An Auto Adjustable Transimpedance Readout System for Wearable Healthcare Devices
Abstract
:1. Introduction
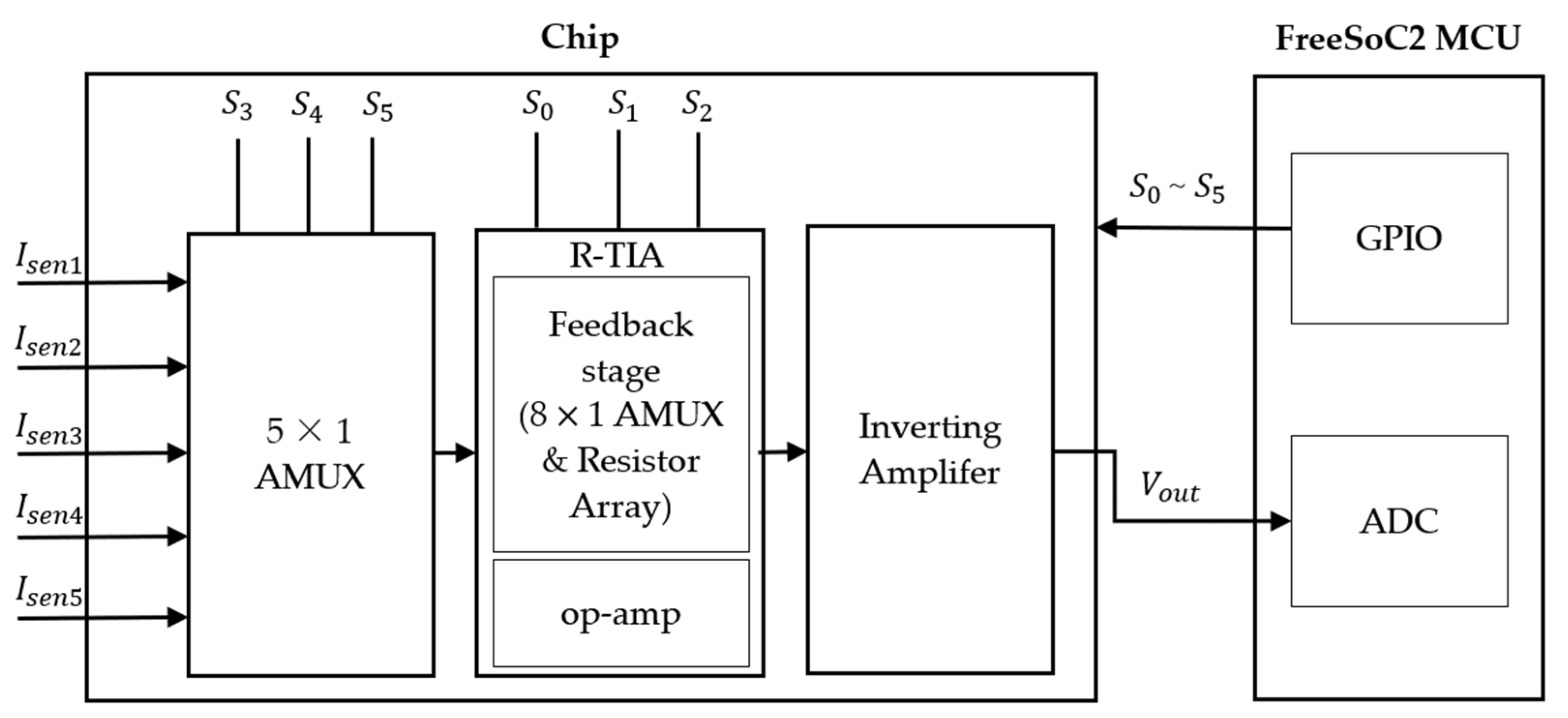
2. Design of Readout Circuit
2.1. Design of Digitally Adjustable TIA
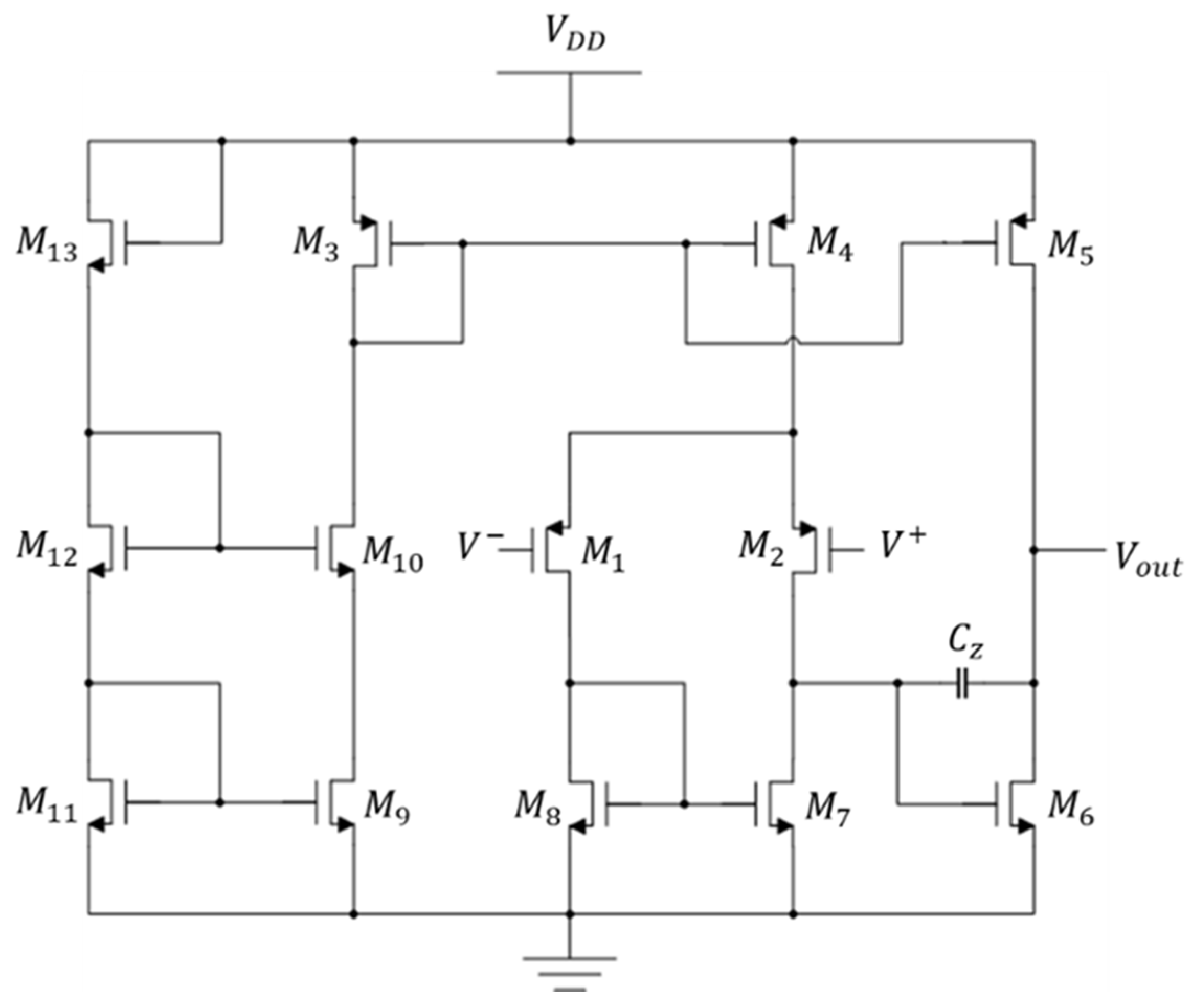
2.1.1. OP–AMP Design
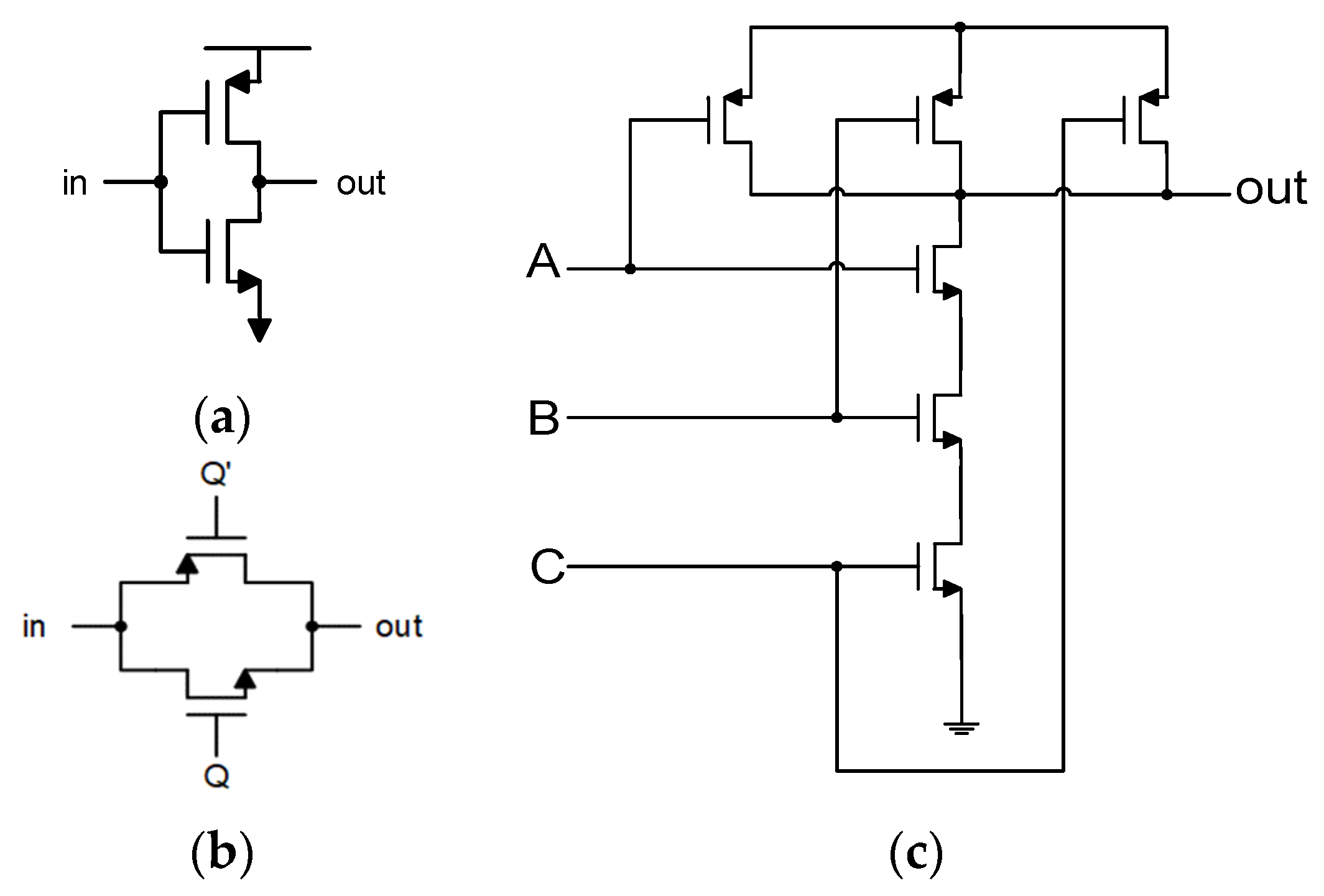
2.1.2. Feedback Stage Design
3. Results and Discussion
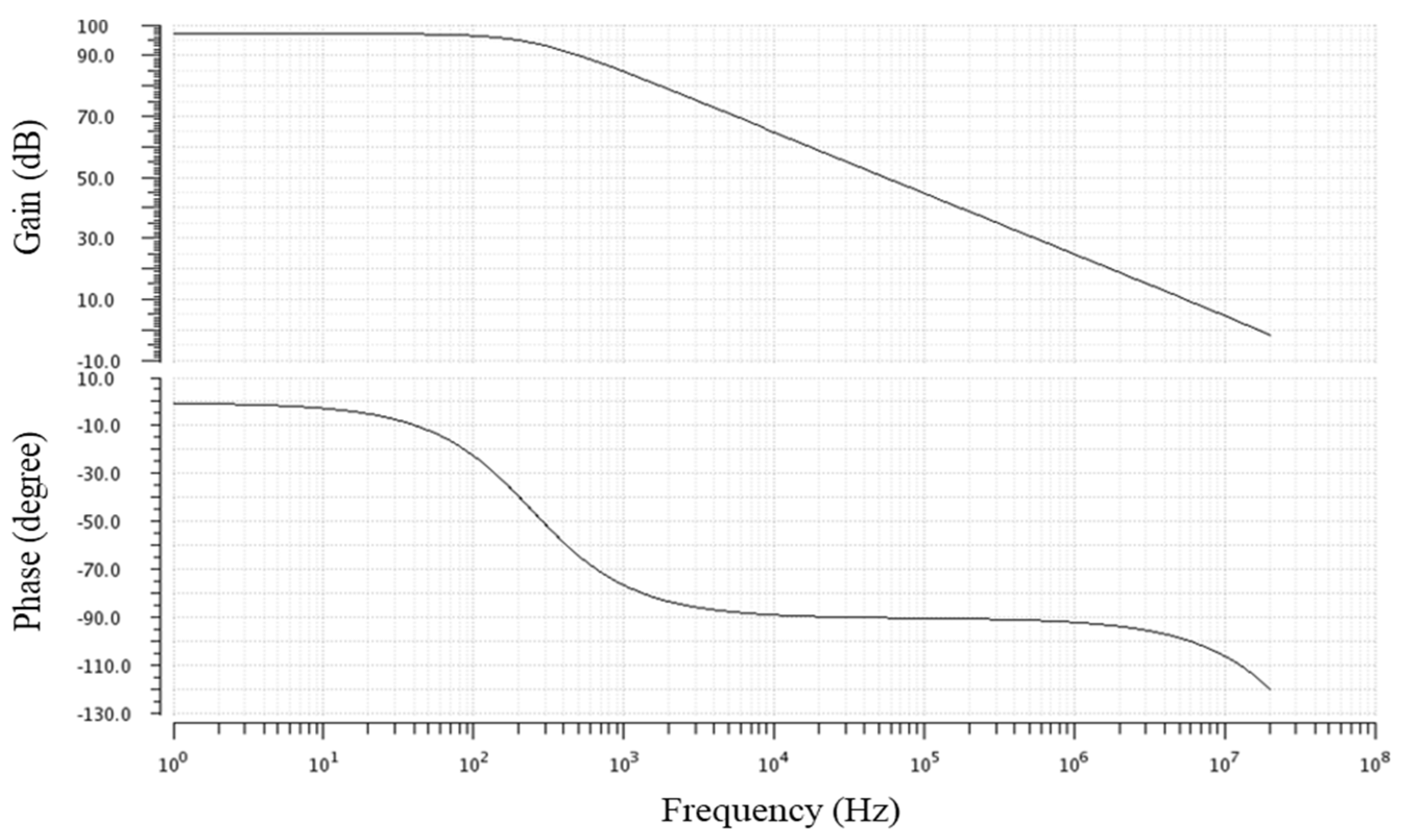
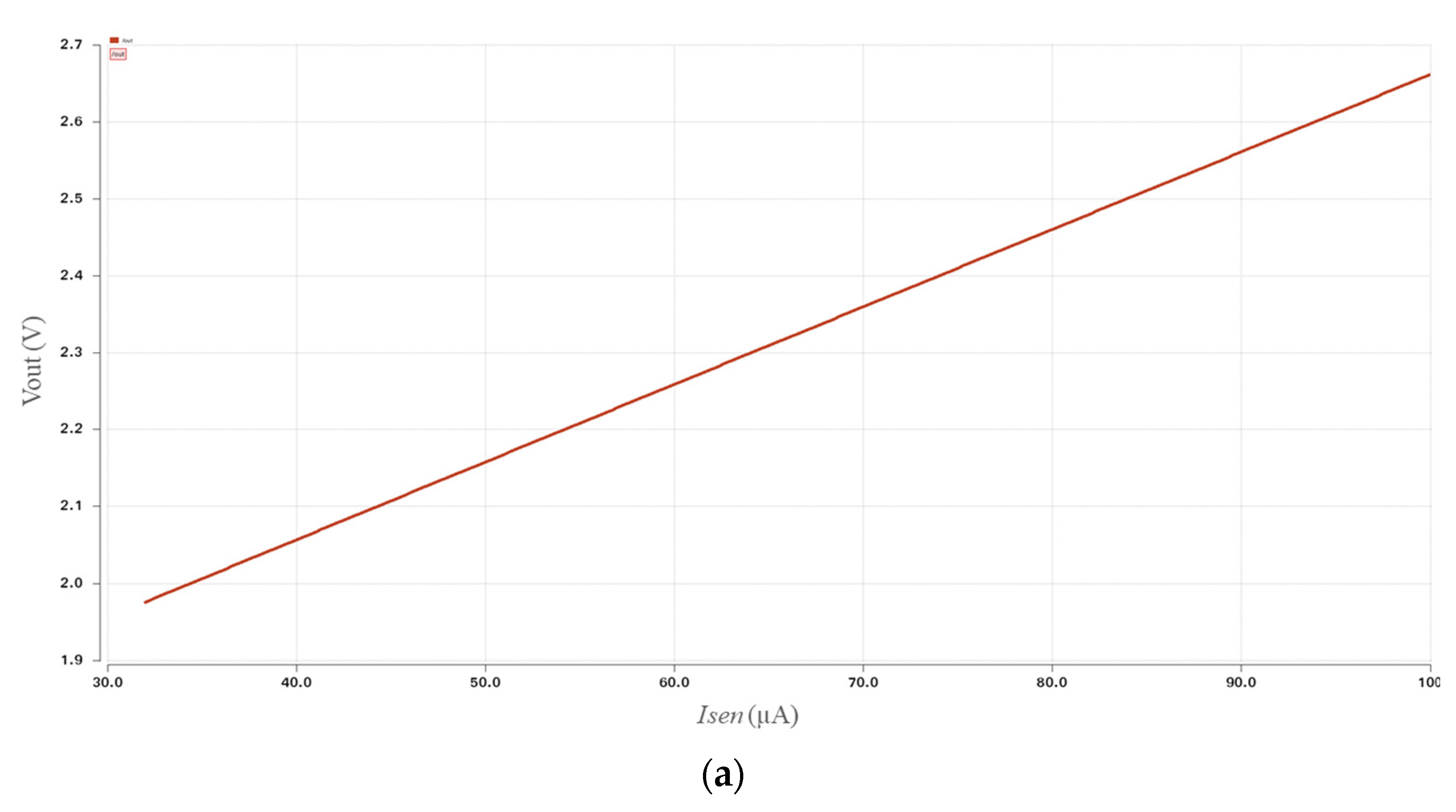
3.1. Simulation Results
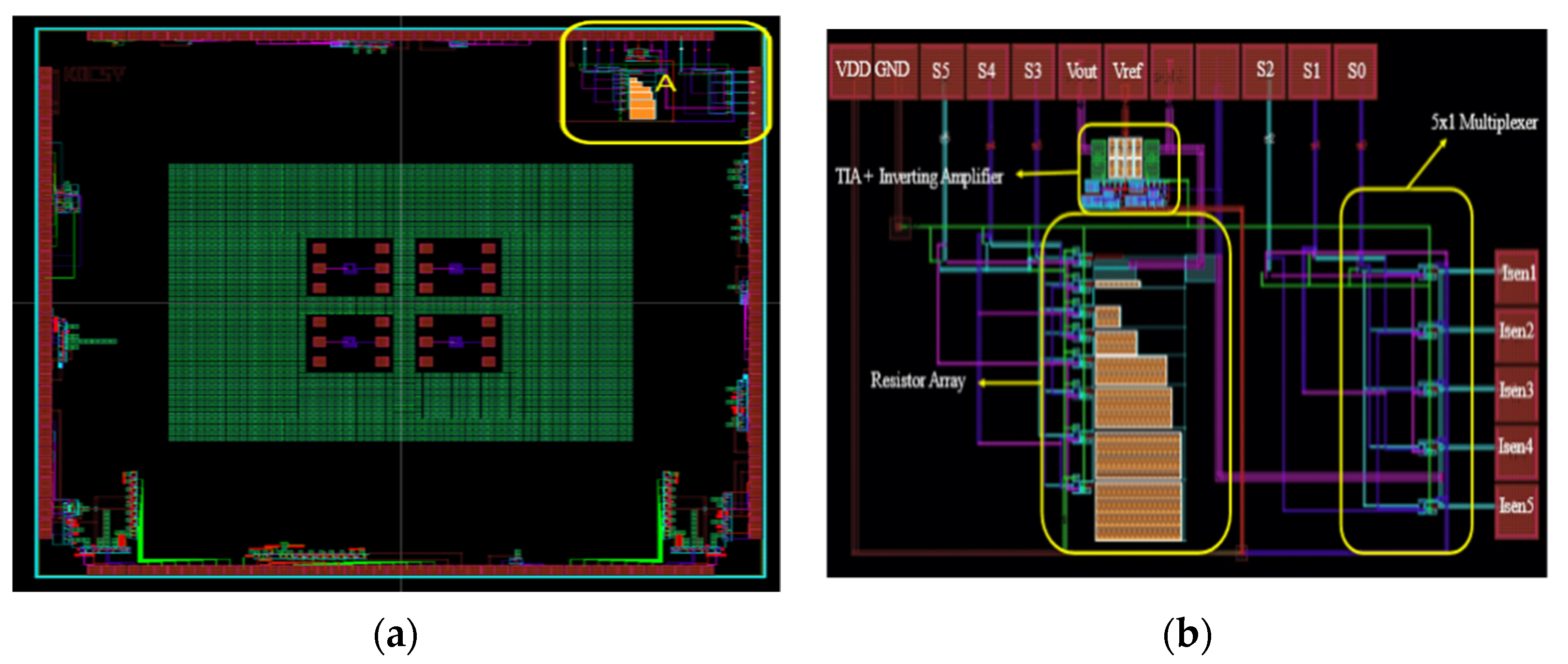
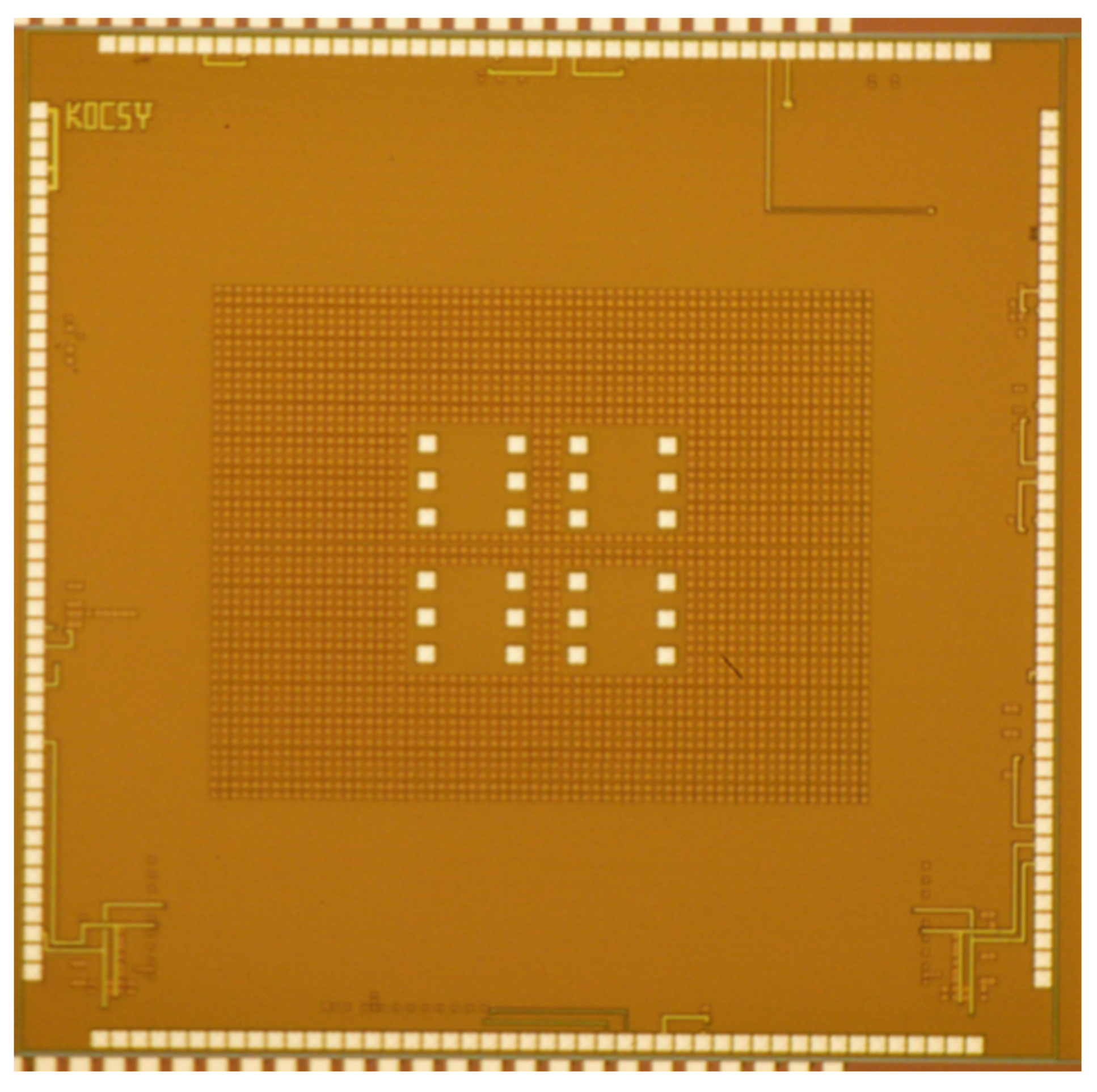
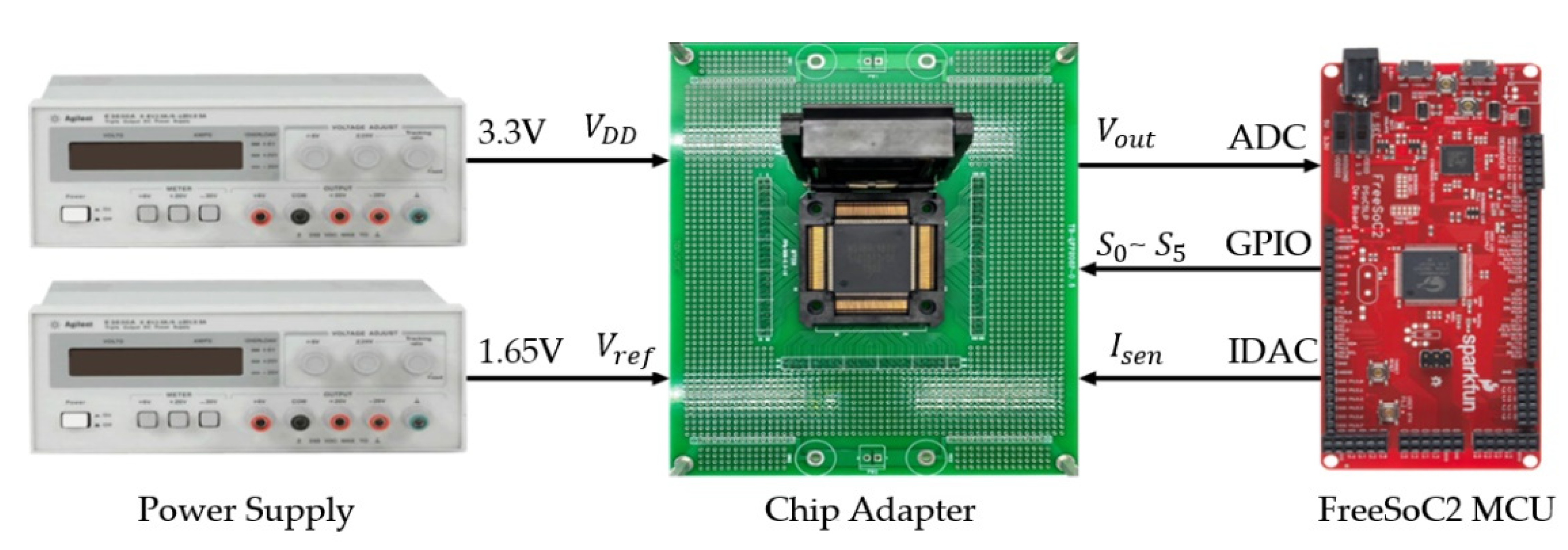
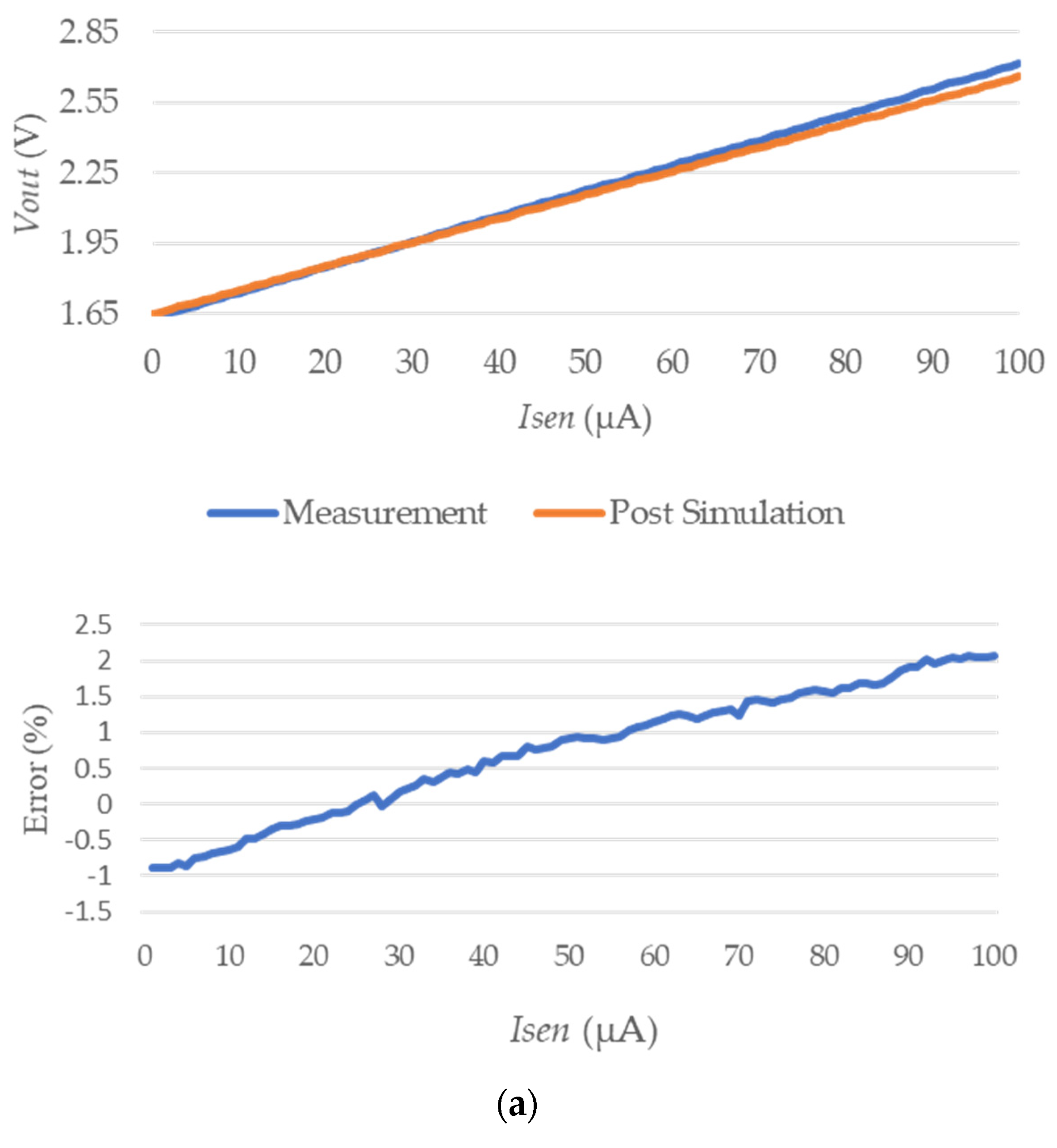
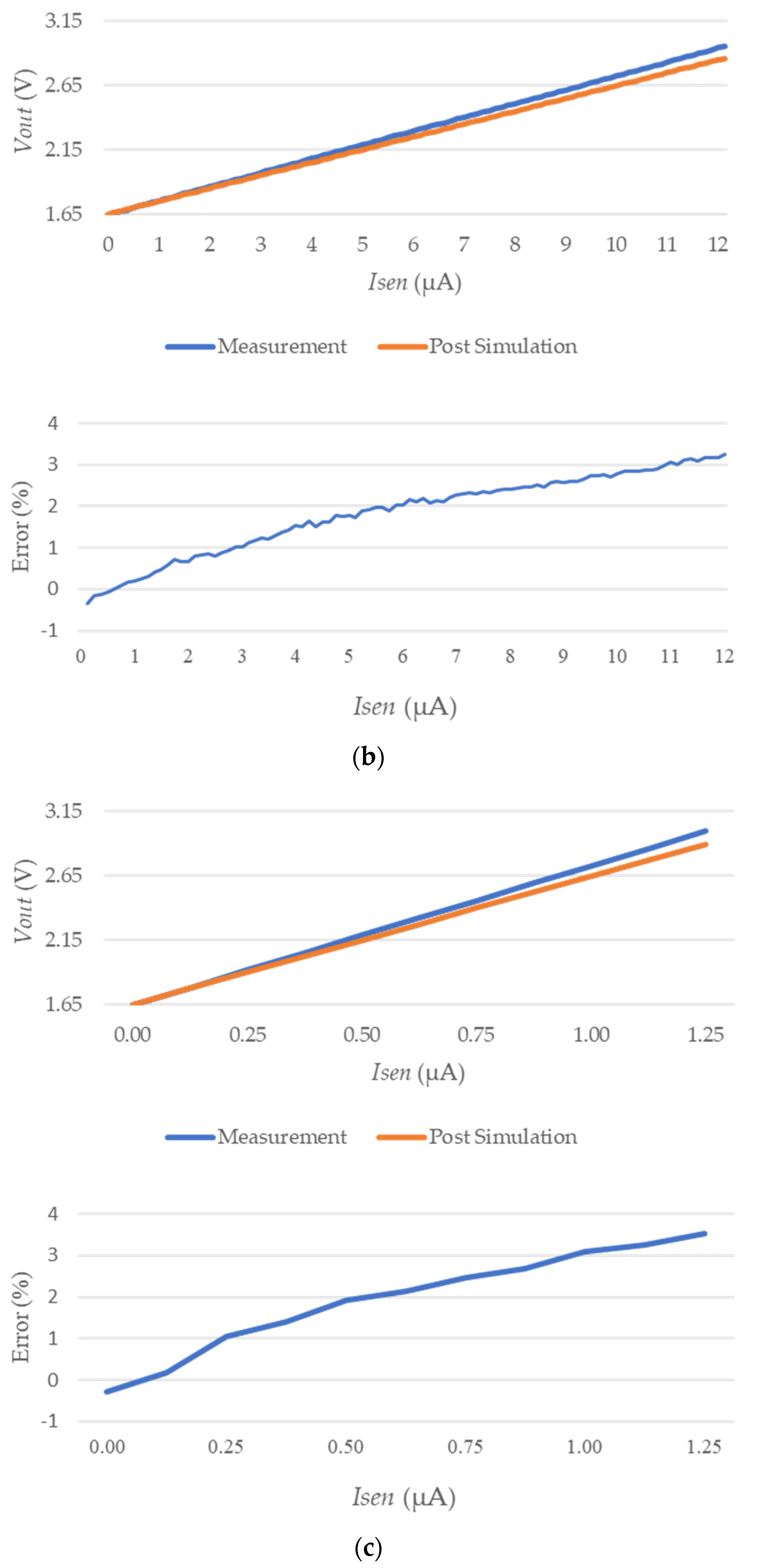
3.2. IC Test Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xie, J.; Chen, Q.; Shen, H.; Li, G. Review—Wearable Graphene Devices for Sensing. J. Electrochem. Soc. 2020, 167, 037541. [Google Scholar] [CrossRef]
- Sweeney, D.; Quinlan, L.R.; Browne, P.; Richardson, M.; Meskell, P.; ÓLaighin, G. A Technological Review of Wearable Cueing Devices Addressing Freezing of Gait in Parkinson’s Disease. Sensors 2019, 19, 1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sana, F.; Isselbacher, E.M.; Singh, J.P.; Heist, E.K.; Pathik, B.; Armoundas, A.A. Wearable Devices for Ambulatory Cardiac Monitoring: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Lutz, W.; Sanderson, W.; Scherbov, S. The coming acceleration of global population ageing. Nature 2008, 451, 716–719. [Google Scholar] [CrossRef]
- Dall, T.M.; Gallo, P.D.; Chakrabarti, R.; West, T.; Semilla, A.P.; Storm, M.V. An Aging Population And Growing Disease Burden Will Require ALarge And Specialized Health Care Workforce By 2025. Health Aff. 2013, 32, 2013–2020. [Google Scholar] [CrossRef] [Green Version]
- Awais, M.; Palmerini, L.; Bourke, A.K.; Ihlen, E.A.F.; Helbostad, J.L.; Chiari, L. Performance Evaluation of State of the Art Systems for Physical Activity Classification of Older Subjects Using Inertial Sensors in a Real Life Scenario: A Benchmark Study. Sensors 2016, 16, 2105. [Google Scholar] [CrossRef] [Green Version]
- Vijayalakshmi, K.; Uma, S.; Bhuvanya, R.; Suresh, A. A demand for wearable devices in health care. Int. J. Eng. Technol. 2018, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Engel, J.M.; Mehta, V.; Fogoros, R.; Chavan, A. Study of Arrhythmia Prevalence in NUVANT Mobile Cardiac Telemetry System Patients. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 2440–2443. [Google Scholar]
- Barrett, P.M.; Komatireddy, R.; Haaser, S.; Topol, S.; Sheard, J.; Encinas, J.; Fought, A.J.; Topol, E.J. Comparison of 24-h Holter Monitoring with 14-day Novel Adhesive Patch Electrocardiographic Monitoring. Am. J. Med. 2014, 127, 95.e11–95.e17. [Google Scholar] [CrossRef] [Green Version]
- Altini, M.; Casale, P.; Penders, J.; ten Velde, G.; Plasqui, G.; Amft, O. Cardiorespiratory fitness estimation using wearable sensors: Laboratory and free-living analysis of context-specific submaximal heart rates. J. Appl. Physiol. 2016, 120, 1082–1096. [Google Scholar] [CrossRef]
- Digiglio, P.; Li, R.; Wang, W.; Pan, T. Microflotronic Arterial Tonometry for Continuous Wearable Non-Invasive Hemodynamic Monitoring. Ann. Biomed. Eng. 2014, 42, 2278–2288. [Google Scholar] [CrossRef]
- Etemadi, M.; Inan, O.T. Wearable ballistocardiogram and seismocardiogram systems for health and performance. J. Appl. Physiol. 2017, 124, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.K.; Lowres, N.; Neubeck, L.; Brieger, D.B.; Sy, R.W.; Galloway, C.D.; Albert, D.E.; Freedman, S.B. iPhone ECG application for community screening to detect silent atrial fibrillation: A novel technology to prevent stroke. Int. J. Cardiol. 2013, 165, 193–194. [Google Scholar] [CrossRef] [PubMed]
- Sohn, K.; Merchant, F.M.; Abohashem, S.; Kulkarni, K.; Singh, J.P.; Heist, E.K.; Owen, C.; Roberts, J.D., Jr.; Isselbacher, E.M.; Sana, F.; et al. Utility of a smartphone based system (cvrphone) to accurately determine apneic events from electrocardiographic signals. PLoS ONE 2019, 14, e0217217. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Sim, J.K.; Cho, Y.H. A Flexible and Wearable Human Stress Monitoring Patch. Sci. Rep. 2016, 6, 23468. [Google Scholar] [CrossRef] [Green Version]
- Lakshminarayana, S.; Park, Y.; Park, H.; Jung, S. High Density Resistive Array Readout System for Wearable Electronics. Sensors 2022, 22, 1878. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Lin, M.; Yin, L.; De la Paz, E.; Pei, K.; Sonsa-Ard, T.; de Loyola Silva, A.N.; Khorshed, A.A.; Zhang, F.; Tostado, N.; et al. An epidermal patch for the simultaneous monitoring of haemodynamic and metabolic biomarkers. Nat. Biomed. Eng. 2021, 5, 737–748. [Google Scholar] [CrossRef]
- Li, X.; He, L.; Li, Y.; Chao, M.; Li, M.; Wan, P.; Zhang, L. Correction to “Healable, Degradable and Conductive MXene Nanocomposite Hydrogel for Multifunctional Epidermal Sensor”. ACS Nano 2021, 15, 12453. [Google Scholar] [CrossRef]
- Xi, W.; Yeo, J.C.; Yu, L.; Zhang, S.; Lim, C.T. Ultrathin and Wearable Microtubular Epidermal Sensor for Real-Time Physiological Pulse Monitoring. Adv. Mater. Technol. 2017, 2, 1700016. [Google Scholar] [CrossRef]
- Yoon, S.; Yoon, H.; Zahed, M.A.; Park, C.; Kim, D.; Park, J.Y. Multifunctional hybrid skin patch for wearable smart healthcare applications. Biosens. Bioelectron. 2022, 196, 113685. [Google Scholar] [CrossRef]
- Imani, S.; Bandodkar, A.J.; Mohan, A.M.V.; Kumar, R.; Yu, S.; Wang, J.; Mercier, P.P. A wearable chemical–electrophysiological hybrid biosensing system for real-time health and fitness monitoring. Nat. Commun. 2016, 7, 11650. [Google Scholar] [CrossRef]
- Mariam El, G.; Fernández-García, R.; Ahyoud, S.; Gil, I. A Review of Flexible Wearable Antenna Sensors: Design, Fabrication Methods, and Applications. Materials 2020, 13, 3781. [Google Scholar]
- Li, R.; Qi, H.; Ma, Y.; Deng, Y.; Liu, S.; Jie, Y.; Jing, J.; He, J.; Zhang, X.; Wheatley, L.; et al. A flexible and physically transient electrochemical sensor for real-time wireless nitric oxide monitoring. Nat. Commun. 2020, 11, 3207. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, W.; Zhang, L.; Yang, J.; Yao, Z.-P.; He, Y.; Li, Y. Integrated hand-held electrochemical sensor for multicomponent detection in urine. Biosens. Bioelectron. 2021, 193, 113534. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zuo, J.; Hou, Z.; Huang, Y.; Huang, C. Preparation of Electrochemical Sensor Based on Zinc Oxide Nanoparticles for Simultaneous Determination of AA, DA, and UA. Front. Chem. 2020, 8, 2538. [Google Scholar] [CrossRef]
- Ma, R.; Liu, M.; Zheng, H.; Zhu, Z. A 66-dB Linear Dynamic Range, 100-dB·Ω Transimpedance Gain TIA With High-Speed PDSH for LiDAR. IEEE Trans. Instrum. Meas. 2020, 69, 1020–1028. [Google Scholar] [CrossRef]
- Micusik, D.; Zimmermann, H. 130dB-DR Transimpedance Amplifier with Monotonic Logarithmic Compression and High-Current Monitor. In Proceedings of the 2008 IEEE International Solid-State Circuits Conference—Digest of Technical Papers, San Francisco, CA, USA, 3–7 February 2008; pp. 78–597. [Google Scholar]
- Mičušík, D.; Zimmermann, H. Transimpedance amplifier with 120 dB dynamic range. Electron. Lett. 2007, 43, 159–160. [Google Scholar] [CrossRef]
- Aznar, F.; Gaberl, W.; Zimmermann, H. A highly sensitive 2.5 Gb/s transimpedance amplifier in CMOS technology. In Proceedings of the 2009 IEEE International Symposium on Circuits and Systems, Taipei, Taiwan, 24–27 May 2009; pp. 189–192. [Google Scholar]
- Atef, M.; Hassan, O.; Awwad, F.; Khan, M.A.B. High Dynamic Range Photocurrent Sensory Circuit with a Multi-Transistor Background Light Cancellation Loop for Photoplethysmography Sensing. Electronics 2021, 10, 2769. [Google Scholar] [CrossRef]
- Wang, W.; Sonkusale, S. An Approach for a Wide Dynamic Range Low-Noise Current Readout Circuit. J. Low Power Electron. Appl. 2020, 10, 23. [Google Scholar] [CrossRef]
- Rad, R.E.; Hejazi, A.; Asl, S.-A.H.; Shehzad, K.; Verma, D.; Kim, S.; Rikan, B.S.; Pu, Y.; Kim, J.T.; Hwang, K.C.; et al. A 77-dB Dynamic-Range Analog Front-End for Fine-Dust Detection Systems with Dual-Mode Ultra-Low Noise TIA. Sensors 2021, 21, 6360. [Google Scholar] [CrossRef]
- Hsu, C.; Hall, D.A. A current-measurement front-end with 160dB dynamic range and 7ppm INL. In Proceedings of the 2018 IEEE International Solid—State Circuits Conference—(ISSCC), San Francisco, CA, USA, 11–15 February 2018; pp. 326–328. [Google Scholar]
- Dai, S.; Perera, R.T.; Yang, Z.; Rosenstein, J.K. A 155-dB Dynamic Range Current Measurement Front End for Electrochemical Biosensing. IEEE Trans. Biomed. Circuits Syst. 2016, 10, 935–944. [Google Scholar] [CrossRef]
- Ferrari, G.; Gozzini, F.; Molari, A.; Sampietro, M. Transimpedance Amplifier for High Sensitivity Current Measurements on Nanodevices. IEEE J. Solid-State Circuits 2009, 44, 1609–1616. [Google Scholar] [CrossRef]
- Hsu, C.L.; Jiang, H.; Venkatesh, A.G.; Hall, D.A. A Hybrid Semi-Digital Transimpedance Amplifier with Noise Cancellation Technique for Nanopore-Based DNA Sequencing. IEEE Trans. Biomed. Circuits Syst. 2015, 9, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.; Kim, D.; Rottigni, A.; Xu, J.; Vanderlick, T.K.; Culurciello, E. CMOS low current measurement system for biomedical applications. IEEE Trans. Biomed. Circuits Syst. 2012, 6, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Maitra, R.; Pedrotti, K.D.; Dunbar, W.B. A Patch-Clamp ASIC for Nanopore-Based DNA Analysis. IEEE Trans. Biomed. Circuits Syst. 2013, 7, 285–295. [Google Scholar] [CrossRef]
- Lu, J.; Holleman, J. A Wideband Ultra-Low-Current On-Chip Ammeter. In Proceedings of the IEEE 2012 Custom Integrated Circuits Conference, San Jose, CA, USA, 9–12 September 2012; pp. 1–4. [Google Scholar]
- Bennati, M.; Thei, F.; Rossi, M.; Crescentini, M.; Avino, G.D.; Baschirotto, A.; Tartagni, M. 20.5 A Sub-pA ΔΣ Current Amplifier for Single-Molecule Nanosensors. In Proceedings of the 2009 IEEE International Solid-State Circuits Conference—Digest of Technical Papers, San Francisco, CA, USA, 8–12 February 2009; pp. 348–349, 349a. [Google Scholar]
- Crescentini, M.; Thei, F.; Bennati, M.; Saha, S.; de Planque, M.R.; Morgan, H.; Tartagni, M. A Distributed Amplifier System for Bilayer Lipid Membrane (BLM) Arrays With Noise and Individual Offset Cancellation. IEEE Trans Biomed. Circuits Syst. 2015, 9, 334–344. [Google Scholar] [CrossRef] [Green Version]
- Qiao, H. Design of a CMOS Two-stage Fully Differential Operation Amplifier. J. Phys. Conf. Ser. 2020, 1449, 012084. [Google Scholar] [CrossRef]
- Ciofi, C.; Crupi, F.; Pace, C.; Scandurra, G.; Patane, M. A New Circuit Topology for the Realization of Very Low-Noise Wide-Bandwidth Transimpedance Amplifier. IEEE Trans. Instrum. Meas. 2007, 56, 1626–1631. [Google Scholar] [CrossRef]
- Chuah, J.H.; Holburn, D. Design of Low-Noise High-Gain CMOS Transimpedance Amplifier for Intelligent Sensing of Secondary Electrons. IEEE Sens. J. 2015, 15, 5997–6004. [Google Scholar] [CrossRef]
- Säckinger, E. Analysis and Design of Transimpedance Amplifiers for Optical Receivers; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 207–278. [Google Scholar] [CrossRef]
- Zihong, L.; Chao, B.; Zhihua, W.; Chun, Z. Full Custom Design of a Two-Stage Fully Differential CMOS Amplifier with High Unity-Gain Bandwidth and Large Dynamic Range at Output. In Proceedings of the 48th Midwest Symposium on Circuits and Systems, Covington, KY, USA, 7–10 August 2005; Volume 982, pp. 984–987. [Google Scholar]
- Ramirez-Angulo, J.; Sawant, M.S.; López-Martín, A.; Carvajal, R.G. Compact implementation of high-performance CMOS current mirror. Electron. Lett. 2005, 41, 570. [Google Scholar] [CrossRef]
- Balestrieri, E.; Daponte, P.; Rapuano, S. A state of the art on ADC error compensation methods. IEEE Trans. Instrum. Meas. 2005, 54, 1388–1394. [Google Scholar] [CrossRef]





| Feedback Resistor (KΩ) | Sensor Current Range (µA) | |||
|---|---|---|---|---|
| 0 | 0 | 0 | 10 | 32~100 |
| 0 | 0 | 1 | 50 | 12~32 |
| 0 | 1 | 0 | 100 | 8~12 |
| 0 | 1 | 1 | 200 | 4~8 |
| 1 | 0 | 0 | 400 | 2.5~4 |
| 1 | 0 | 1 | 600 | 2~2.5 |
| 1 | 1 | 0 | 800 | 1.5~2 |
| 1 | 1 | 1 | 1000 | 0.1~1.5 |
| Expected
(A) | Measured with Conventional TIA (A) | Measured with Proposed TIA (A) | Error Rate of Conventional TIA (%) | Error Rate of Proposed TIA (%) |
|---|---|---|---|---|
| 100 µ | 100.48 µ | 100.48 µ | 0.48 | 0.48 |
| 10 µ | 13.32 µ | 10.069 µ | 33.278 | 0.698 |
| 1 µ | 2.253 µ | 1.005 µ | 125.3 | 0.5 |
| Ref. | Input Current Range (A) | Input Dynamic Range (dB) | Power Consumption (mW) | Transimpedance Gain Range (dBΩ) | Supply Voltage (V) | Technology | Area mm2 | Error Rate (%) |
|---|---|---|---|---|---|---|---|---|
| Our work | 0.1 µ–100 µ | 60 | 0.38 | 60–120 | 3.3 | 0.18 µm CMOS | 0.282 | 1 |
| [26] | 1 µ–2 m | 66 | 21 | 54–100 | 3.3 | 0.18 µm CMOS | 0.356 | 0.40 |
| [31] | 100 f–1 μ | 140 | 10.3 | N/A | 1.8 | 0.18 µm CMOS | 1.015 | N/A |
| [32] | N/A | 77 | 2.71 | N/A | 3.3 | 0.18 µm CMOS | 1.21 | N/A |
| [38] | 4.21 p–369 n | 98.9 | 0.5 | 138–168 | 1.5 | 0.35 µm CMOS | 0.3 | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Lakshminarayana, S.; Pan, C.; Chung, H.-J.; Jung, S. An Auto Adjustable Transimpedance Readout System for Wearable Healthcare Devices. Electronics 2022, 11, 1181. https://doi.org/10.3390/electronics11081181
Park H, Lakshminarayana S, Pan C, Chung H-J, Jung S. An Auto Adjustable Transimpedance Readout System for Wearable Healthcare Devices. Electronics. 2022; 11(8):1181. https://doi.org/10.3390/electronics11081181
Chicago/Turabian StylePark, Hyusim, Shanthala Lakshminarayana, Chenyun Pan, Hoon-Ju Chung, and Sungyong Jung. 2022. "An Auto Adjustable Transimpedance Readout System for Wearable Healthcare Devices" Electronics 11, no. 8: 1181. https://doi.org/10.3390/electronics11081181
APA StylePark, H., Lakshminarayana, S., Pan, C., Chung, H.-J., & Jung, S. (2022). An Auto Adjustable Transimpedance Readout System for Wearable Healthcare Devices. Electronics, 11(8), 1181. https://doi.org/10.3390/electronics11081181







