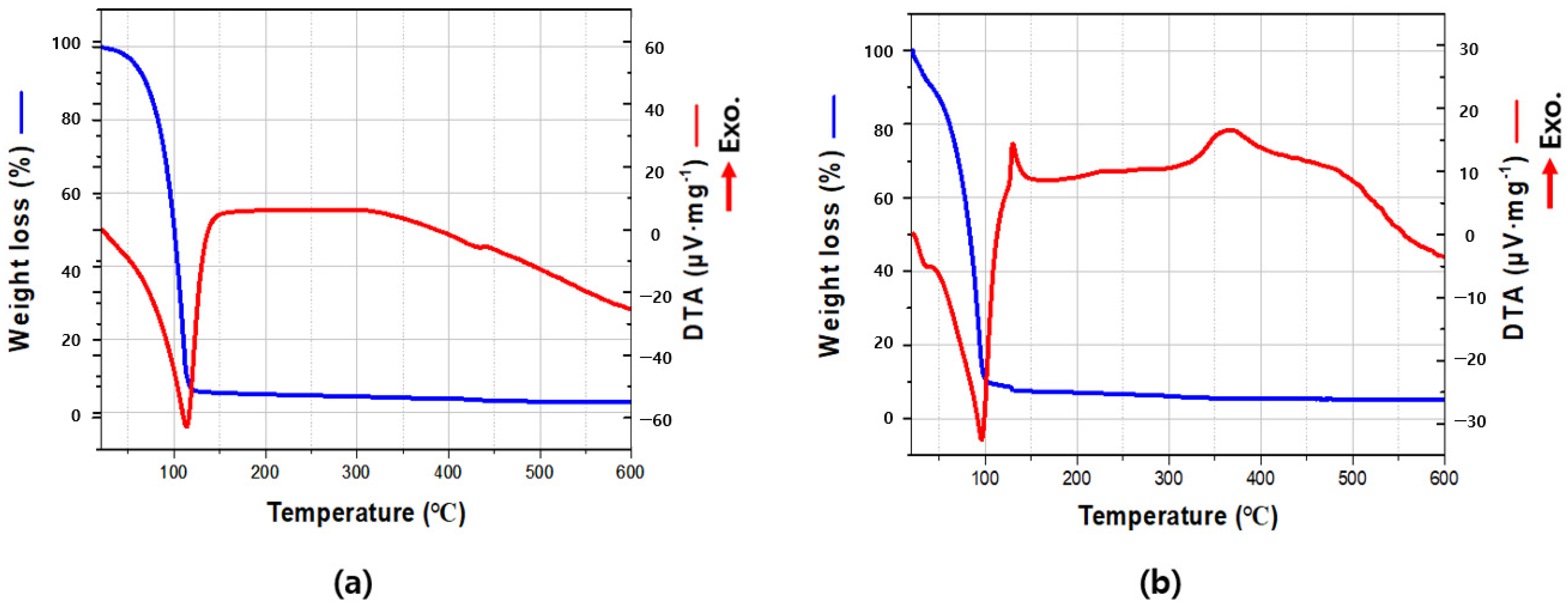
3.2. TG-DTA Analyses
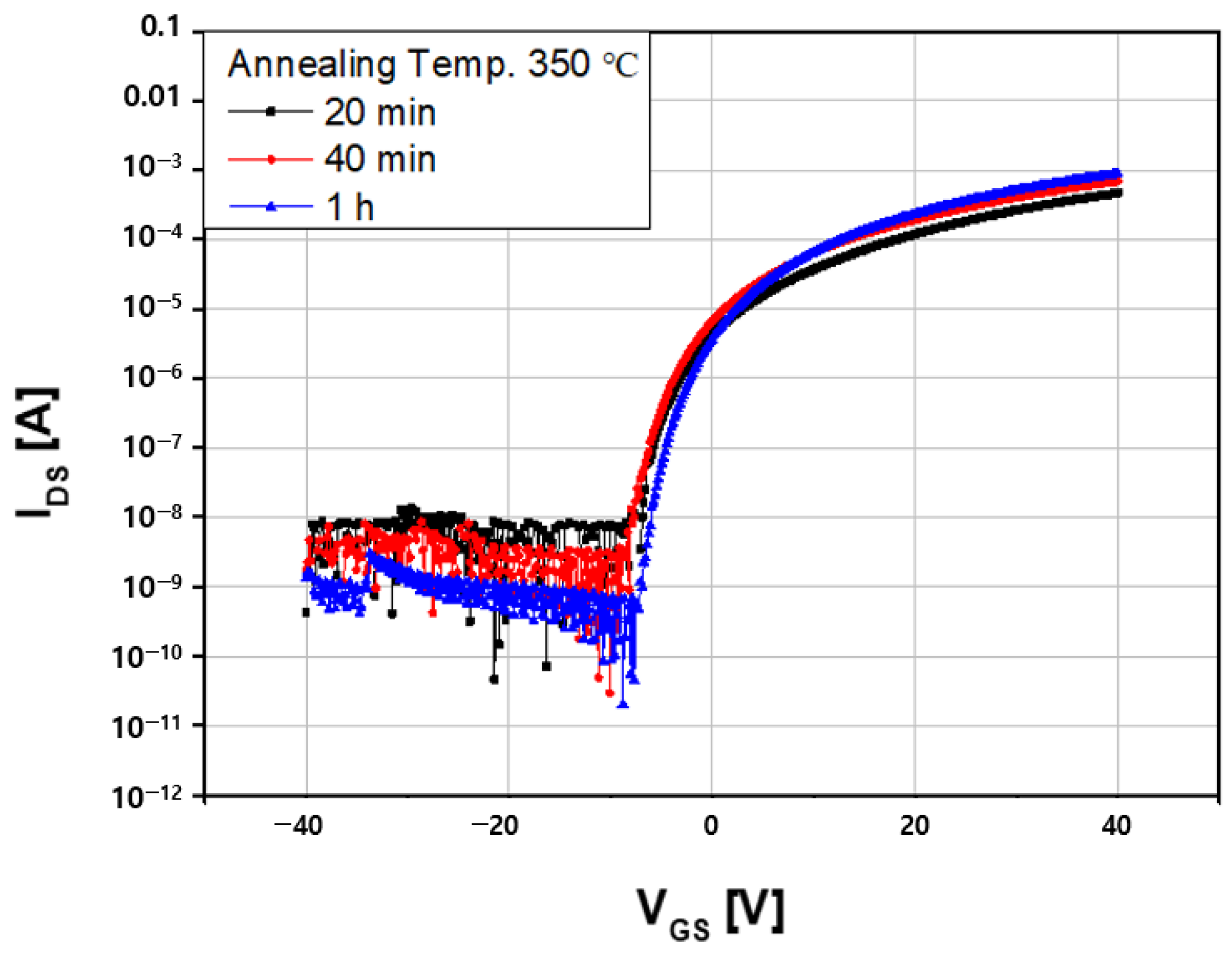
3.3. Self-ZTO Properties at Various Annealing Time
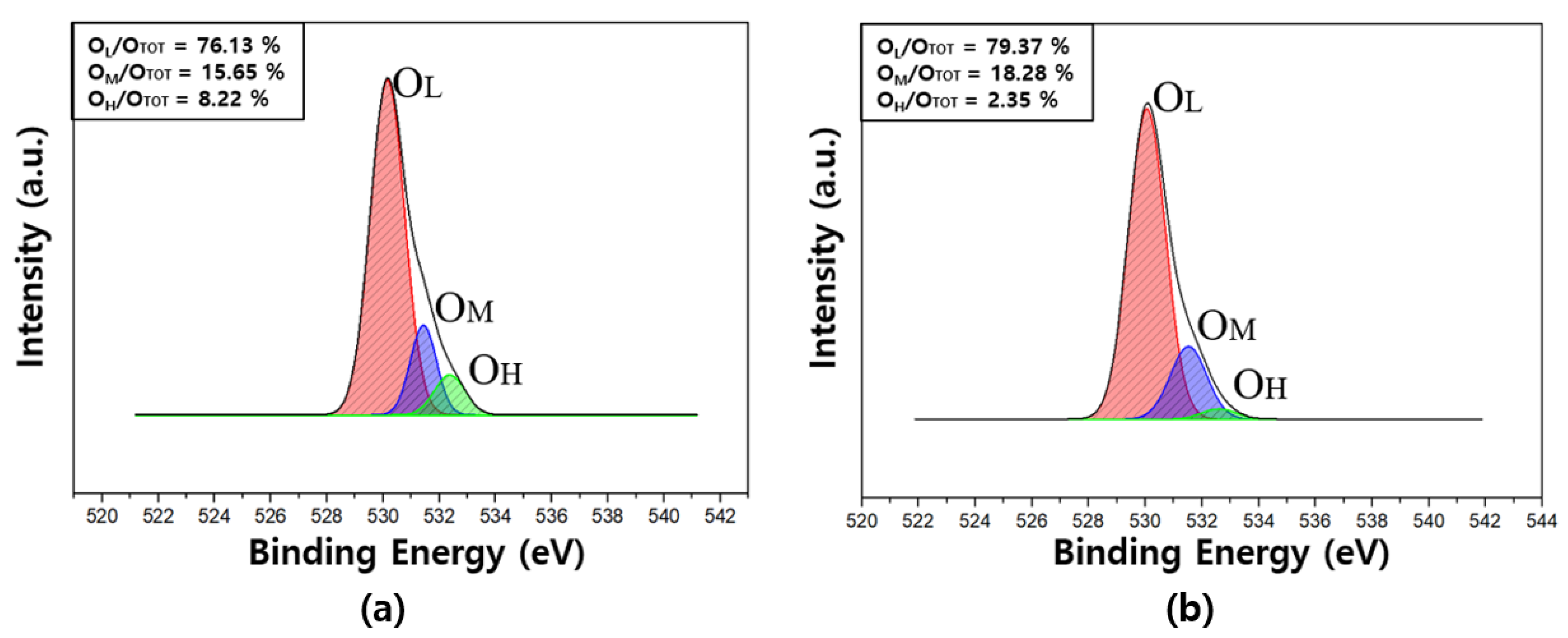
3.4. XPS Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nomura, K.; Ohta, H.; Takagi, A.; Kamina, T.; Hirano, M.; Hosono, H. Room-temperature fabrication of transparent flexible thin-film transistors using amorphous oxide semiconductors. Nature 2004, 432, 488–492. [Google Scholar] [CrossRef]
- Kamiya, T.; Nomura, K.; Hosono, H. Present status of amorphous In-Ga-Zn-O thin-film transistors. Sci. Technol. Adv. Mater. 2010, 11, 044305. [Google Scholar] [CrossRef]
- Park, J.S.; Maeng, W.J.; Kim, H.S.; Park, J.S. Review of recent developments in amorphous oxide semiconductor thin-film transistor devices. Thin Solid Films 2012, 520, 1679–1693. [Google Scholar] [CrossRef]
- Han, D.S.; Kang, Y.J.; Park, J.H.; Jeon, H.T.; Park, J.W. Influence of molybdenum source/drain electrode contact resistance in amorphous zinc-tin-oxide (a-ZTO) thin film transistors. Mater. Res. Bull. 2014, 58, 174–177. [Google Scholar] [CrossRef]
- Cho, S.W.; Kim, D.E.; Kang, W.J.; Kim, B.; Yoon, D.H.; Kim, K.S.; Cho, H.K.; Kim, Y.H.; Kim, Y. Chemical durability engineering of solution-processed oxide thin films and its application in chemically-robust patterned oxide thin-film transistors. J. Mater. Chem. C 2017, 5, 339–349. [Google Scholar] [CrossRef]
- Kim, S.J.; Yoon, S.; Kim, H.J. Review of solution-processed oxide thin-film transistors. Jpn. J. Appl. Phys. 2014, 53, 02BA02. [Google Scholar] [CrossRef]
- Lim, K.H.; Lee, J.; Huh, J.E.; Park, J.; Lee, J.H.; Lee, S.E.; Kim, Y.S. A systematic study on effects of precursors and solvents for optimization of solution-processed oxide semiconductor thin-film transistors. J. Mater. Chem. C 2017, 5, 7768–7776. [Google Scholar] [CrossRef]
- Lim John, R.A.; Nguyen, A.C.; Chen, Y.; Shukla, S.; Chen, S.; Mathews, N. Modulating Cationic Ratios for High-Performance Transparent Solution-Processed Electronics, ACS Appl. Mater. Interfaces 2016, 8, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.G.; Lee, N.H.; Kwon, Y.K.; Kang, B. Effect of film thickness and Sn concentration on electrical properties of solution-processed zinc tin oxide thin film transistors. Thin Solid Films 2013, 554, 129–133. [Google Scholar] [CrossRef]
- Ruzgar, S.; Caglar, M. The effect of Sn on electrical performance of zinc oxide based thin film transistor. J. Mater. Sci. Mater. Electron. 2019, 30, 485–490. [Google Scholar] [CrossRef]
- Yoon, S.; Tak, Y.J.; Yoon, D.H.; Choi, U.H.; Park, J.S.; Ahn, B.D.; Kim, H.J. Study of Nitrogen High-Pressure Annealing on InGaZnO Thin-Film Transistors. ACS Appl. Mater. Interfaces 2014, 6, 13496–13501. [Google Scholar] [CrossRef]
- Kim, M.G.; Kanatzidis, M.G.; Facchetti, A.; Marks, T.J. Low-temperature fabrication of high-performance metal oxide thin-film electronics via combustion processing. Nat. Mater. 2011, 10, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.H.; Jeong, S.; Ko, J.M.; Lee, J.Y.; Choi, Y.; Lee, C.; Cho, S.Y. Two-component solution processing of oxide semiconductors for thin-film transistors via self-combustion reaction. J. Mater. Chem. C 2014, 2, 4247–4256. [Google Scholar] [CrossRef]
- Jiang, Q.; Feong, L.; Wu, C.; Sun, R.; Li, X.; Lu, B.; Ye, Z.; Lu, J. Amorphous ZnAlSnO thin-film transistors by a combustion solution process for future displays. Appl. Phys. Lett. 2015, 106, 053503. [Google Scholar] [CrossRef]
- Yu, X.; Zhou, N.; Smith, J.; Lin, H.; Stallings, K.; Yu, J.; Marks, T.J.; Facchetti, A. Synergistic Approach to High-Performance Oxide Thin Film Transistors Using a Bilayer Channel Architecture. ACS Appl. Mater. Interfaces 2013, 5, 7983–7988. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.J.; Kang, Y.H.; Han, M.; Lee, C.; Cho, S.Y. Soluble oxide gate dielectrics prepared using the self-combustion reaction for high-performance thin-film transistors. J. Mater. Chem. C 2014, 2, 5695–5703. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, S.; Woo, K.; Kim, Y.J.; Bak, S.Y.; Han, Y.J.; Kim, S.; Han, T.H.; Yi, M. Effects of thermal annealing time and molar ratio of channel layers on solution-processed ZnO/SnO2 thin-film transistors. Solid State Electron. 2020, 165, 107765. [Google Scholar] [CrossRef]
- Young, D.L.; Moutinho, H.; Yan, Y.; Coutts, T.J. Growth and characterization of radio frequency magnetron sputter-deposited zinc stannate, Zn2SnO4, thin films. J. Appl. Phys. 2002, 92, 310–319. [Google Scholar] [CrossRef]
- Chiang, H.Q.; Wager, J.F. High mobility transparent thin-film transistors with amorphous zinc tin oxide channel layer. Appl. Phys. Lett. 2005, 86, 013503. [Google Scholar] [CrossRef]
- Kim, D.E.; Cho, S.W.; Kim, Y.B.; Kim, K.S.; Yoon, D.H.; Jung, S.H.; Kang, W.J.; Cho, H.K. Electrical and chemical stability engineering of solution-processed indium zinc oxide thin film transistors via a synergistic approach of annealing duration and self-combustion process. Ceram. Int. 2017, 43, 8956–8962. [Google Scholar] [CrossRef]
- Kim, J.; Bang, J.; Nakamura, N.; Hosono, H. Ultra-wide bandgap amorphous oxide semiconductors for NBIS-free thin-film transistors. APL Mater. 2019, 7, 022501. [Google Scholar] [CrossRef]

| Temperature [°C] | μsat [cm2/V∙s] | Vth [V] | ION/OFF | SS [V/dec] | |
|---|---|---|---|---|---|
| Con-ZTO DAL | 350 | 1.37 | +12.16 | 3.72 × 105 | 0.73 |
| 400 | 2.80 | +11.36 | 1.58 × 106 | 0.56 | |
| 470 | 5.67 | +2.40 | 2.51 × 106 | 0.46 | |
| Self-ZTO DAL | 330 | 2.91 | +1.74 | 9.33 × 105 | 0.97 |
| 350 | 4.72 | −1.28 | 1.70 × 106 | 0.86 | |
| 370 | 5.60 | −0.32 | 1.95 × 106 | 0.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.-J.; Lee, S.H.; Bak, S.-Y.; Han, T.-H.; Kim, S.; Yi, M. Performance Improvement of ZnSnO Thin-Film Transistors with Low-Temperature Self-Combustion Reaction. Electronics 2021, 10, 1099. https://doi.org/10.3390/electronics10091099
Han Y-J, Lee SH, Bak S-Y, Han T-H, Kim S, Yi M. Performance Improvement of ZnSnO Thin-Film Transistors with Low-Temperature Self-Combustion Reaction. Electronics. 2021; 10(9):1099. https://doi.org/10.3390/electronics10091099
Chicago/Turabian StyleHan, Ye-Ji, Se Hyeong Lee, So-Young Bak, Tae-Hee Han, Sangwoo Kim, and Moonsuk Yi. 2021. "Performance Improvement of ZnSnO Thin-Film Transistors with Low-Temperature Self-Combustion Reaction" Electronics 10, no. 9: 1099. https://doi.org/10.3390/electronics10091099
APA StyleHan, Y.-J., Lee, S. H., Bak, S.-Y., Han, T.-H., Kim, S., & Yi, M. (2021). Performance Improvement of ZnSnO Thin-Film Transistors with Low-Temperature Self-Combustion Reaction. Electronics, 10(9), 1099. https://doi.org/10.3390/electronics10091099





