Introduction of a Novel Image-Based and Non-Invasive Method for the Estimation of Local Elastic Properties of Great Vessels
Abstract
:1. Introduction
2. Materials and Methods
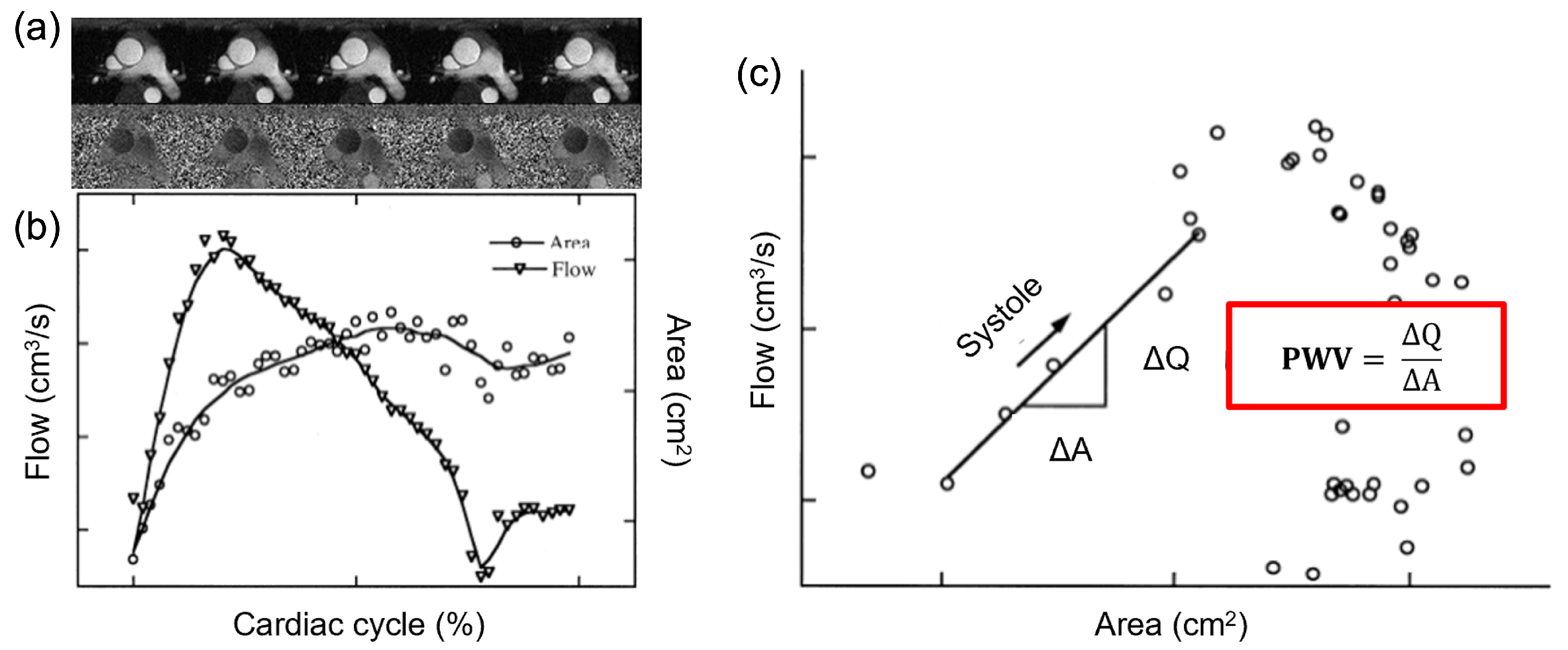
2.1. Theoretical Background
2.2. Definition of the Image-Based Formulation
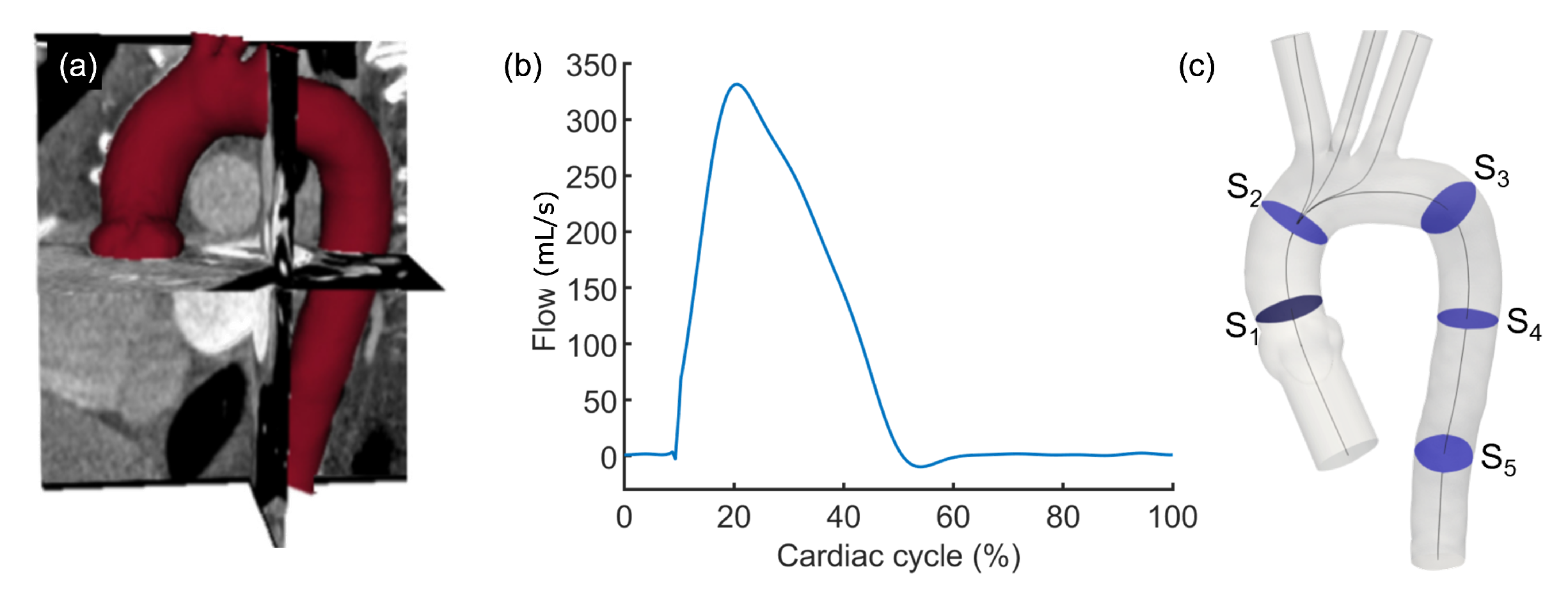
2.3. Fluid-Structure Interaction Simulation Workflow
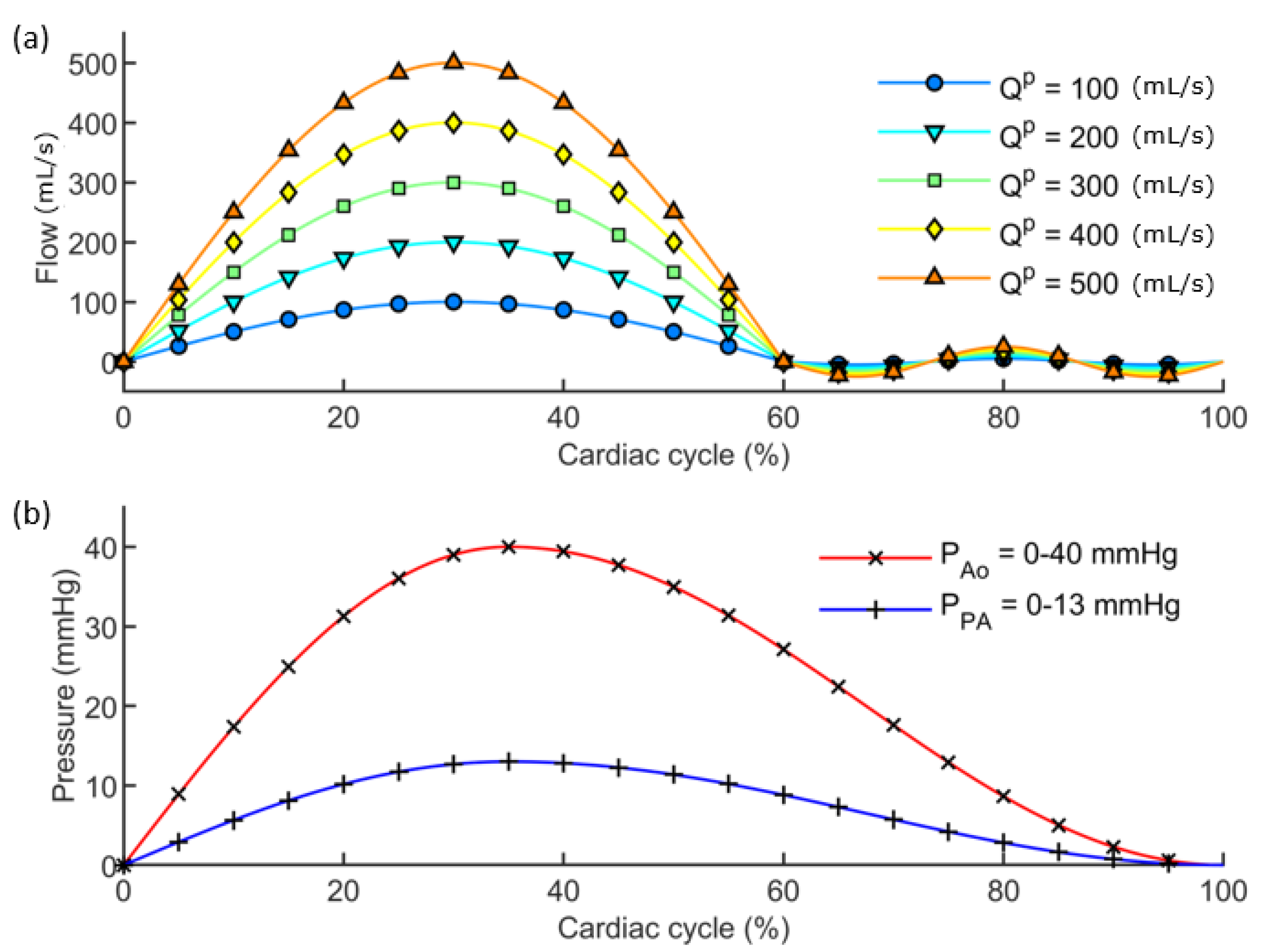
2.4. Application of the Image-Based Method to Virtual Models
3. Results
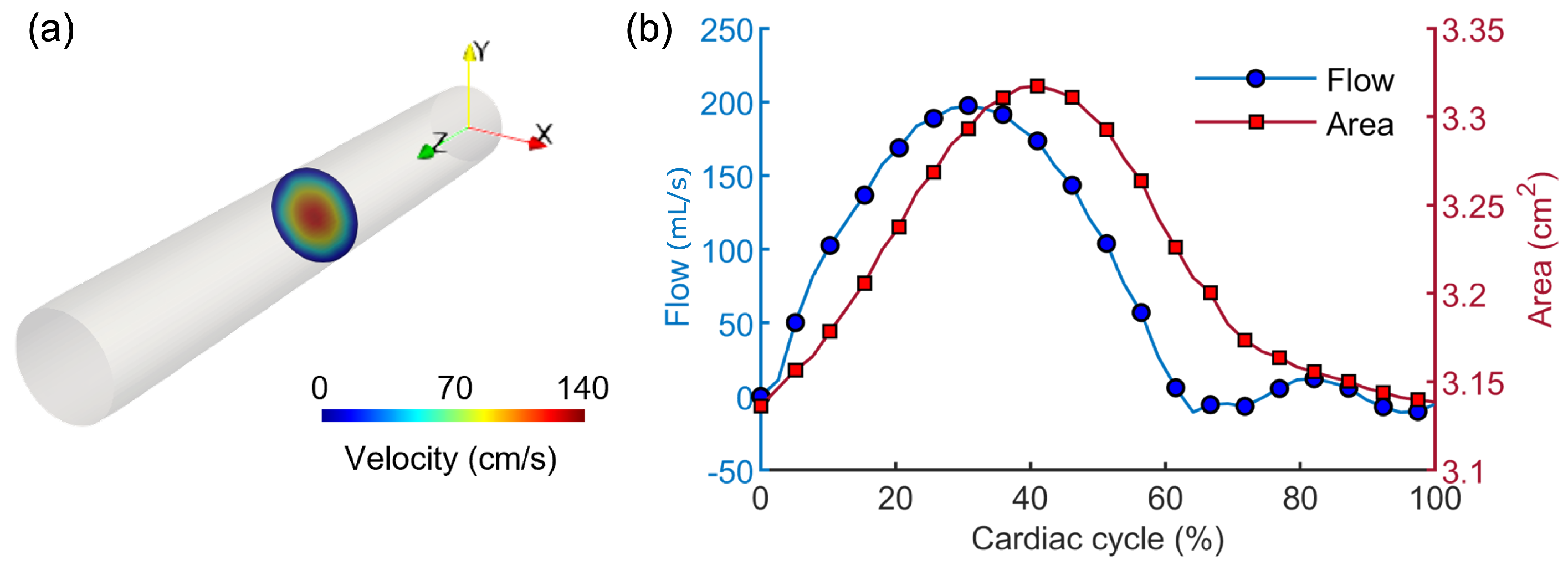
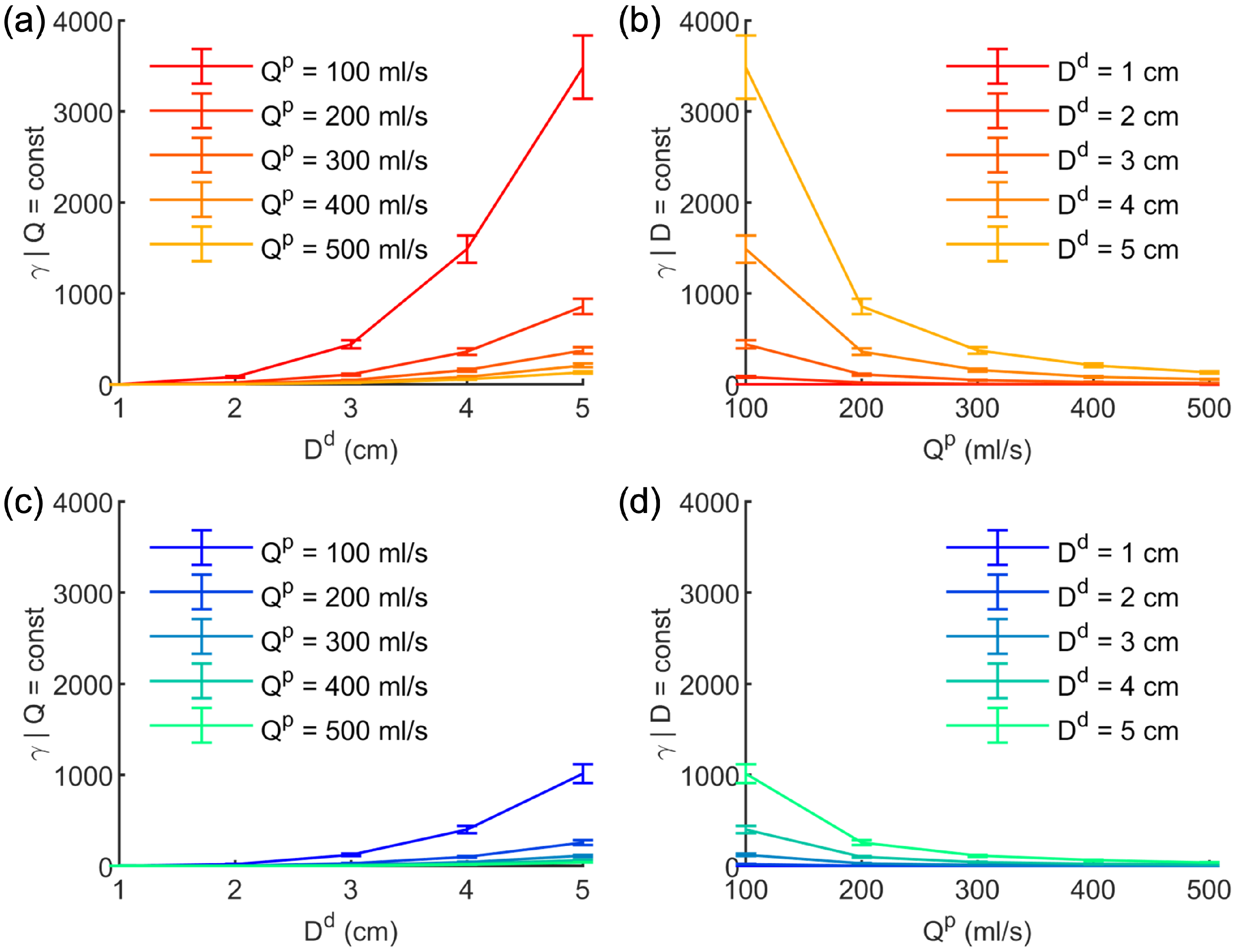
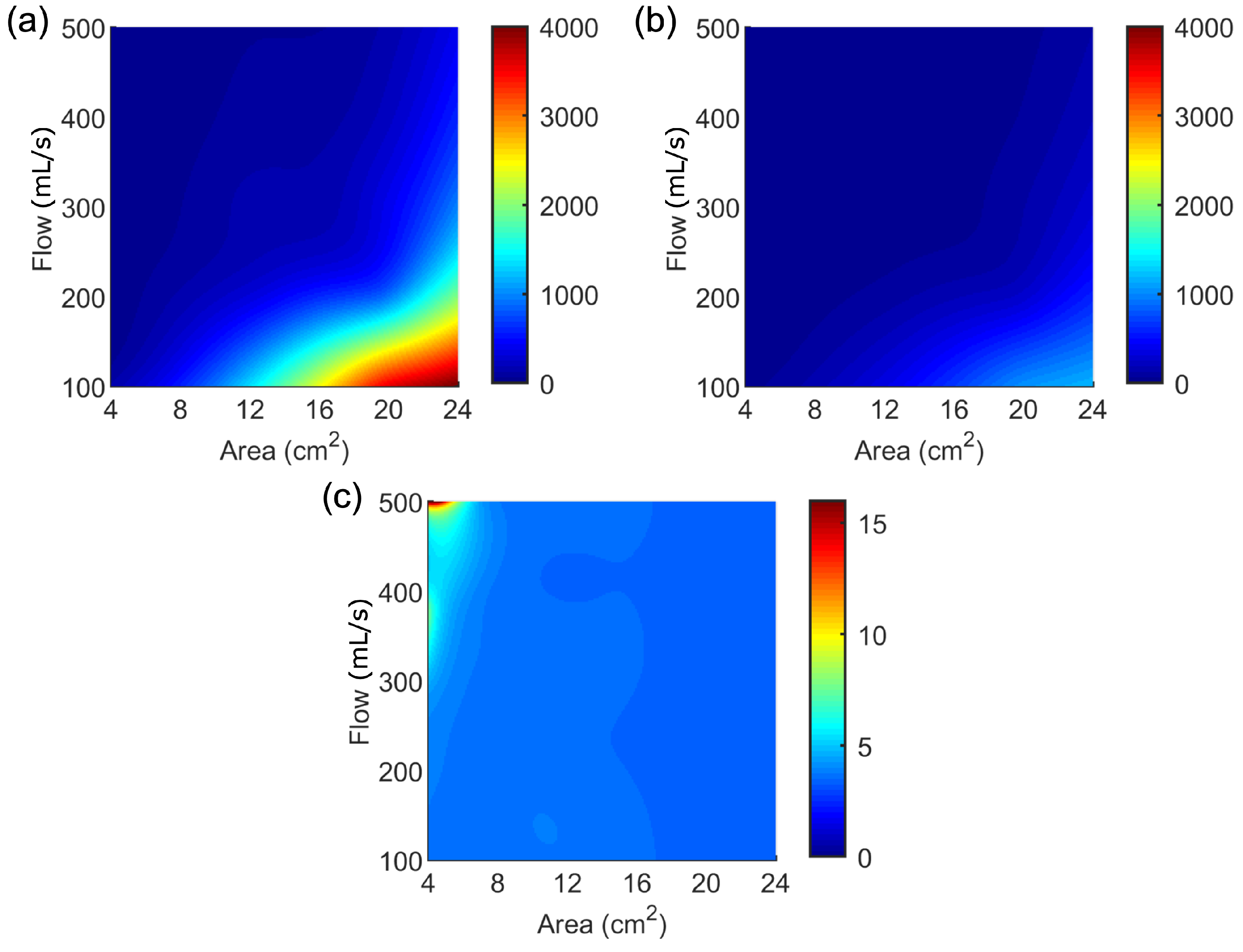
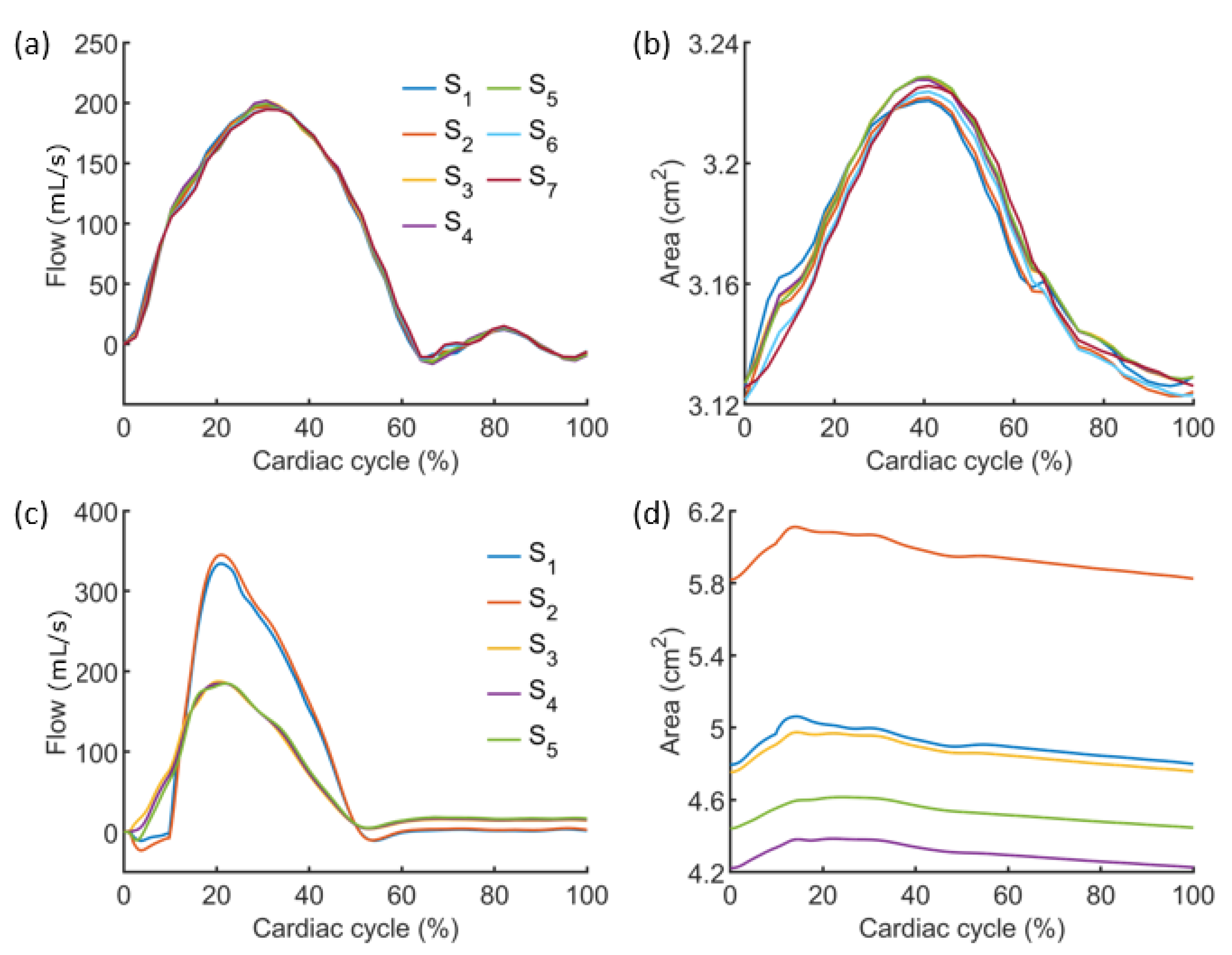
3.1. Results from the Simulations Workflow
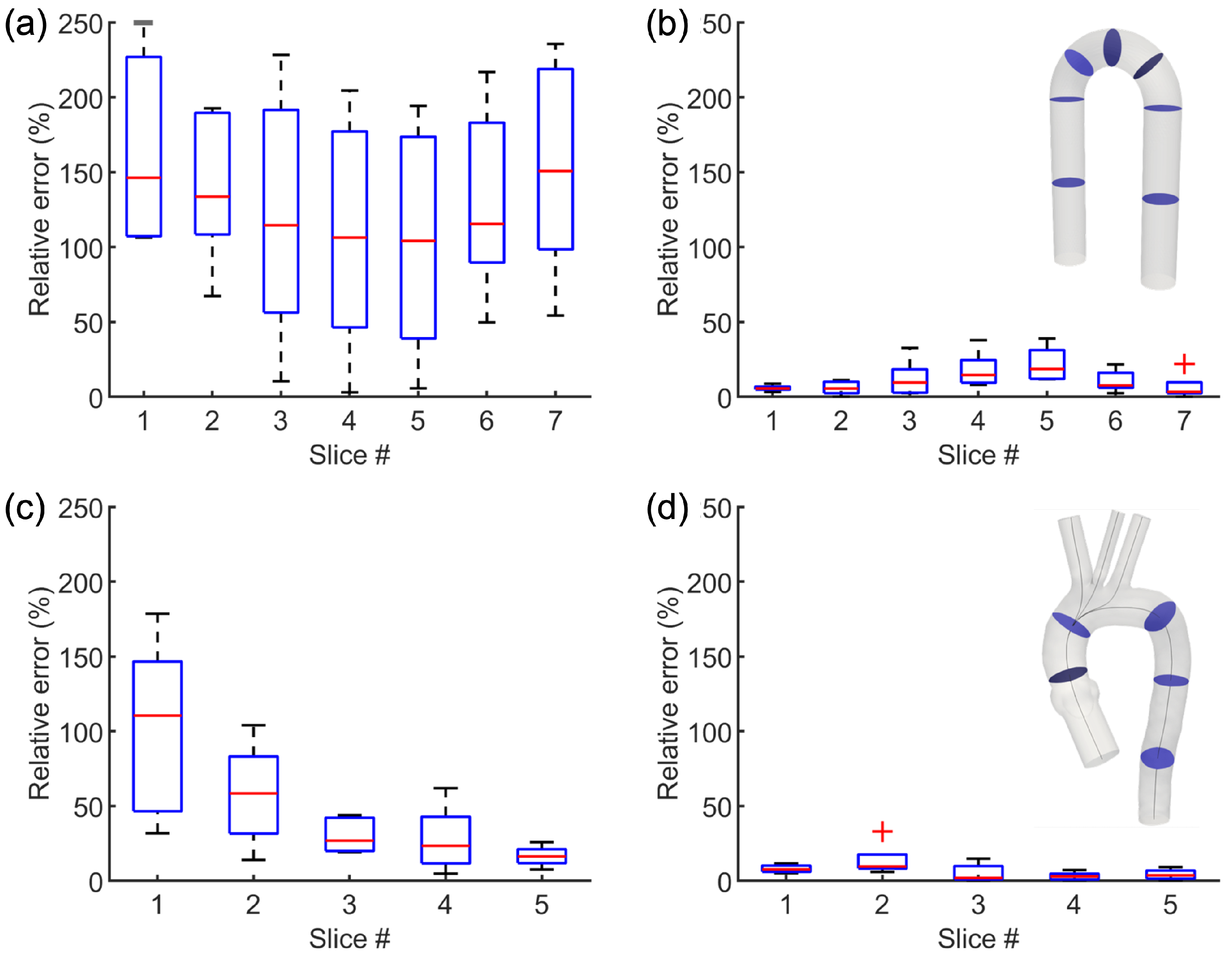
3.2. Results from the Application of the Image-Based Method
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Capellini, K.; Gasparotti, E.; Cella, U.; Costa, E.; Fanni, B.M.; Groth, C.; Porziani, S.; Biancolini, M.E.; Celi, S. A novel formulation for the study of the ascending aortic fluid dynamics with in vivo data. Med. Eng. Phys. 2020, 91, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wu, W.; Samant, S.; Khan, B.; Kassab, G.S.; Watanabe, Y.; Murasato, Y.; Sharzehee, M.; Makadia, J.; Zolty, D.; et al. Patient-specific computational simulation of coronary artery bifurcation stenting. Sci. Rep. 2021, 11, 16486. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.; Kerfoot, E.; Mauger, C.; Ismail, T.F.; Young, A.A.; Nordsletten, D.A. An Implementation of Patient-Specific Biventricular Mechanics Simulations With a Deep Learning and Computational Pipeline. Front. Physiol. 2021, 12, 716597. [Google Scholar] [CrossRef] [PubMed]
- Viceconti, M.; Pappalardo, F.; Rodriguez, B.; Horner, M.; Bischoff, J.; Musuamba Tshinanu, F. In silico trials: Verification, validation and uncertainty quantification of predictive models used in the regulatory evaluation of biomedical products. Methods 2021, 185, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Antonini, L.; Mandelli, L.; Berti, F.; Pennati, G.; Petrini, L. Validation of the computational model of a coronary stent: A fundamental step towards in silico trials. J. Mech. Behav. Biomed. Mater. 2021, 122, 104644. [Google Scholar] [CrossRef] [PubMed]
- Huberts, W.; Heinen, S.G.H.; Zonnebeld, N.; van den Heuvel, D.A.F.; de Vries, J.P.M.; Tordoir, J.H.M.; Hose, D.R.; Delhaas, T.; van de Vosse, F.N. What is needed to make cardiovascular models suitable for clinical decision support? A viewpoint paper. J. Comput. Sci. 2018, 24, 68–84. [Google Scholar] [CrossRef]
- Gray, R.A.; Pathmanathan, P. Patient-Specific Cardiovascular Computational Modeling: Diversity of Personalization and Challenges. J. Cardiovasc. Transl. Res. 2018, 11, 80–88. [Google Scholar] [CrossRef] [Green Version]
- Spronck, B.; Humphrey, J.D. Arterial Stiffness: Different Metrics, Different Meanings. J. Biomech. Eng. 2019, 141, 091004. [Google Scholar] [CrossRef] [Green Version]
- Brault, A.; Dumas, L.; Lucor, D. Uncertainty quantification of inflow boundary condition and proximal arterial stiffness-coupled effect on pulse wave propagation in a vascular network: UQ of pulse wave propagation in a vascular network. Int. J. Numer. Methods Biomed. Eng. 2017, 33, e2859. [Google Scholar] [CrossRef] [Green Version]
- Rego, B.V.; Weiss, D.; Bersi, M.R.; Humphrey, J.D. Uncertainty quantification in subject-specific estimation of local vessel mechanical properties. Int. J. Numer. Methods Biomed. Eng. 2021, 37, e3535. [Google Scholar] [CrossRef]
- Romarowski, R.M.; Lefieux, A.; Morganti, S.; Veneziani, A.; Auricchio, F. Patient-specific CFD modelling in the thoracic aorta with PC-MRI–based boundary conditions: A least-square three-element Windkessel approach. Int. J. Numer. Methods Biomed. Eng. 2018, 34, e3134. [Google Scholar] [CrossRef] [PubMed]
- Antonuccio, M.N.; Mariotti, A.; Fanni, B.M.; Capellini, K.; Capelli, C.; Sauvage, E.; Celi, S. Effects of Uncertainty of Outlet Boundary Conditions in a Patient-Specific Case of Aortic Coarctation. Ann. Biomed. Eng. 2021, 49, 3494–3507. [Google Scholar] [CrossRef] [PubMed]
- Swanson, L.; Owen, B.; Keshmiri, A.; Deyranlou, A.; Aldersley, T.; Lawrenson, J.; Human, P.; De Decker, R.; Fourie, B.; Comitis, G.; et al. A Patient-Specific CFD Pipeline Using Doppler Echocardiography for Application in Coarctation of the Aorta in a Limited Resource Clinical Context. Front. Bioeng. Biotechnol. 2020, 8, 409. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, D.R.; Gutmark, E.; Andersen, N.; Nielsen, D.; Mortensen, K.; Gravholt, C.; Backeljauw, P.; Gutmark-Little, I. Influence of Material Model and Aortic Root Motion in Finite Element Analysis of Two Exemplary Cases of Proximal Aortic Dissection. J. Biomech. Eng. 2021, 143, 014504. [Google Scholar] [CrossRef] [PubMed]
- Celi, S.; Berti, S. Biomechanics and FE Modelling of Aneurysm: Review and Advances in Computational Models. In Aneurysm; InTech: London, UK, 2012. [Google Scholar] [CrossRef] [Green Version]
- Boccadifuoco, A.; Mariotti, A.; Capellini, K.; Celi, S.; Salvetti, M.V. Validation of numerical simulations of thoracic aorta hemodynamics: Comparison with in vivo measurements and stochastic sensitivity analysis. Cardiovasc. Eng. Technol. 2018, 9, 688–706. [Google Scholar] [CrossRef]
- De Nisco, G.; Tasso, P.; Calò, K.; Mazzi, V.; Gallo, D.; Condemi, F.; Farzaneh, S.; Avril, S.; Morbiducci, U. Deciphering ascending thoracic aortic aneurysm hemodynamics in relation to biomechanical properties. Med. Eng. Phys. 2020, 82, 119–129. [Google Scholar] [CrossRef]
- Vignali, E.; Gasparotti, E.; Celi, S.; Avril, S. Fully-Coupled FSI Computational Analyses in the Ascending Thoracic Aorta Using Patient-Specific Conditions and Anisotropic Material Properties. Front. Physiol. 2021, 12, 732561. [Google Scholar] [CrossRef]
- Vignali, E.; Gasparotti, E.; Capellini, K.; Fanni, B.M.; Landini, L.; Positano, V.; Celi, S. Modeling biomechanical interaction between soft tissue and soft robotic instruments: Importance of constitutive anisotropic hyperelastic formulations. Int. J. Robot. Res. 2021, 40, 224–235. [Google Scholar] [CrossRef]
- Cebull, H.L.; Rayz, V.L.; Goergen, C.J. Recent Advances in Biomechanical Characterization of Thoracic Aortic Aneurysms. Front. Cardiovasc. Med. 2020, 7, 75. [Google Scholar] [CrossRef]
- Vignali, E.; di Bartolo, F.; Gasparotti, E.; Malacarne, A.; Concistré, G.; Chiaramonti, F.; Murzi, M.; Positano, V.; Landini, L.; Celi, S. Correlation between micro and macrostructural biaxial behavior of ascending thoracic aneurysm: A novel experimental technique. Med. Eng. Phys. 2020, 86, 78–85. [Google Scholar] [CrossRef]
- Liu, M.; Liang, L.; Sulejmani, F.; Lou, X.; Iannucci, G.; Chen, E.; Leshnower, B.; Sun, W. Identification of in vivo nonlinear anisotropic mechanical properties of ascending thoracic aortic aneurysm from patient-specific CT scans. Sci. Rep. 2019, 9, 12983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flamini, V.; Creane, A.P.; Kerskens, C.M.; Lally, C. Imaging and finite element analysis: A methodology for non-invasive characterization of aortic tissue. Med. Eng. Phys. 2015, 37, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Wittek, A.; Derwich, W.; Karatolios, K.; Fritzen, C.P.; Vogt, S.; Schmitz-Rixen, T.; Blase, C. A finite element updating approach for identification of the anisotropic hyperelastic properties of normal and diseased aortic walls from 4D ultrasound strain imaging. J. Mech. Behav. Biomed. Mater. 2016, 58, 122–138. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, G.A.; Taylor, M.D.; Banerjee, R.K. Evaluation of pulmonary artery wall properties in congenital heart disease patients using cardiac magnetic resonance. Prog. Pediatr. Cardiol. 2017, 47, 49–57. [Google Scholar] [CrossRef]
- Zambrano, B.A.; McLean, N.A.; Zhao, X.; Tan, J.; Zhong, L.; Figueroa, C.A.; Lee, L.C.; Baek, S. Image-based computational assessment of vascular wall mechanics and hemodynamics in pulmonary arterial hypertension patients. J. Biomech. 2018, 68, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, R.M.; Liau, J.; Kaffas, A.E.; Chammas, M.C.; Willmann, J.K. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, B.; Ewertsen, C.; Carlsen, J.; Nielsen, M. Ultrasound Vascular Elastography as a Tool for Assessing Atherosclerotic Plaques – A Systematic Literature Review. Ultrasound Int. Open 2016, 2, E106–E112. [Google Scholar] [CrossRef] [Green Version]
- Low, G. General review of magnetic resonance elastography. World J. Radiol. 2016, 8, 59. [Google Scholar] [CrossRef]
- Pruijssen, J.T.; de Korte, C.L.; Voss, I.; Hansen, H.H.G. Vascular Shear Wave Elastography in Atherosclerotic Arteries: A Systematic Review. Ultrasound Med. Biol. 2020, 46, 2145–2163. [Google Scholar] [CrossRef]
- Huang, C.; Lan, Y.; Xu, G.; Zhai, X.; Wu, J.; Lin, F.; Zeng, N.; Hong, Q.; Ng, E.Y.K.; Peng, Y.; et al. A Deep Segmentation Network of Multi-Scale Feature Fusion Based on Attention Mechanism for IVOCT Lumen Contour. IEEE/ACM Trans. Comput. Biol. Bioinform. 2021, 18, 62–69. [Google Scholar] [CrossRef]
- Celi, S.; Gasparotti, E.; Capellini, K.; Vignali, E.; Fanni, B.M.; Ait-Ali, L.; Cantinotti, M.; Murzi, M.; Berti, S.; Santoro, G.; et al. 3D Printing in Modern Cardiology. Curr. Pharm. Des. 2021, 27, 1918–1930. [Google Scholar] [CrossRef] [PubMed]
- Fanni, B.M.; Sauvage, E.; Celi, S.; Norman, W.; Vignali, E.; Landini, L.; Schievano, S.; Positano, V.; Capelli, C. A Proof of Concept of a Non-Invasive Image-Based Material Characterization Method for Enhanced Patient-Specific Computational Modeling. Cardiovasc. Eng. Technol. 2020, 11, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Milan, A.; Zocaro, G.; Leone, D.; Tosello, F.; Buraioli, I.; Schiavone, D.; Veglio, F. Current assessment of pulse wave velocity: Comprehensive review of validation studies. J. Hypertens. 2019, 37, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Vulliémoz, S.; Stergiopulos, N.; Meuli, R. Estimation of local aortic elastic properties with MRI: Estimation of Local Aortic Elastic Properties. Magn. Reson. Med. 2002, 47, 649–654. [Google Scholar] [CrossRef]
- Rabben, S.I.; Stergiopulos, N.; Hellevik, L.R.; Smiseth, O.A.; Slørdahl, S.; Urheim, S.; Angelsen, B. An ultrasound-based method for determining pulse wave velocity in superficial arteries. J. Biomech. 2004, 37, 1615–1622. [Google Scholar] [CrossRef]
- Boonyasirinant, T.; Rajiah, P.; Flamm, S.D. Abnormal aortic stiffness in patients with bicuspid aortic valve: Phenotypic variation determined by magnetic resonance imaging. Int. J. Cardiovasc. Imaging 2019, 35, 133–141. [Google Scholar] [CrossRef]
- Tiwari, K.K.; Bevilacqua, S.; Aquaro, G.; Festa, P.; Ait-Ali, L.; Solinas, M. Evaluation of Distensibility and Stiffness of Ascending Aortic Aneurysm using Magnetic Resonance Imaging. J. Nepal Med. Assoc. 2016, 55, 67–71. [Google Scholar] [CrossRef]
- Sugawara, J.; Tomoto, T.; Tanaka, H. Heart-to-Brachium Pulse Wave Velocity as a Measure of Proximal Aortic Stiffness: MRI and Longitudinal Studies. Am. J. Hypertens. 2019, 32, 146–154. [Google Scholar] [CrossRef]
- Bramwell, J.C.; Hill, A.V. The velocity of pulse wave in man. Proc. R. Soc. London. Ser. B Contain. Pap. Biol. Character 1922, 93, 298–306. [Google Scholar] [CrossRef] [Green Version]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H.; et al. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef] [Green Version]
- Ahrens, J.; Geveci, B.; Law, C. ParaView: An End-User Tool for Large-Data Visualization. In Visualization Handbook; Elsevier: Amsterdam, The Netherlands, 2005; pp. 717–731. [Google Scholar] [CrossRef]
- Westerhof, N.; Lankhaar, J.; Westerhof, B.E. The arterial Windkessel. Med. Biol. Eng. Comput. 2009, 47, 131–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vatner, S.F.; Zhang, J.; Vyzas, C.; Mishra, K.; Graham, R.M.; Vatner, D.E. Vascular Stiffness in Aging and Disease. Front. Physiol. 2021, 12, 762437. [Google Scholar] [CrossRef] [PubMed]
- Segers, P.; Rietzschel, E.R.; Chirinos, J.A. How to Measure Arterial Stiffness in Humans. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1034–1043. [Google Scholar] [CrossRef] [Green Version]
- Grotenhuis, H.B.; Westenberg, J.J.M.; Steendijk, P.; van der Geest, R.J.; Ottenkamp, J.; Bax, J.J.; Jukema, J.W.; de Roos, A. Validation and reproducibility of aortic pulse wave velocity as assessed with velocity-encoded MRI. J. Magn. Reson. Imaging 2009, 30, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Bertoglio, C.; Barber, D.; Gaddum, N.; Valverde, I.; Rutten, M.; Beerbaum, P.; Moireau, P.; Hose, R.; Gerbeau, J.F. Identification of artery wall stiffness: In vitro validation and in vivo results of a data assimilation procedure applied to a 3D fluid–structure interaction model. J. Biomech. 2014, 47, 1027–1034. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Yang, Y.; Yuan, L.j.; Liu, J.; Duan, Y.y.; Cao, T.s. Noninvasive Method for Measuring Local Pulse Wave Velocity by Dual Pulse Wave Doppler: In Vitro and In Vivo Studies. PLoS ONE 2015, 10, e0120482. [Google Scholar] [CrossRef]
- Chen, Z.W.; Joli, P.; Feng, Z.Q. Anisotropic hyperelastic behavior of soft biological tissues. Comput. Methods Biomech. Biomed. Eng. 2015, 18, 1436–1444. [Google Scholar] [CrossRef]
- Nolan, D.R.; Gower, A.L.; Destrade, M.; Ogden, R.W.; McGarry, J.P. A robust anisotropic hyperelastic formulation for the modelling of soft tissue. J. Mech. Behav. Biomed. Mater. 2014, 39, 48–60. [Google Scholar] [CrossRef] [Green Version]


| (cm) | (mL/s) | (MPa) |
|---|---|---|
| 0.78 | 100 | 0.5 |
| 3.14 | 200 | 1.0 |
| 7.07 | 300 | 2.0 |
| 12.57 | 400 | 4.0 |
| 19.64 | 500 | 8.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fanni, B.M.; Pizzuto, A.; Santoro, G.; Celi, S. Introduction of a Novel Image-Based and Non-Invasive Method for the Estimation of Local Elastic Properties of Great Vessels. Electronics 2022, 11, 2055. https://doi.org/10.3390/electronics11132055
Fanni BM, Pizzuto A, Santoro G, Celi S. Introduction of a Novel Image-Based and Non-Invasive Method for the Estimation of Local Elastic Properties of Great Vessels. Electronics. 2022; 11(13):2055. https://doi.org/10.3390/electronics11132055
Chicago/Turabian StyleFanni, Benigno Marco, Alessandra Pizzuto, Giuseppe Santoro, and Simona Celi. 2022. "Introduction of a Novel Image-Based and Non-Invasive Method for the Estimation of Local Elastic Properties of Great Vessels" Electronics 11, no. 13: 2055. https://doi.org/10.3390/electronics11132055
APA StyleFanni, B. M., Pizzuto, A., Santoro, G., & Celi, S. (2022). Introduction of a Novel Image-Based and Non-Invasive Method for the Estimation of Local Elastic Properties of Great Vessels. Electronics, 11(13), 2055. https://doi.org/10.3390/electronics11132055








