Investigating the Effect of Cross-Conjugation Patterns on the Optoelectronic Properties of 7,7′Isoindigo-Based Materials
Abstract
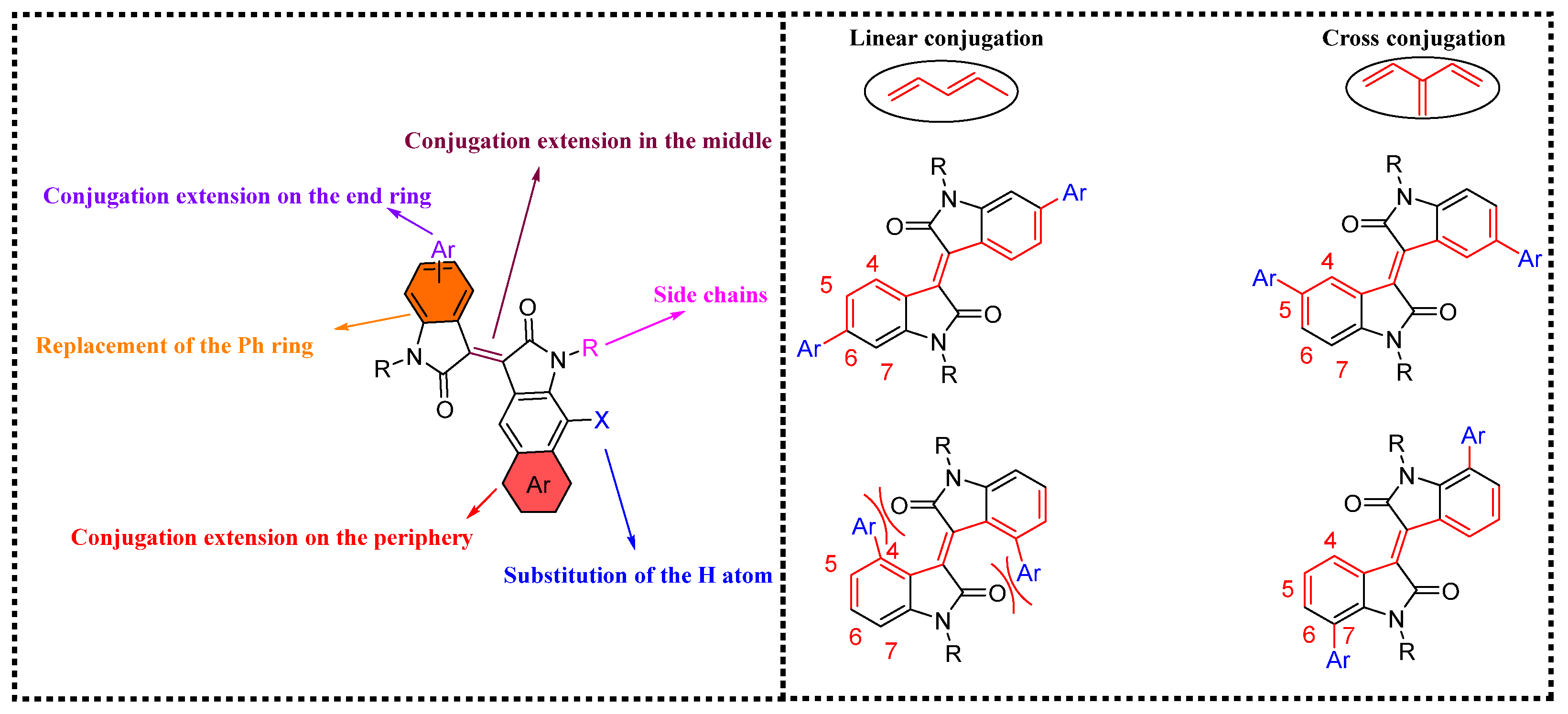
1. Introduction
2. Materials and Methods
3. Results
3.1. Synthesis
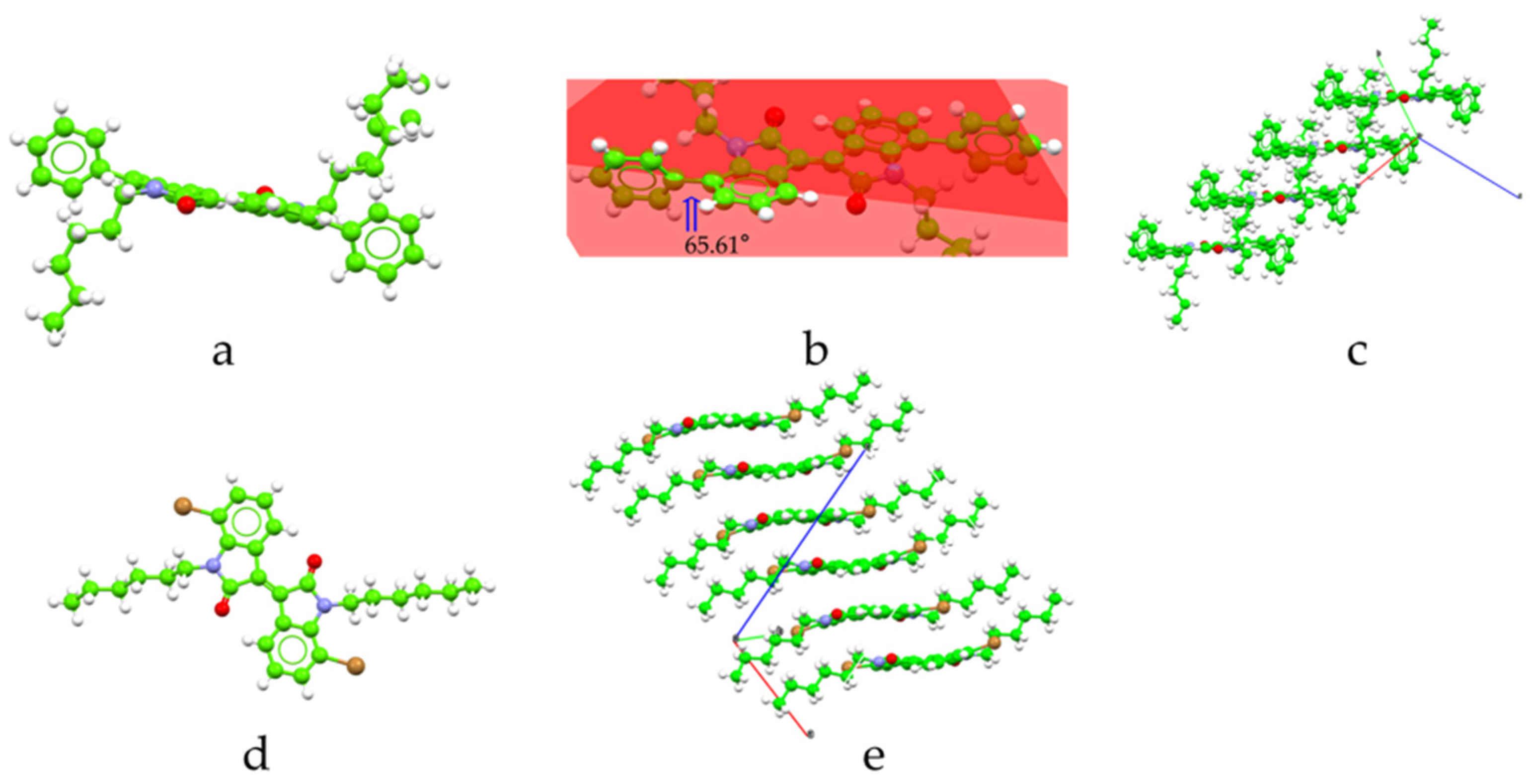
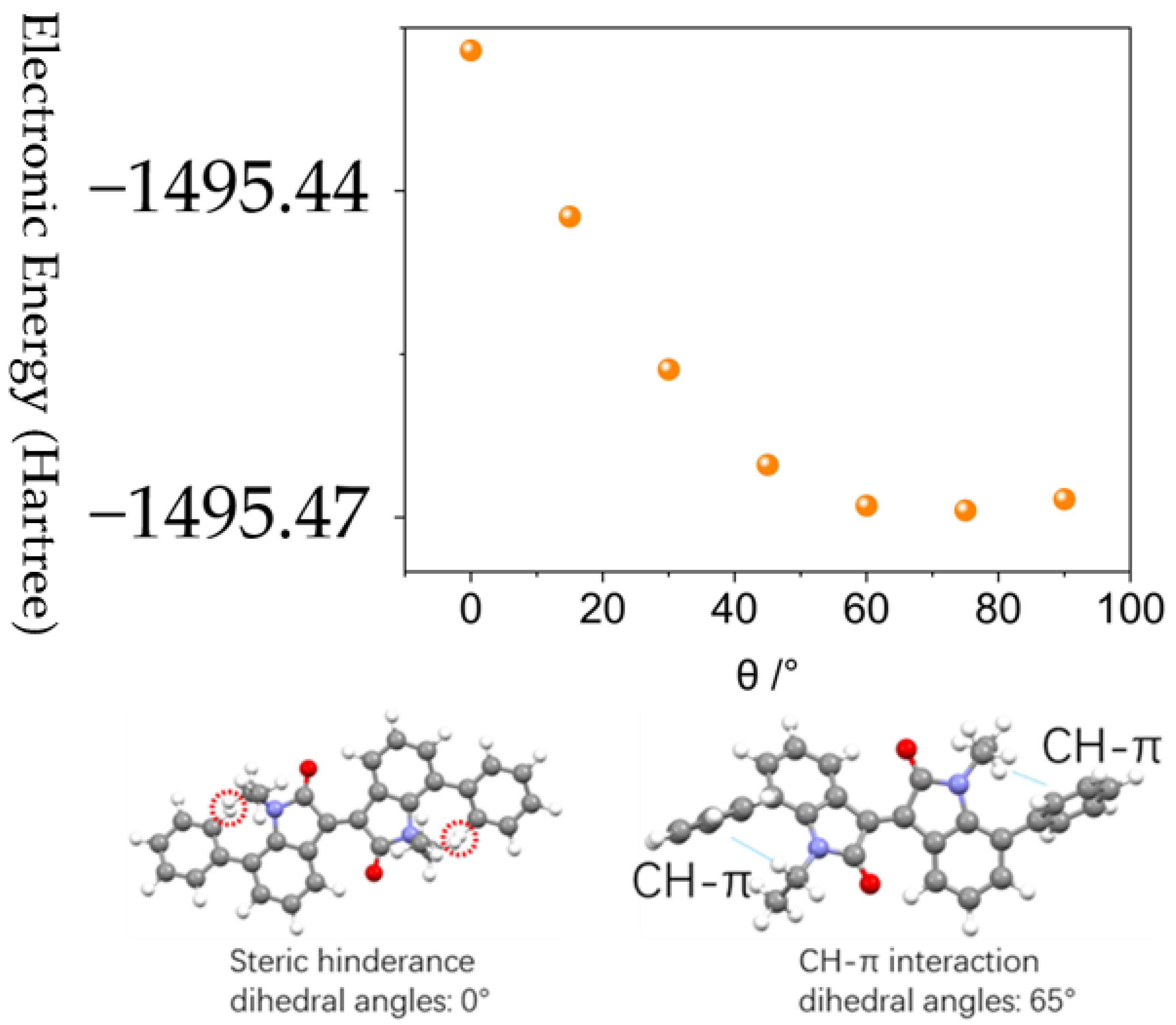
3.2. Crystal Structure and Molecular Packing
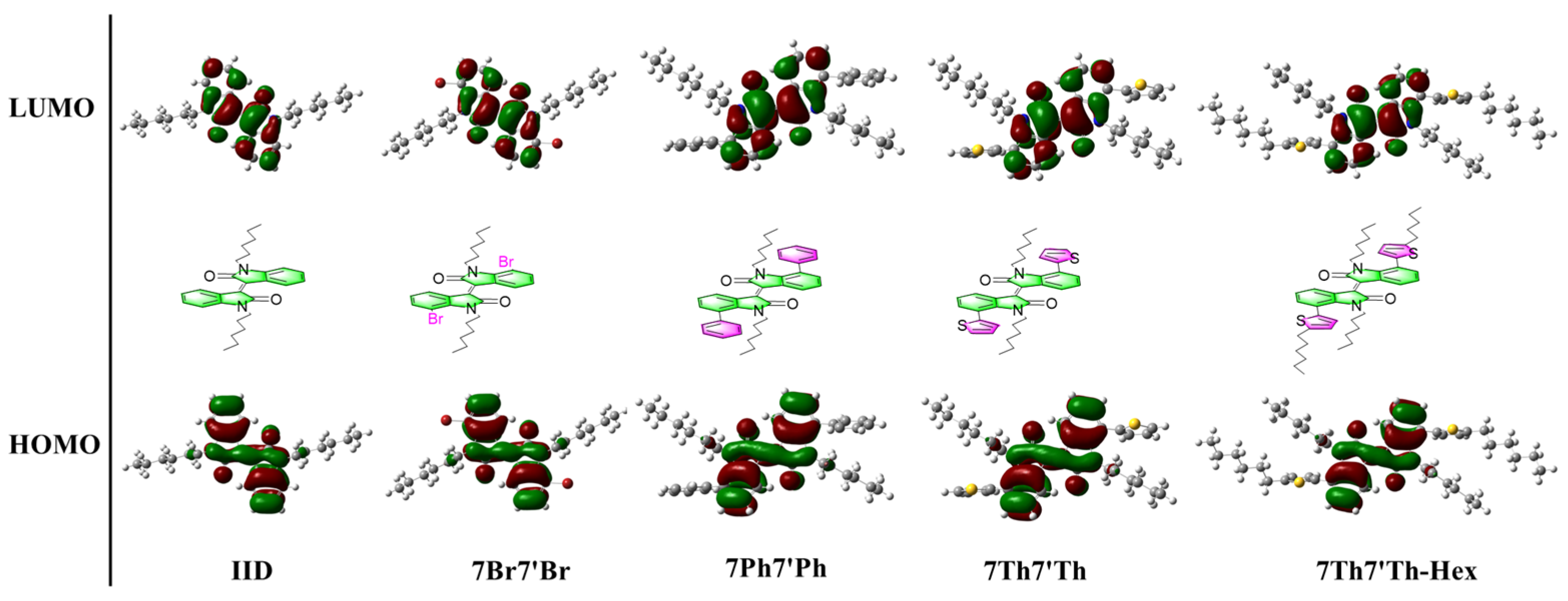
3.3. Density Functional Theory Calculations
3.4. Optical Properties
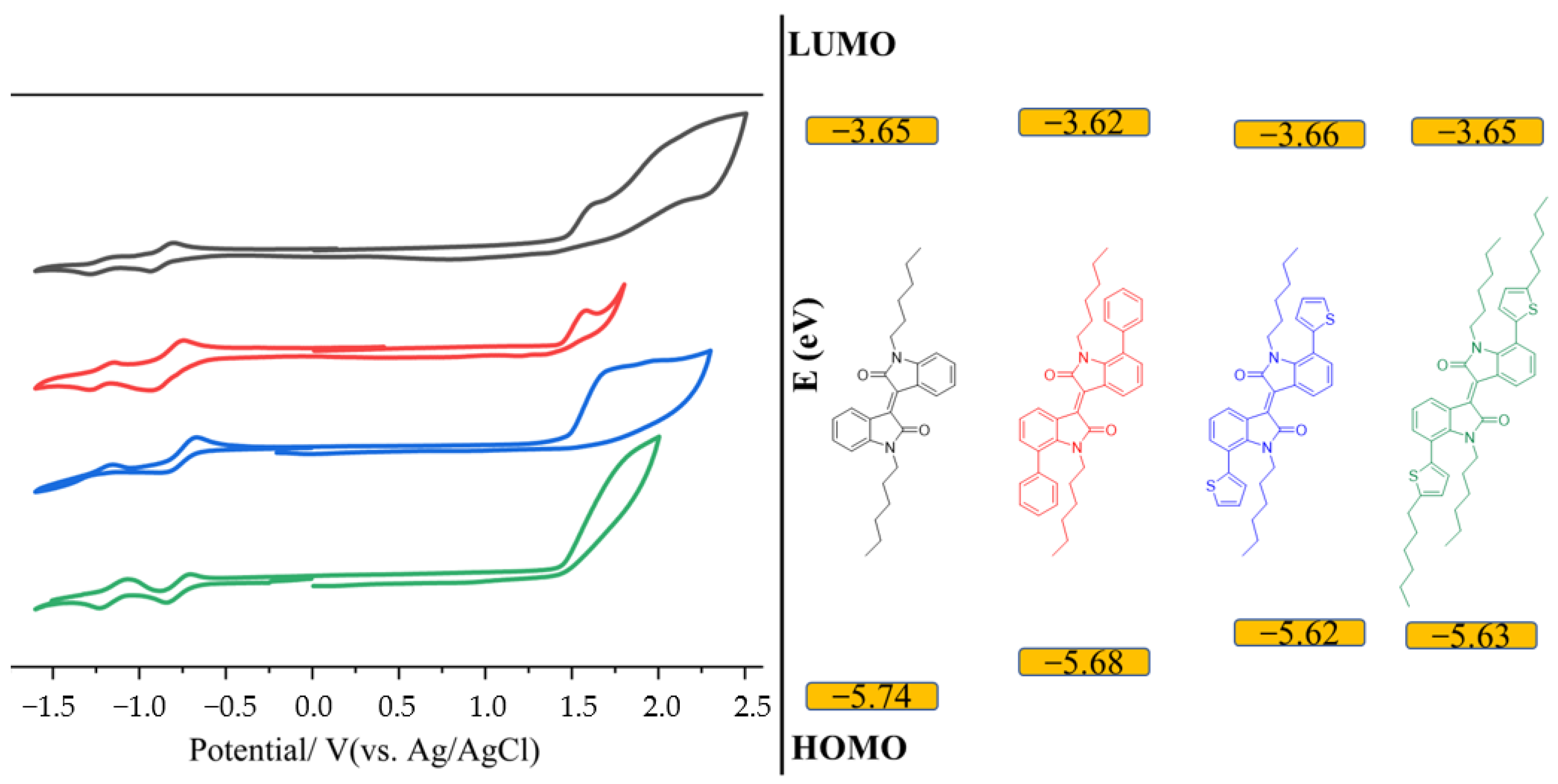
3.5. Electrochemical Properties
3.6. Electrical Characterization and Photovoltaic Devices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, W.; Shi, K.; Lai, J.; Zhou, Y.; Wei, X.; Che, Q.; Wei, J.; Wang, L.; Yu, G. Record-High Electron Mobility Exceeding 16 cm2 V−1 s−1 in Bisisoindigo-Based Polymer Semiconductor with a Fully Locked Conjugated Backbone. Adv. Mater. 2023, 35, e2300145. [Google Scholar] [CrossRef]
- Li, J.-L.; Cao, J.-J.; Duan, L.-L.; Zhang, H.-L. Evolution of Isoindigo-Based Electron-Deficient Units for Organic Electronics: From Natural Dyes to Organic Semiconductors. Asian J. Org. Chem. 2018, 7, 2147–2160. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, W.; Yu, G. Semiconducting Polymers Based on Isoindigo and Its Derivatives: Synthetic Tactics, Structural Modifications, and Applications. Adv. Funct. Mater. 2021, 31, 2010979. [Google Scholar] [CrossRef]
- Jung, J.W. A low band gap conjugated small molecule based on isoindigo flanked with diketopyrrolopyrrole for efficient organic solar cells. Dye. Pigment. 2017, 137, 512–517. [Google Scholar] [CrossRef]
- Zhu, M.; Guo, Y.; Liu, Y. A thriving decade: Rational design, green synthesis, and cutting-edge applications of isoindigo-based conjugated polymers in organic field-effect transistors. Sci. China Chem. 2022, 65, 1225–1264. [Google Scholar] [CrossRef]
- Ngai, J.H.L.; Polena, J.; Afzal, D.; Gao, X.; Kapadia, M.; Li, Y. Green Solvent-Processed Hemi-Isoindigo Polymers for Stable Temperature Sensors. Adv. Funct. Mater. 2022, 32, 2110995. [Google Scholar] [CrossRef]
- Parr, Z.S.; Borges-Gonzalez, J.; Rashid, R.B.; Thorley, K.J.; Meli, D.; Paulsen, B.D.; Strzalka, J.; Rivnay, J.; Nielsen, C.B. From p- to n-Type Mixed Conduction in Isoindigo-Based Polymers through Molecular Design. Adv. Mater. 2022, 34, e2107829. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Du, C.Z.; Chen, X.Y.; Fu, L.; Gao, R.R.; Yao, Z.F.; Wang, J.Y.; Hu, W.; Pei, J.; Wang, X.Y. BN-Anthracene for High-Mobility Organic Optoelectronic Materials through Periphery Engineering. Angew. Chem. Int. Ed. Engl. 2022, 61, e202201464. [Google Scholar] [CrossRef]
- Ren, S.; Habibi, A.; Ni, P.; Nahdi, H.; Bouanis, F.Z.; Bourcier, S.; Clavier, G.; Frigoli, M.; Yassar, A. Synthesis and characterization of solution-processed indophenine derivatives for function as a hole transport layer for perovskite solar cells. Dye. Pigment. 2023, 213, 111136. [Google Scholar] [CrossRef]
- Lei, T.; Wang, J.Y.; Pei, J. Design, synthesis, and structure-property relationships of isoindigo-based conjugated polymers. Acc. Chem. Res. 2014, 47, 1117–1126. [Google Scholar] [CrossRef]
- Ren, S.; Yassar, A. Recent Research Progress in Indophenine-Based-Functional Materials: Design, Synthesis, and Optoelectronic Applications. Materials 2023, 16, 2474. [Google Scholar] [CrossRef] [PubMed]
- Randell, N.M.; Kelly, T.L. Recent Advances in Isoindigo-Inspired Organic Semiconductors. Chem. Rec. 2019, 19, 973–988. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ha, J.; Khan, M.F.; Im, C.; Park, J.Y.; Yoo, S.H.; Rehman, M.A.; Kang, K.; Lee, S.H.; Jun, S.C. Pronounced Optoelectronic Effect in n–n ReS2 Homostructure. ACS Appl. Electron. Mater. 2022, 4, 4306–4315. [Google Scholar] [CrossRef]
- Rabeel, M.; Javed, S.; Khan, R.; Akram, M.A.; Rehman, S.; Kim, D.K.; Khan, M.F. Controlling the Wettability of ZnO Thin Films by Spray Pyrolysis for Photocatalytic Applications. Materials 2022, 15, 3364. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, M.F.; Raza, M.A.; Rehman, Z.U.; Tayyeb, A.; Makhdoom, M.A.; Ghafoor, F.; Latif, U.; Khan, M.F. Role of Solvent Used in Development of Graphene Oxide Coating on AZ31B Magnesium Alloy: Corrosion Behavior and Biocompatibility Analysis. Nanomaterials 2022, 12, 3745. [Google Scholar] [CrossRef]
- Estrada, L.A.; Stalder, R.; Abboud, K.A.; Risko, C.; Brédas, J.-L.; Reynolds, J.R. Understanding the Electronic Structure of Isoindigo in Conjugated Systems: A Combined Theoretical and Experimental Approach. Macromolecules 2013, 46, 8832–8844. [Google Scholar] [CrossRef]
- Deng, P.; Zhang, Q. Recent developments on isoindigo-based conjugated polymers. Polym. Chem. 2014, 5, 3298–3305. [Google Scholar] [CrossRef]
- Ganguly, A.; Zhu, J.; Kelly, T.L. Effect of Cross-Conjugation on Derivatives of Benzoisoindigo, an Isoindigo Analogue with an Extended π-System. J. Phys. Chem. C 2017, 121, 9110–9119. [Google Scholar] [CrossRef]
- Yao, H.; Ye, L.; Hou, J.; Jang, B.; Han, G.; Cui, Y.; Su, G.M.; Wang, C.; Gao, B.; Yu, R.; et al. Achieving Highly Efficient Nonfullerene Organic Solar Cells with Improved Intermolecular Interaction and Open-Circuit Voltage. Adv. Mater. 2017, 29, 1700254. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B Condens. Matter 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Miehlich, B.; Savin, A.; Stoll, H.; Preuss, H. Results obtained with the correlation energy density functionals of becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989, 157, 200–206. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian: Wallingford, CT, USA, 2016. [Google Scholar]
- Elsawy, W.; Lee, C.L.; Cho, S.; Oh, S.H.; Moon, S.H.; Elbarbary, A.; Lee, J.S. Isoindigo-based small molecules for high-performance solution-processed organic photovoltaic devices: The electron donating effect of the donor group on photo-physical properties and device performance. Phys. Chem. Chem. Phys. 2013, 15, 15193–15203. [Google Scholar] [CrossRef] [PubMed]
- Perez, G.S.; Dasgupta, S.; Żuraw, W.; Pineda, R.F.; Wojciechowski, K.; Jagadamma, L.K.; Samuel, I.; Robertson, N. Solution-processable perylene diimide-based electron transport materials as non-fullerene alternatives for inverted perovskite solar cells. J. Mater. Chem. A 2022, 10, 11046–11053. [Google Scholar] [CrossRef]
- Jung, E.H.; Jeon, N.J.; Park, E.Y.; Moon, C.S.; Shin, T.J.; Yang, T.Y.; Noh, J.H.; Seo, J. Efficient, stable and scalable perovskite solar cells using poly(3-hexylthiophene). Nature 2019, 567, 511–515. [Google Scholar] [CrossRef] [PubMed]



| ε (cm−1 M−1) | HOMO (eV) | LUMO (eV) | fosc | ΔEc (eV) | |
|---|---|---|---|---|---|
| IID | 16,387 | −5.87 | −3.01 | 0.11 | 2.86 |
| 7Br7′Br | 12,278 | −6.10 | −3.29 | 0.11 | 2.81 |
| 7Ph7′Ph | 16,104 | −5.86 | −3.06 | 0.15 | 2.80 |
| 7Th7′Th | 14,827 | −5.93 | −3.09 | 0.14 | 2.84 |
| 7Th7′Th-Hex | 15,699 | −5.90 | −3.08 | 0.13 | 2.82 |
| 5Ph5′Ph | 3830 | −5.74 | −3.06 | 0.04 | 2.68 |
| 5Th5′Th | 3900 | −5.62 | −3.10 | 0.07 | 2.52 |
| 6Ph6′Ph | 28,656 | −5.71 | −3.06 | 0.48 | 2.65 |
| 6Th6′Th | 32,976 | −5.54 | −3.10 | 0.77 | 2.44 |
| 6Ph7′Th | 15,154 | −5.80 | −3.08 | 0.31 | 2.72 |
| 6Th7′Ph | 20,351 | −5.67 | −3.08 | 0.43 | 2.59 |
| Low Energy | High Energy | ε | λonset | Egopt | |
|---|---|---|---|---|---|
| λmax soln (nm) a | λmax soln (nm) a | (cm−1 M−1) a | (nm) a | (eV) b | |
| IID | 484 | 365 | 1515 | 608 | 2.04 |
| 7Ph7′Ph | 505 | 408 | 5100 | 636 | 1.95 |
| 7Th7′Th | 528 | 411 | 4600 | 655 | 1.89 |
| 7Th7′Th-Hex | 530 | 415 | 4700 | 660 | 1.88 |
| 5Th5′Th | 518 | 420 | 3500 | 665 | 1.86 |
| 6Th6′Th | 533 | 410 | 20,000 | 682 | 1.81 |
(V) | HOMO (eV) c | Ered (V) | (V) | LUMO (eV) d | (eV) e | |
|---|---|---|---|---|---|---|
| IID | 1.47 | −5.74 | −0.96 | −0.62 | −3.65 | 2.09 |
| 7Ph7′Ph | 1.41 | −5.68 | −0.87 | −0.64 | −3.62 | 2.06 |
| 7Th7′Th | 1.35 | −5.62 | −0.84 | −0.61 | −3.66 | 1.96 |
| 7Th7′Th-Hex | 1.36 | −5.63 | −0.85 | −0.62 | −3.65 | 1.98 |
| Materials | PCE (%) | Voc (V) | Jsc (mA/cm2) | FF (%) |
|---|---|---|---|---|
| 7 | 5.70 | 0.84 | 15.31 | 43.09 |
| 8 | 6.07 | 0.82 | 17.34 | 42.06 |
| P3HT | 8.14 | 0.85 | 15.73 | 60.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, S.; Habibi, A.; Wang, Y.; Yassar, A. Investigating the Effect of Cross-Conjugation Patterns on the Optoelectronic Properties of 7,7′Isoindigo-Based Materials. Electronics 2023, 12, 3313. https://doi.org/10.3390/electronics12153313
Ren S, Habibi A, Wang Y, Yassar A. Investigating the Effect of Cross-Conjugation Patterns on the Optoelectronic Properties of 7,7′Isoindigo-Based Materials. Electronics. 2023; 12(15):3313. https://doi.org/10.3390/electronics12153313
Chicago/Turabian StyleRen, Shiwei, Amirhossein Habibi, Yujie Wang, and Abderrahim Yassar. 2023. "Investigating the Effect of Cross-Conjugation Patterns on the Optoelectronic Properties of 7,7′Isoindigo-Based Materials" Electronics 12, no. 15: 3313. https://doi.org/10.3390/electronics12153313
APA StyleRen, S., Habibi, A., Wang, Y., & Yassar, A. (2023). Investigating the Effect of Cross-Conjugation Patterns on the Optoelectronic Properties of 7,7′Isoindigo-Based Materials. Electronics, 12(15), 3313. https://doi.org/10.3390/electronics12153313








