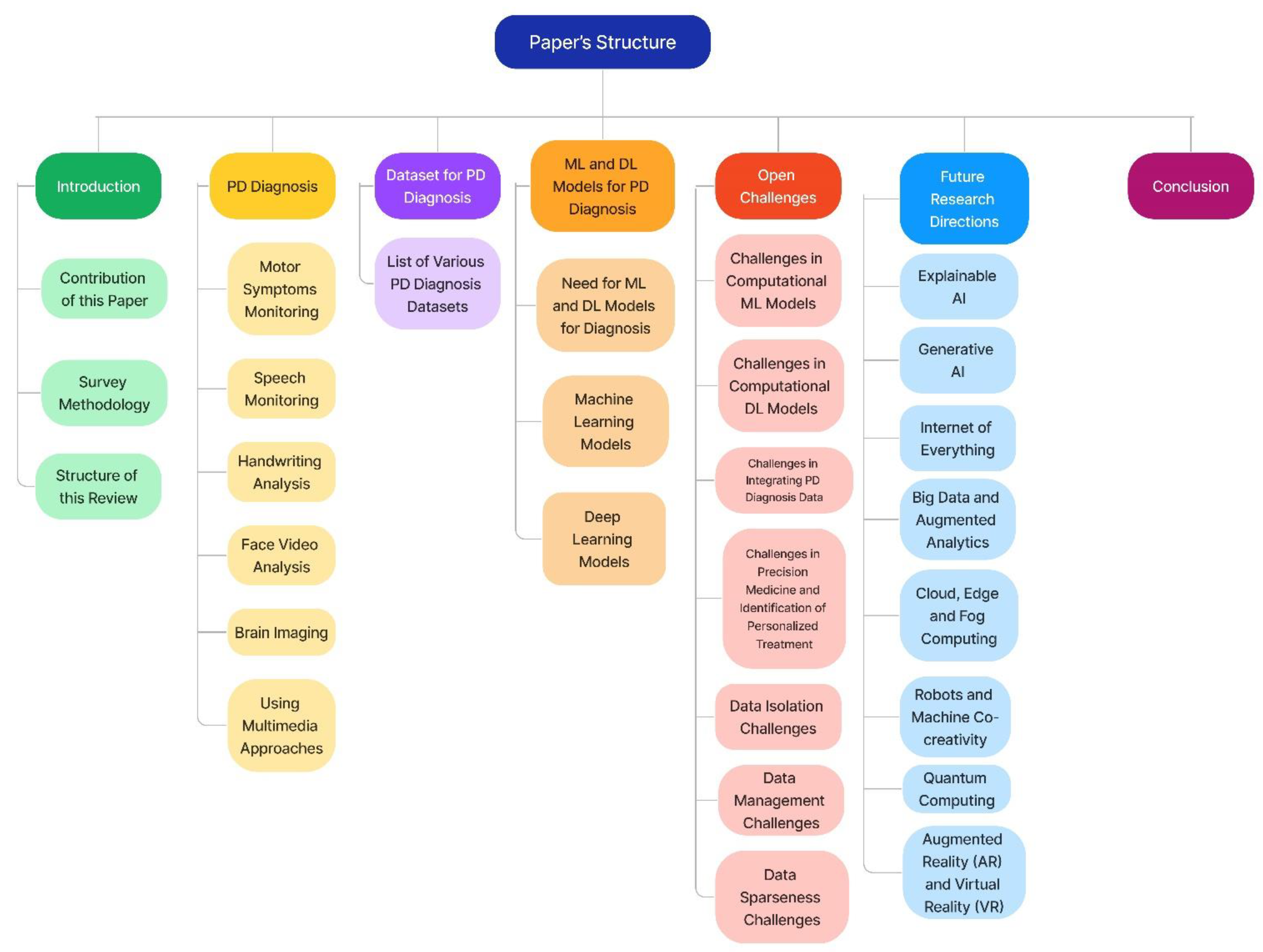
A Comprehensive Review on AI-Enabled Models for Parkinson’s Disease Diagnosis
Abstract
1. Introduction
1.1. Motivation
1.2. Contribution of This Survey
- Review of new techniques such as extreme learning machine, DBN, the deep generative model, and others, as well as older computational intelligence techniques such as Random Forest, ANN, DNN, KNN, and others to identify early traces of Parkinson’s disease.
- The studies on ML and DL techniques in PD are summarized in a thorough tabular format. The model, major contributions, and model constraints are all provided in the summary.
- We have also included the latest mobile technology and applications which can be used for assessment as well as for identification of PD. This review explicitly discusses the open challenges and future directions in Parkinson’s diagnosis and disease management.
1.3. Survey Methodology
1.3.1. Search Strategy and Literature Sources
1.3.2. Inclusion Criteria
1.3.3. Elimination Criteria
1.3.4. Results
2. Parkinson’s Disease Diagnosis
2.1. Motor Symptoms Monitoring
2.1.1. Gait and Posture
2.1.2. Bradykinesia
2.1.3. Freezing of Gait
2.1.4. Tremor
2.1.5. Dyskinesia
2.2. Speech Monitoring
2.3. Handwriting Analysis
2.4. Face Video Analysis
2.5. Brain Imaging
2.6. Using Multimedia Approaches
2.7. Stage-Wise Prediction of Parkinson
3. Datasets for Parkinson’s Disease Diagnosis
4. Machine Learning and Deep Learning Models for Parkinson’s Disease Diagnosis
4.1. Need for Machine Learning and Deep Learning Models for Parkinson’s Disease Diagnosis
4.2. Machine Learning Techniques
4.2.1. Artificial Neural Network
4.2.2. Naïve Bayes
4.2.3. Decision Tree
4.2.4. K-Nearest Neighbor
4.2.5. K-Mean Clustering
4.2.6. Random Forest
4.2.7. Support Vector Machine
4.2.8. Ensemble Models
4.2.9. Limitations of the ML Models
4.2.10. Inference of ML Models
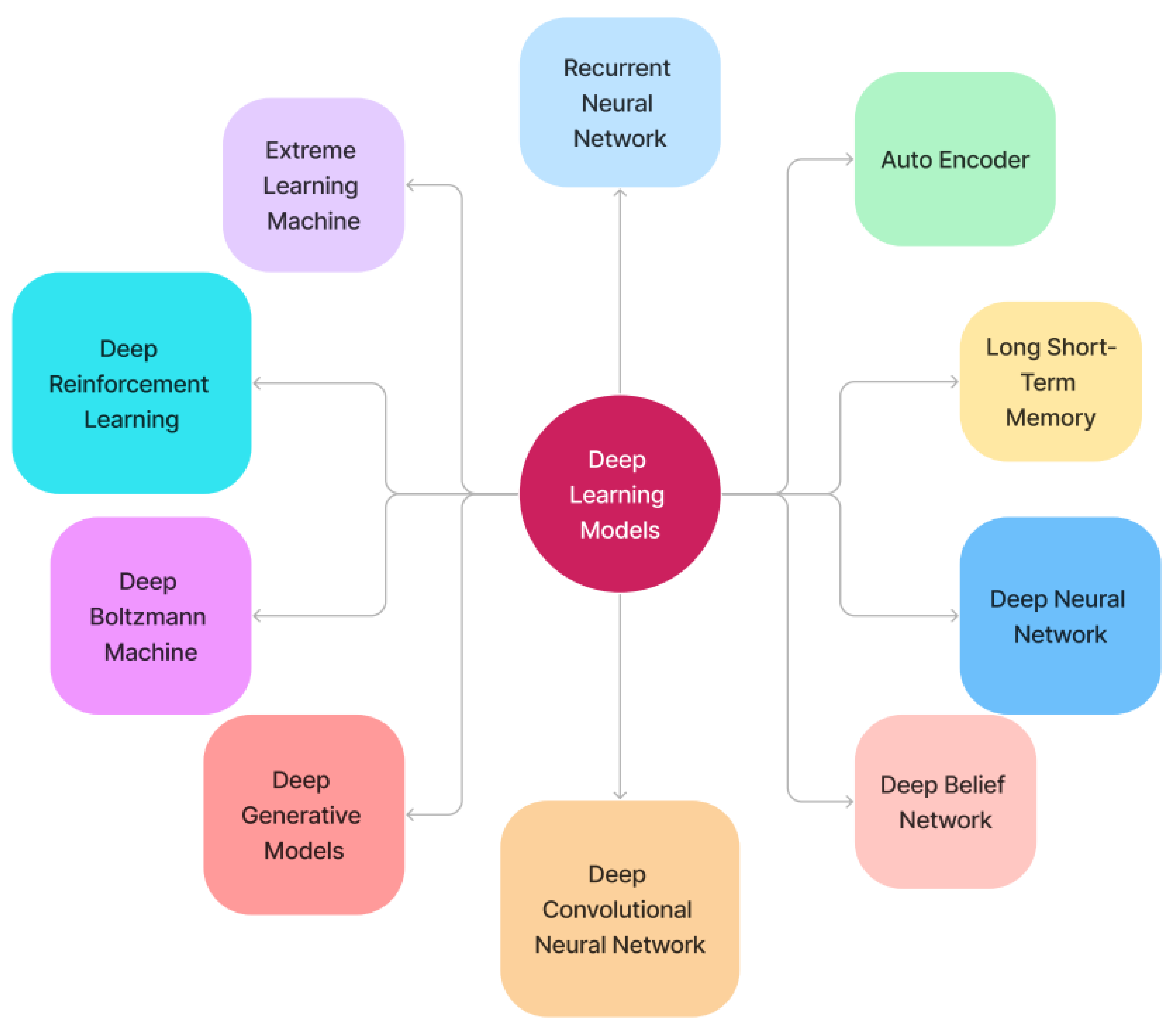
4.3. Deep Learning Models
4.3.1. Recurrent Neural Networks
4.3.2. Deep Autoencoder
4.3.3. Long Short-Term Memory
4.3.4. Deep Neural Network
- The suggested DNN classification model can uncover latent characteristics, significantly improving the classifier’s execution.
- This classification model can be used to remotely diagnose and monitor Parkinson’s disease. As a result, PWPs only need to visit the clinic once in a while.
- It could be capable of monitoring and treating PWDs in creating useful biomarkers for diagnosing PD at a preliminary phase because speech difficulties are one of the earliest indications of PD.
- Due to its high selectivity and responsiveness, the DNN model may be employed as a trustworthy PD sorter [59].
4.3.5. Deep Belief Network
4.3.6. Deep Convolutional Neural Network
4.3.7. Deep Generative Models
4.3.8. Deep Boltzmann Machine
4.3.9. Deep Reinforcement Learning
4.3.10. Extreme Learning Machine
4.3.11. Limitations of the DL Models
- Given that the imbalanced dataset today influences the results, handling it is quite difficult.
- In addition, due to advancements in deep learning techniques combined with nature-inspired methodologies, there is a latent potential to leverage multimodal datasets to enhance PD’s prediction accuracy.
- Although using the right criteria to assess ML models’ performance in PD classification is important, there is still room for improvement.
4.3.12. Inferences of DL Models
5. Open Challenges
5.1. Challenges in Computational ML Models
5.2. Challenges in Computational DL Models
- A DL model is a closed system that trains from data that can be used to imitate the dataset acquisition. As a result, explanations are frequently insufficient to fully comprehend its mechanism. Images from different datasets have diverse appearances due to non-standardized reference sources. This is a significant difficulty when using DL to analyze brain imaging.
- The use of large training datasets is critical for generating better results with DL approaches, and the lack of them is among the major hurdles in the application process. It is used in neural mapping to protect patients’ confidentiality. At the same time, labeling those data is a major challenge that requires professional guidance.
5.3. Challenges in Integrating Parkinson’s Disease Diagnosis Data
- Medical data consists of patient longitudinal records that span from a few months to years during their regular visits. Dealing with conflicting patient records is one of the most difficult aspects of working with longitudinal data. Because many patients leave out or fail to show up for evaluations, there is a discrepancy in data, causing statistics to be skewed. Another issue is that patient data is missing for a few medical practitioner assessment exams that are not provided at the time. Lipton et al. [142] also employed forward and backward filling within a one-hour window for each visit to resample all missing values. When the whole variable record was lacking, they substituted a clinically normal value as determined by specialists.
- Realigning and combining complex multi-source and multi-site PD databases is also a challenge. In Parkinson’s, collecting such data is difficult since there is such a scarcity of cohorts having extensive, well-curated information. As a result, one major requirement is the growth or duplication of projects like PPMI or PDBP, ideally with a model that provides an unrestricted approach to the underlying information; the expense of this data type gathering is high, but it is an essential resource in their attempts to understand PD.
5.4. Challenges in Merging Omics Data with Various other Sources of Information, Such as Electronic Health Records and Wearable Sensors Data
- The evolution of wearable devices to monitor people with PD has largely emphasized the motor elements of the condition, which are also assessed by clinical scales, but with less sensitivity and specificity. Even though there have been recent improvements in quantifying motor symptoms like tremors, these outcomes frequently only show limited quantitative consistency with evaluations of life quality.
- Non-motor impairments are frequently sources of disability and patient priorities (e.g., depression, anxiety). The majority of health data are now kept on paper and are controlled by medical centers, many of which have poor communication capabilities. Some health files have already transitioned from paper charts to electronic health records, but these EHRs are primarily digital copies of their paper-based forebears and do not include all of the technical alternatives that are presently available to help clinical decision-making. Strong and comprehensive data protection laws and regulations are also expected to lower the danger of data leakage to a minimum and raise the user acceptability of EHRs.
5.5. Challenges in Precision Medicine and Identification of Personalized Treatment
5.6. Data Isolation Challenges
5.7. Data Management Challenges
- The BEAT-PD data challenge was created to test innovative strategies for predicting PD development. Its goal was to see if illness intensity and development could be determined using passive sensor data collected in everyday life. Participants had access to raw sensor time-series data that might be utilized to forecast individual medication status and symptom intensity. Cleaning and curating data is difficult, but discovering patterns from it is much more difficult. Quoc V [120] outlined the problems of big data, as well as the importance of big data technologies in the biomedical field. It is challenging to create a stereotype MRI database because it is a remnant of a training method that might end in a statistical product. The issue can be alleviated by introducing a large dataset into the system, assessing the relationship between retrieved characteristics, and fine-tuning the system’s variables. It is still a work in progress to predict NLD in actual time from visual observation.
- Stream processing, on the other hand, is a parallel computer approach for processing substantial amounts of data. When we use ML techniques to gain value from large data, maintaining data quality is a major difficulty. According to researchers, unbalanced data is a prevalent difficulty in categorization. The most difficult task is the cleaning and curating of the data. Each file must be separately examined, and any duplicate or administrative data not necessary for the research should be eliminated. When we combine all of these broad properties, we obtain a massive sparse matrix. The goal of the research should be to uncover and display the connections between different traits.
- Because it is time series data, the complication is raised even further. Compiling and layering data are difficult tasks. The information is skewed, unbalanced, and contradictory. There are many missing values when data is pooled. Only 30% of the data is accessible. Databases from healthcare research and behavioral investigations of PD are now quickly developing, with little awareness or integration of the qualities obtained. Recognizing the significance of each characteristic collected through PD identification and therapy is critical, and the research serves to emphasize the data’s reliability difficulties and ways to address them. We can expand their study in the future by allowing them to add more qualities and identify their involvement in PD [143].
5.8. Data Sparseness Challenges
- The major goal of [8] was to enhance the reliability of the current state-of-the-art in-patient diagnosis and avoid patient misinterpretation, and the experimental findings showed that the goal was met. However, because the diagnosis may be conducted in a variety of ways, there is still a lot of room for advancement in technology. The findings of this study advised against using less accurate approaches for diagnosing Parkinson’s and the usefulness of telemonitoring apps.
- The PPMI is a pioneering prospective in clinical research that examines PD cohorts using a variety of data sources, including sophisticated imaging, biologic samples, and clinical and behavioral evaluations, to determine the circumstances of PD development in individuals. The data is scarce, inconsistent, and continuous, with a lot of temporal facts encoded in a clinical situation that supports the lengthy progress route of PD, making learning even more challenging.
- Che et al.[111] dealt with data anomalies and imputed the bulk of incomplete data. For the bulk of the missing data, they used the latest occurrence carry forward method. They substituted the patient’s first observed record if the patient’s initial record was missing. Table 7 presents the open challenges for Parkinson’s disease diagnosis. Figure 7 depicts the open challenges for Parkinson’s disease diagnosis.
6. Parkinson’s Disease Diagnosis using Sensors, Smartphone Devices, and Web Applications
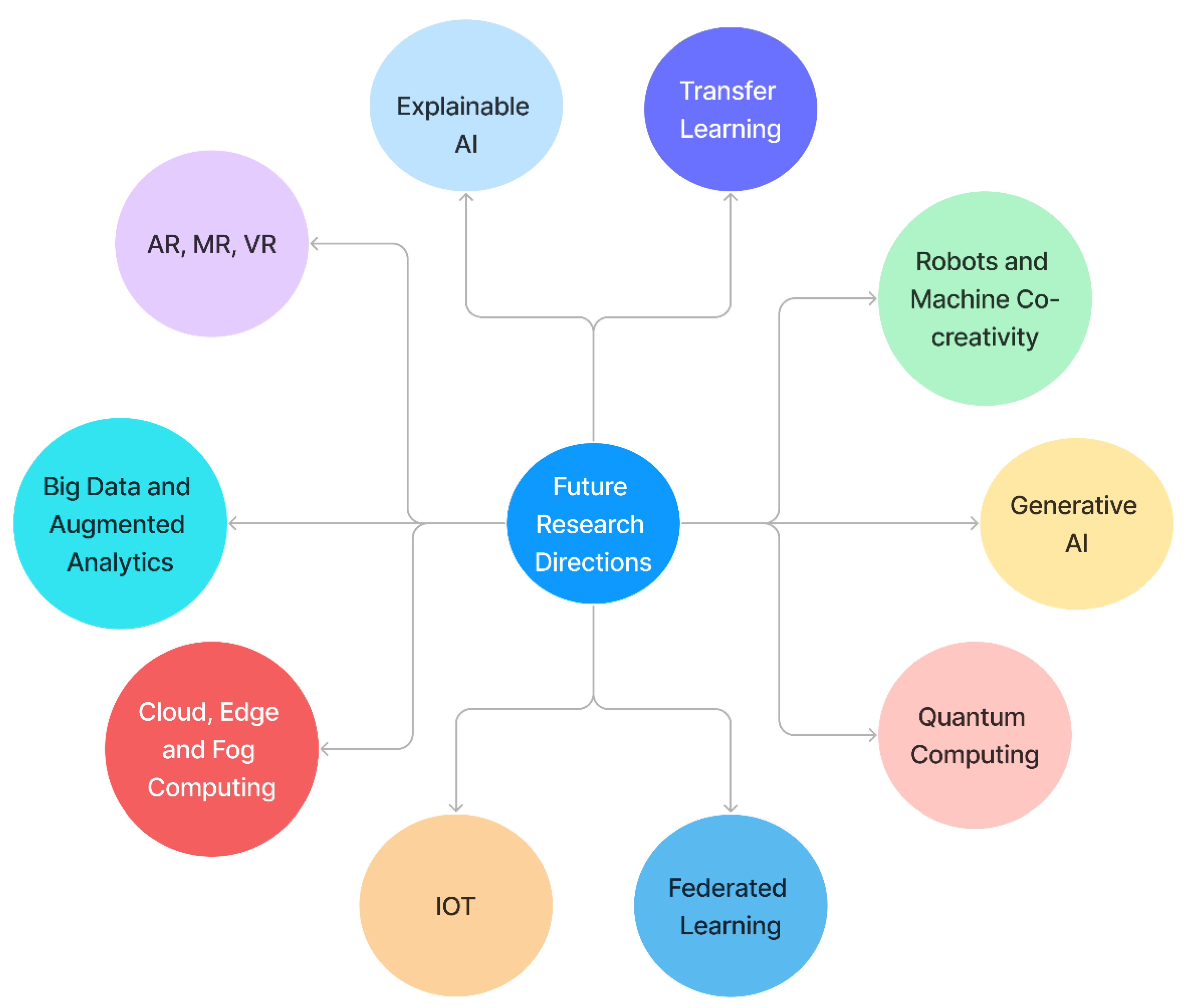
7. Future Research Directions
- Even if rather high accuracies have previously been achieved, the reported results indicate that there is still room for development. For identifying the presence of PD movement disorders in time series data, scientists have suggested using an uncommon combination of algorithms, including the log algorithm. The main goal is to see if these methods can match, if not exceed, the mentioned papers in terms of accuracy. MOSIS, a relevant framework for assessing various techniques, is currently being developed. Future research should focus on this topic, particularly using longitudinal approaches. Dopamine bioavailability, on the other hand, can influence speech results and other communicating abilities. It should also incorporate new processes or at-risk carrier states to see if central mediators can anticipate symptomology more closely linked to PD risks, such as olfaction and sleep disruption, which did not develop much in the established PD cohort.
- There is a need to develop better robust models which will improve PD identification while maintaining the accuracy of the results and developing models’ impartial behavior. Feature selection approaches and DL models can be combined to achieve this. A bigger database is needed, and the algorithm will be tweaked and refined for other classification tasks important to PD monitoring (e.g., dyskinesia, tremor). Finally, data collected in the home and community can be used to test these strategies.
7.1. Explainable AI
7.2. Generative AI
- It is recommended that, in the future, more data augmentation techniques based on various AI paradigms and architectural frameworks be investigated to create a smart model for voice recognition with sparse data. Ref. [151] provided a unique technique for selecting the most exclusionary feature for differential diagnosis of PD and SWEDD, utilizing machine learning methods such as KSOM, LSSVM, and WAT as statistical measurements in the study. Clinically significant ROIs were discovered to be identified by employing MRIs and KSOM-based feature extraction. This technology might be utilized not only to diagnose PD early on, but also for exploratory study into brain regions. This paradigm might hasten the emergence of evidence-based prognosis in this environment. The limited size of the group under research is, of course, the study’s fundamental part, making the findings less generalizable. Regrettably, there is currently a void in the scientific community working on this issue in terms of the accessibility of a large benchmark dataset.
- Impedovo et al. [99] findings suggested that a diagnostic assessment based on such technology might appropriately exclude illness in healthy individuals’ communities, making it helpful for ruling in disease when a satisfactory reaction is obtained. Even though usage of DL frameworks is on the rise, there are no articles relating to DL in the large and diverse scientific databases. The exploration of this specific field, PD, in conjunction with a well-known, optimized, and robust DL architecture, like Caffe, might be fruitful. In the future, research on new databases only focusing on the DL and DP might be carried out for the recipient to comprehend this difference and, as a result, other research possibilities in the field [134].
7.3. Internet of Everything
7.4. Big Data and Augmented Analytics
7.5. Cloud, Edge, and Fog Computing
7.6. Robots and Machine Co-Creativity
7.7. Quantum Computing
7.8. Transfer Learning
7.9. Federated Learning
7.10. Augmented Reality (AR) and Virtual Reality (VR)
7.10.1. Augmented Reality
7.10.2. Virtual Reality
- Virtual reality has been developed as a feasible method for investigating and treating people with PD who have complex deficiencies. In a regulated laboratory or clinical setting, the goal of using VR in stroke recovery is to evoke and/or prepare neurobiological responses that are analogous to the few that happen in real life. The extent to which a user is completely absorbed in a digital environment is known as immersion, which is a major feature of VR.
- Scientists are encouraged to build interactive virtual applications with combined evaluation and training programs that are tailored to the needs of persons with PD and healthcare professionals to maximize the potential of VR rehabilitation and improve rehabilitation results. By immersing persons with PD in an enhanced and highly tailored environment that resembles real-world events, while avoiding risk, VR offers the potential to improve knowledge and treatment of complicated PD impairments. However, its full potential for PD rehabilitation has yet to be realized. When provided in a fully supervised format, both are preferable to no treatment, although there is no indication that VR treatment is better than non-VR therapy in terms of gait and balanced outcomes. Virtual reality enables the secure detection of a person’s particular FOG triggers and equilibrium deficits, resulting in specific training targets [147]. Figure 8 illustrates the future research directions for Parkinson’s disease diagnosis.
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Parkinson Association of the Carolinas. Understanding Parkinson’s Disease—Parkinson’s Association of Carolinas. 2022. Available online: https://www.parkinsonassociation.org/understanding-parkinsons-disease/ (accessed on 20 December 2022).
- Dorsey, E.R.; Constantinescu, R.; Thompson, J.P.; Biglan, K.M.; Holloway, R.G.; Kieburtz, K.; Marshall, F.J.; Ravina, B.M.; Schifitto, G.; Siderowf, A.; et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 2007, 68, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Frid, A.; Hazan, H.; Hilu, D.; Manevitz, L.; Ramig, L.O.; Sapir, S. Computational Diagnosis of Parkinson’s Disease Directly from Natural Speech Using Machine Learning Techniques. In Proceedings of the 2014 IEEE International Conference on Software Science, Technology and Engineering, Ramat Gan, Israel, 11–12 June 2014; pp. 50–53. [Google Scholar]
- Srivastava, S. Genetic Algorithm Optimized Deep Learning Model for Parkinson Disease Severity Detection. Ph.D. Thesis, National College of Ireland, Dublin, Ireland, 2021. [Google Scholar]
- Ahmadi Rastegar, D.; Ho, N.; Halliday, G.M.; Dzamko, N. Parkinson’s progression prediction using machine learning and serum cytokines. NPJ Park. Dis. 2019, 5, 14. [Google Scholar] [CrossRef]
- Miljkovic, D.; Aleksovski, D.; Podpečan, V.; Lavrač, N.; Malle, B.; Holzinger, A. Machine Learning and Data Mining Methods for Managing Parkinson’s Disease. In Machine Learning for Health Informatics; Springer: Cham, Switzerland, 2016; pp. 209–220. [Google Scholar]
- Pereira, C.R.; Pereira, D.R.; Weber, S.A.; Hook, C.; De Albuquerque VH, C.; Papa, J.P. A survey on computer-assisted Parkinson’s disease diagnosis. Artif. Intell. Med. 2019, 95, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Noor MB, T.; Zenia, N.Z.; Kaiser, M.S.; Mamun, S.A.; Mahmud, M. Application of deep learning in detecting neurological disorders from magnetic resonance images: A survey on the detection of Alzheimer’s disease, Parkinson’s disease and schizophrenia. Brain Inform. 2020, 7, 11. [Google Scholar] [CrossRef]
- Latella, D.; Maggio, M.G.; Maresca, G.; Saporoso, A.F.; Le Cause, M.; Manuli, A.; Milardi, D.; Bramanti, P.; De Luca, R.; Calabrò, R.S. Impulse control disorders in Parkinson’s disease: A systematic review on risk factors and pathophysiology. J. Neurol. Sci. 2019, 398, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Pahuja, G.; Nagabhushan, T.N. A comparative study of existing machine learning approaches for Parkinson’s disease detection. IETE J. Res. 2021, 67, 4–14. [Google Scholar] [CrossRef]
- Alzubaidi, M.S.; Shah, U.; Dhia Zubaydi, H.; Dolaat, K.; Abd-Alrazaq, A.A.; Ahmed, A.; Househ, M. The role of neural network for the detection of Parkinson’s disease: A scoping review. Healthcare 2021, 9, 740. [Google Scholar] [CrossRef]
- Mei, J.; Desrosiers, C.; Frasnelli, J. Machine learning for the diagnosis of Parkinson’s disease: A review of literature. Front. Aging Neurosci. 2021, 13, 633752. [Google Scholar] [CrossRef]
- Myszczynska, M.A.; Ojamies, P.N.; Lacoste, A.M.B.; Neil, D.; Saffari, A.; Mead, R.; Hautbergue, G.M.; Holbrook, J.D.; Ferraiuolo, L. Applications of machine learning to diagnosis and treatment of neurodegenerative diseases. Nat. Rev. Neurol. 2020, 16, 440–456. [Google Scholar] [CrossRef]
- Krokidis, M.G.; Dimitrakopoulos, G.N.; Vrahatis, A.G.; Tzouvelekis, C.; Drakoulis, D.; Papavassileiou, F.; Exarchos, T.P.; Vlamos, P. A Sensor-Based Perspective in Early-Stage Parkinson’s Disease: Current State and the Need for Machine Learning Processes. Sensors 2022, 22, 409. [Google Scholar] [CrossRef]
- Rana, A.; Dumka, A.; Singh, R.; Panda, M.K.; Priyadarshi, N. A Computerized Analysis with Machine Learning Techniques for the Diagnosis of Parkinson’s Disease: Past Studies and Future Perspectives. Diagnostics 2022, 12, 2708. [Google Scholar] [CrossRef]
- Bind, S.; Tiwari, A.K.; Sahani, A.K. A survey of machine learning based approaches for Parkinson disease prediction. Int. J. Comput. Sci. Inf. Technol. 2015, 6, 1648–1655. [Google Scholar]
- Armstrong, M.J.; Okun, M.S. Time for a new image of Parkinson disease. JAMA Neurol. 2020, 77, 1345–1346. [Google Scholar] [CrossRef] [PubMed]
- García, A.M.; Arias-Vergara, T.; CVasquez-Correa, J.; Nöth, E.; Schuster, M.; Welch, A.E.; Bocanegra, Y.; Baena, A.; Orozco-Arroyave, J.R. Cognitive determinants of dysarthria in Parkinson’s disease: An automated machine learning approach. Mov. Disord. 2021, 36, 2862–2873. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.W.; Chao, W.H.; Lin, S.H.; Chen, Y.Y. A vision-based analysis system for gait recognition in patients with Parkinson’s disease. Expert Syst. Appl. 2009, 36, 7033–7039. [Google Scholar] [CrossRef]
- Johri, A.; Tripathi, A. Parkinson Disease Detection Using Deep Neural Networks. In Proceedings of the 2019 Twelfth International Conference on Contemporary Computing (IC3), Noida, India, 8–10 August 2019; pp. 1–4. [Google Scholar]
- El Maachi, I.; Bilodeau, G.A.; Bouachir, W. Deep 1D-Convnet for accurate Parkinson disease detection and severity prediction from gait. Expert Syst. Appl. 2020, 143, 113075. [Google Scholar] [CrossRef]
- Camps, J.; Sama, A.; Martin, M.; Rodriguez-Martin, D.; Perez-Lopez, C.; Arostegui, J.M.M.; Cabestany, J.; Català, A.; Alcaine, S.; Mestre, B.; et al. Deep learning for FOG detection in Parkinson’s disease patients in their homes using a waist-worn inertial measurement unit. Knowl.-Based Syst. 2018, 139, 119–131. [Google Scholar] [CrossRef]
- Thomas, M.; Lenka, A.; Kumar Pal, P. Handwriting analysis in Parkinson’s disease: Current status and future directions. Mov. Disord. Clin. Pract. 2017, 4, 806–818. [Google Scholar] [CrossRef]
- Kubota, K.J.; Chen, J.A.; Little, M.A. Machine learning for large-scale wearable sensor data in Parkinson’s disease: Concepts, promises, pitfalls, and futures. Mov. Disord. 2016, 31, 1314–1326. [Google Scholar] [CrossRef]
- Abdulhay, E.; Arunkumar, N.; Narasimhan, K.; Vellaiappan, E.; Venkatraman, V. Gait and tremor investigation using machine learning techniques for the diagnosis of Parkinson disease. Future Gener. Comput. Syst. 2018, 83, 366–373. [Google Scholar] [CrossRef]
- Aich, S.; Kim, H.C.; Hui, K.L.; Al-Absi, A.A.; Sain, M. A Supervised Machine Learning Approach Using Different Feature Selection Techniques on Voice Datasets for Prediction of Parkinson’s Disease. In Proceedings of the 2019 21st International Conference on Advanced Communication Technology (ICACT), PyeongChang, Republic of Korea, 17–20 February 2019; pp. 1116–1121. [Google Scholar]
- Bryant, M.S.; Rintala, D.H.; Hou, J.G.; Lai, E.C.; Protas, E.J. Effects of levodopa on forward and backward gait patterns in persons with Parkinson’s disease. NeuroRehabilitation 2011, 29, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Pistacchi, M.; Gioulis, M.; Sanson, F.; De Giovannini, E.; Filippi, G.; Rossetto, F.; Marsala, S.Z. Gait analysis and clinical correlations in early Parkinson’s disease. Funct. Neurol. 2017, 32, 28. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Yang, M.; Wang, H.; McClean, S. Machine Learning and Statistical Approaches to Support the Discrimination of Neuro-degenerative Diseases Based on Gait Analysis. Intell. Patient Manag. 2009, 189, 57–70. [Google Scholar]
- Ahlrichs, C.; Lawo, M. Parkinson’s disease motor symptoms in machine learning: A review. arXiv 2013, arXiv:1312.3825. [Google Scholar] [CrossRef]
- Sherrill, D.M.; Hughes, R.; Salles, S.S.; Lie-Nemeth, T.; Akay, M.; Standaert, D.G.; Bonato, P. Advanced Analysis of Wearable Sensor Data to Adjust Medication Intake in Patients with Parkinson’s Disease. In Proceedings of the 2005 Neural Engineering—Conference Proceedings: 2nd International IEEE EMBS Conference, Arlington, VA, USA, 16–19 March 2005; pp. 202–205. [Google Scholar]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J.Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. Available online: http://jnnp.bmj.com/content/79/4/368.abstract (accessed on 18 December 2022). [CrossRef]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Cook, D.J.; Schmitter-Edgecombe, M.; Dawadi, P. Analyzing activity behavior and movement in a naturalistic environment using smart home techniques. IEEE J. Biomed. Health Inform. 2015, 19, 1882–1892. [Google Scholar] [CrossRef]
- Passos, L.A.; Pereira, C.R.; Rezende, E.R.; Carvalho, T.J.; Weber, S.A.; Hook, C.; Papa, J.P. Parkinson Disease Identification Using Residual Networks and Optimum-Path Forest. In Proceedings of the 2018 IEEE 12th International Symposium on Applied Computational Intelligence and Informatics (SACI), Timisoara, Romania, 17–19 May 2018; pp. 325–330. [Google Scholar]
- Mazilu, S.; Hardegger, M.; Zhu, Z.; Roggen, D.; Tröster, G.; Plotnik, M.; Hausdorff, J.M. Online Detection of FOG with Smartphones and Machine Learning Techniques. In Proceedings of the 2012 6th International Conference on Pervasive Computing Technologies for Healthcare (PervasiveHealth) and Workshops, San Diego, CA, USA, 21–24 May 2012; pp. 123–130. [Google Scholar]
- Bloem, B.R.; Hausdor, J.M.; Visser, J.E.; Giladi, N. Falls and FOG in Parkinson’s disease: A review of two interconnected, episodic phenomena. Mov. Disord. J. 2004, 19, 871–884. [Google Scholar] [CrossRef]
- Giladi, N.; Tal, J.; Azulay, T.; Rascol, O.; Brooks, D.J.; Melamed, E.; Oertel, W.; Poewe, W.H.; Stocchi, F.; Tolosa, E. Validation of the FOG questionnaire in patients with Parkinson’s disease. Mov. Disord. J. 2009, 24, 655–661. [Google Scholar] [CrossRef]
- Heremans, E.; Nackaerts, E.; Broeder, S.; Vervoort, G.; Swinnen, S.P.; Nieuwboer, A. Handwriting impairments in people with Parkinson’s disease and FOG. Neurorehabilit. Neural Repair 2016, 30, 911–919. [Google Scholar] [CrossRef]
- Lopez, I.C.; Ruiz, P.J.; Del Pozo, S.V.; Bernardos, V.S. Motor complications in Parkinson’s disease: Ten year follow-up study. Mov. Disord. 2010, 25, 2735–2739. [Google Scholar] [CrossRef] [PubMed]
- Rehman RZ, U.; Del Din, S.; Guan, Y.; Yarnall, A.J.; Shi, J.Q.; Rochester, L. Selecting clinically relevant gait characteristics for classification of early Parkinson’s disease: A comprehensive machine learning approach. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.T.; MacDougall, H.G.; Ondo, W.G. Ambulatory monitoring of 1010 FOG in Parkinson’s disease. J. Neurosci. Methods 2008, 167, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Marquez, J.S.; Hasan, S.S.; Siddiquee, M.R.; Luca, C.C.; Mishra, V.R.; Mari, Z.; Bai, O. Neural correlates of freezing of gait in Parkinson’s disease: An electrophysiology mini-review. Front. Neurol. 2020, 11, 571086. [Google Scholar] [CrossRef]
- Nieuwboer, A.; Vercruysse, S.; Feys, P.; Levin, O.; Spildooren, J.; Swinnen, S. Upper limb movement interruptions are correlated to FOG in Parkinson’s disease. Eur. J. Neurosci. 2009, 29, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Ziv, I.; Avraham, M.; Dabby, R.; Zoldan, J.; Djaldetti, R.; Melamed, E. Early-occurrence of manual motor blocks in Parkinson’s disease: A quantitative assessment. Acta Neurol. Scand. 1999, 99, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Abd El Aal, H.A.; Taie, S.A.; El-Bendary, N. An optimized RNN-LSTM approach for Parkinson’s disease early detection using speech features. Bull. Electr. Eng. Inform. 2021, 10, 2503–2512. [Google Scholar] [CrossRef]
- Bazgir, O.; Frounchi, J.; Habibi SA, H.; Palma, L.; Pierleoni, P. A Neural Network System for Diagnosis and Assessment of Tremor in Parkinson Disease Patients. In Proceedings of the 2015 22nd Iranian Conference on Biomedical Engineering (ICBME), Tehran, Iran, 25–27 November 2015; pp. 1–5. [Google Scholar]
- Shetty, S.; Rao, Y.S. SVM Based Machine Learning Approach to Identify Parkinson’s Disease Using Gait Analysis. In Proceedings of the 2016 International Conference on Inventive Computation Technologies (ICICT), Coimbatore, India, 26–27 August 2016; Volume 2, pp. 1–5. [Google Scholar]
- Balaji, E.; Brindha, D.; Balakrishnan, R. Supervised machine learning based gait classification system for early detection and stage classification of Parkinson’s disease. Appl. Soft Comput. 2020, 94, 106494. [Google Scholar]
- Hammerla, N.Y.; Fisher, J.; Andras, P.; Rochester, L.; Walker, R.; Plötz, T. PD Disease State Assessment in Naturalistic Environments Using Deep Learning. In Proceedings of the Twenty-Ninth AAAI Conference on Artificial Intelligence, Austin, TX, USA, 25–30 January 2015. [Google Scholar]
- Scherzer, C.R.; Eklund, A.C.; Morse, L.J.; Liao, Z.; Locascio, J.J.; Fefer, D.; Schwarzschild, M.A.; Schlossmacher, M.G.; Hauser, M.A.; Vance, J.M.; et al. Molecular markers of early Parkinsons disease based on gene expression in blood. Proc. Natl. Acad. Sci. USA 2007, 104, 955960. [Google Scholar] [CrossRef] [PubMed]
- Oung, Q.W.; Hariharan, M.; Lee, H.L.; Basah, S.N.; Sarillee, M.; Lee, C.H. Wearable Multimodal Sensors for Evaluation of Patients with Parkinson Disease. In Proceedings of the 2015 IEEE International Conference on Control System, Computing and Engineering (ICCSCE), Penang, Malaysia, 27–29 November 2015; pp. 269–274. [Google Scholar]
- Zhang, Y.N. Can a smartphone diagnose Parkinson disease? A deep neural network method and telediagnosis system implementation. Park. Dis. 2017, 2017, 6209703. [Google Scholar] [CrossRef]
- Abayomi-Alli, O.O.; Damaševičius, R.; Maskeliūnas, R.; Abayomi-Alli, A. BiLSTM with Data Augmentation Using Inter Ion Methods to Improve Early Detection of Parkinson Disease. In Proceedings of the 2020 15th Conference on Computer Science and Information Systems (FedCSIS), Sofia, Bulgaria, 6–9 September 2020; pp. 371–380. [Google Scholar]
- Al-Fatlawi, A.H.; Jabardi, M.H.; Ling, S.H. Efficient Diagnosis System for Parkinson’s Disease Using Deep Belief Network. In Proceedings of the 2016 IEEE Congress on Evolutionary Computation (CEC), Vancouver, BC, Canada, 24–29 July 2016; pp. 1324–1330. [Google Scholar]
- Hazan, H.; Hilu, D.; Manevitz, L.; Ramig, L.O.; Sapir, S. Early Diagnosis of Parkinson’s Disease via Machine Learning on Speech Data. In Proceedings of the 2012 IEEE 27th Convention of Electrical and Electronics Engineers in Israel, Eilat, Israel, 14–17 November 2012; pp. 1–4. [Google Scholar]
- McNamara, P.; Obler, L.K.; Au, R.; Durso, R.; Albert, M.L. Speech monitoring skills in Alzheimer’s disease, Parkinson’s disease, and normal aging. Brain Lang. 1992, 42, 38–51. [Google Scholar] [CrossRef]
- Karan, B.; Sahu, S.S.; Mahto, K. Parkinson disease prediction using intrinsic mode function-based features from speech signal. Biocybern. Biomed. Eng. 2020, 40, 249–264. [Google Scholar] [CrossRef]
- Caliskan, A.; Badem, H.; Basturk, A.; Yuksel, M.E. Diagnosis of the Parkinson disease by using deep neural network classifier. IU-J. Electr. Electron. Eng. 2017, 17, 3311–3318. [Google Scholar]
- Mandal, I.; Sairam, N. New machine-learning algorithms for prediction of Parkinson’s disease. Int. J. Syst. Sci. 2014, 45, 647–666. [Google Scholar] [CrossRef]
- McLennan, J.E.; Nakano, K.; Tyler, H.R.; Schwab, R.S. Micrographia in Parkinson’s disease. J. Neurol. Sci. 1972, 15, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Ranzato, M.; Poultney, C.; Chopra, S.; LeCun, Y. Efficient Learning of Sparse Representations with an Energy-Based Model. Proc. Neural Inf. Process. Syst. 2006, 19. [Google Scholar] [CrossRef]
- Kotsenas, A.L.; Vernooij, M.W.; Port, J.D. Advances in neurodegenerative and psychiatric imaging: Introductory editorial. Br. J. Radiol. 2019, 92, 20199003. [Google Scholar] [CrossRef]
- Pereira, C.R.; Weber, S.A.; Hook, C.; Rosa, G.H.; Papa, J.P. Deep Learning-Aided Parkinson’s Disease Diagnosis from Handwritten Dynamics. In Proceedings of the 2016 29th SIBGRAPI Conference on Graphics, Patterns and Images (SIBGRAPI), Sao Paulo, Brazil, 4–7 October 2016; pp. 340–346. [Google Scholar]
- Jin, B.; Qu, Y.; Zhang, L.; Gao, Z. Diagnosing Parkinson disease through facial expression recognition: Video analysis. J. Med. Internet Res. 2020, 22, e18697. [Google Scholar] [CrossRef] [PubMed]
- Nieuwboer, A.; Dom, R.; De Weerdt, W.; Desloovere, K.; Janssens, L.; Stijn, V. Electromyographic profiles of gait prior to onset of freezing episodes in patients with Parkinson’s disease. Brain 2004, 127, 1650–1660. [Google Scholar] [CrossRef] [PubMed]
- Hausdor, J.M.; Schaafsma, J.D.; Balash, Y.; Bartels, A.L.; Gurevich, T.; Giladi, N. Impaired regulation of stride variability in Parkinson’s disease subjects with FOG. Exp. Brain Res. 2003, 149, 187–194. [Google Scholar] [CrossRef]
- Misiaszek, G.; Riconscente, M.; Henke, M.; Walsh, J.P. Online multimedia teaching tool for Parkinson’s disease. J. Undergrad. Neurosci. Educ. 2008, 6, A68. [Google Scholar] [PubMed]
- Faulkner, T.P.; Sprague, J.E. Application of several multimedia approaches to the teaching of CNS pharmacology: Parkinson’s disease and antiparkinsonism drugs. Am. J. Pharm. Educ. 1996, 60, 417–421. [Google Scholar]
- Yu, W.; Vuong, C.; Ingalls, T. An Interactive Multimedia System for Parkinson’s Patient Rehabilitation. In International Conference on Virtual and Mixed Reality; Springer: Berlin/Heidelberg, Germany, 2020; pp. 129–137. [Google Scholar]
- Nagasubramanian, G.; Sankayya, M. Multi-variate vocal data analysis for detection of Parkinson disease using deep learning. Neural Comput. Appl. 2021, 33, 4849–4864. [Google Scholar] [CrossRef]
- Drotár, P.; Mekyska, J.; Rektorová, I.; Masarová, L.; Smékal, Z.; Faundez-Zanuy, M. Evaluation of handwriting kinematics and pressure for differential diagnosis of Parkinson’s disease. Artif. Intell. Med. 2016, 67, 39–46. [Google Scholar] [CrossRef]
- Loh, H.W.; Hong, W.; Ooi, C.P.; Chakraborty, S.; Barua, P.D.; Deo, R.C.; Soar, J.; Palmer, E.E.; Acharya, U.R. Application of deep learning models for automated identification of Parkinson’s disease: A review (2011–2021). Sensors 2021, 21, 7034. [Google Scholar] [CrossRef] [PubMed]
- Heinzel, S.; Roeben, B.; Ben-Shlomo, Y.; Lerche, S.; Alves, G.; Barone, P.; Behnke, S.; Berendse, H.W.; Bloem, B.R.; Burn, D.; et al. Prodromal markers in Parkinson’s disease: Limitations in longitudinal studies and lessons learned. Front. Aging Neurosci. 2016, 8, 147. [Google Scholar] [CrossRef]
- Butt, A.H.; Rovini, E.; Dolciotti, C.; De Petris, G.; Bongioanni, P.; Carboncini, M.C.; Cavallo, F. Objective and automatic classification of Parkinson disease with Leap Motion controller. Biomed. Eng. Online 2018, 17, 168. [Google Scholar] [CrossRef]
- Tiwari, H.; Shridhar, S.K.; Patil, P.V.; Sinchana, K.R.; Aishwarya, G. Early prediction of Parkinson disease using machine learning and deep learning approaches. EasyChair Prepr. 2021, 4889, 1–14. [Google Scholar]
- Bourouhou, A.; Jilbab, A.; Nacir, C.; Hammouch, A. Comparison of Classification Methods to Detect the Parkinson Disease. In Proceedings of the 2016 International Conference on Electrical and Information Technologies (ICEIT), Tangiers, Morocco, 4–7 May 2016; pp. 421–424. [Google Scholar]
- Sachnev, V.; Kim, H.J. Parkinson Disease Classification Based on Binary Coded Genetic Algorithm and Extreme Learning Machine. In Proceedings of the 2014 IEEE Ninth International Conference on Intelligent Sensors, Sensor Networks and Information Processing (ISSNIP), Singapore, 21–24 April 2014; pp. 1–6. [Google Scholar]
- Parisi, L.; RaviChandran, N.; Manaog, M.L. Feature-driven machine learning to improve early diagnosis of Parkinson’s disease. Expert Syst. Appl. 2018, 110, 182–190. [Google Scholar] [CrossRef]
- Tiwari, A.K. Machine learning based approaches for prediction of Parkinson’s disease. Mach. Learn. Appl. 2016, 3, 33–39. [Google Scholar] [CrossRef]
- Armañanzas, R.; Bielza, C.; Chaudhuri, K.R.; Martinez-Martin, P.; Larrañaga, P. Unveiling relevant non-motor Parkinson’s disease severity symptoms using a machine learning approach. Artif. Intell. Med. 2013, 58, 195–202. [Google Scholar] [CrossRef]
- Zhang, L.; Qu, Y.; Jin, B.; Jing, L.; Gao, Z.; Liang, Z. An intelligent mobile-enabled system for diagnosing Parkinson disease: Development and validation of a speech impairment detection system. JMIR Med. Inform. 2020, 8, e18689. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, D.R.; Nissar, I.; Masood, S.; Ahmed, M.; Ahmad, F. An LSTM based Deep learning model for voice-based detection of Parkinson’s disease. Int. J. Adv. Sci. Technol. 2020, 29, 337–343. [Google Scholar]
- Reyes, J.F.; Montealegre, J.S.; Castano, Y.J.; Urcuqui, C.; Navarro, A. LSTM and Convolution Networks Exploration for Parkinson’s Diagnosis. In Proceedings of the 2019 IEEE Colombian Conference on Communications and Computing (COLCOM), Barranquilla, Colombia, 5–7 June 2019; pp. 1–4. [Google Scholar]
- Gunduz, H. Deep learning-based Parkinson’s disease classification using vocal feature sets. IEEE Access 2019, 7, 115540–115551. [Google Scholar] [CrossRef]
- Darnall, N.D.; Donovan, C.K.; Aktar, S.; Tseng, H.Y.; Barthelmess, P.; Cohen, P.R.; Lin, D.C. Application of machine learning and numerical analysis to classify tremor in patients affected with essential tremor or Parkinson’s disease. Gerontechnology 2012, 10, 208–219. [Google Scholar] [CrossRef]
- Zham, P.; Arjunan, S.P.; Raghav, S.; Kumar, D.K. Efficacy of guided spiral drawing in the classification of Parkinson’s disease. IEEE J. Biomed. Health Inform. 2017, 22, 1648–1652. [Google Scholar] [CrossRef] [PubMed]
- Marar, S.; Swain, D.; Hiwarkar, V.; Motwani, N.; Awari, A. Predicting the Occurrence of Parkinson’s Disease Using Various Classification Models. In Proceedings of the 2018 International Conference on Advanced Computation and Telecommunication (ICACAT), Bhopal, India, 28–29 December 2018; pp. 1–5. [Google Scholar]
- Ghanad, N.K.; Ahmadi, S. Combination of PSO algorithm and naive Bayesian classification for Parkinson disease diagnosis. Adv. Comput. Sci. Int. J. 2015, 4, 119–125. [Google Scholar]
- Shamir, R.R.; Dolber, T.; Noecker, A.M.; Walter, B.L.; McIntyre, C.C. Machine learning approach to optimizing combined stimulation and medication therapies for Parkinson’s disease. Brain Stimul. 2015, 8, 1025–1032. [Google Scholar] [CrossRef]
- Lahmiri, S.; Dawson, D.A.; Shmuel, A. Performance of machine learning methods in diagnosing Parkinson’s disease based on dysphonia measures. Biomed. Eng. Lett. 2018, 8, 29–39. [Google Scholar] [CrossRef]
- Alemami, Y.; Almazaydeh, L. Detection of Parkinson disease through voice signal features. J. Am. Sci. 2014, 10, 44–47. [Google Scholar]
- Sriram, T.V.; Rao, M.V.; Narayana, G.V.; Kaladhar, D.S.V.G.K. Diagnosis of Parkinson Disease Using Machine Learning and Data Mining Systems from Voice Dataset. In Proceedings of the 3rd International Conference on Frontiers of Intelligent Computing: Theory and Applications (FICTA), Odisha, India, 4–15 November 2014; Springer: Cham, Switzerland, 2015; pp. 151–157. [Google Scholar]
- Halawani, S.M.; Ahmad, A. Ensemble Methods for Prediction of Parkinson Disease. In Proceedings of the International Conference on Intelligent Data Engineering and Automated Learning, Natal, Brazil, 29–31 August 2012; Springer: Berlin/Heidelberg, Germany; pp. 516–521. [Google Scholar]
- Nilashi, M.; bin Ibrahim, O.; Ahmadi, H.; Shahmoradi, L. An analytical method for diseases prediction using machine learning techniques. Comput. Chem. Eng. 2017, 106, 212–223. [Google Scholar] [CrossRef]
- Kim, Y.; Suescun, J.; Schiess, M.C.; Jiang, X. Computational medication regimen for Parkinson’s disease using reinforcement learning. Sci. Rep. 2021, 11, 9313. [Google Scholar] [CrossRef]
- Dinesh, A.; He, J. Using Machine Learning to Diagnose Parkinson’s Disease from Voice Recordings. In Proceedings of the 2017 IEEE MIT Undergraduate Research Technology Conference (URTC), Cambridge, MA, USA, 3–5 November 2017; pp. 1–4. [Google Scholar]
- Betrouni, N.; Delval, A.; Chaton, L.; Defebvre, L.; Duits, A.; Moonen, A.; Leentjens, A.F.G.; Dujardin, K. Electroencephalography-based machine learning for cognitive profiling in Parkinson’s disease: Preliminary results. Mov. Disord. 2019, 34, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Impedovo, D.; Pirlo, G.; Vessio, G. Dynamic handwriting analysis for supporting earlier Parkinson’s disease diagnosis. Information 2018, 9, 247. [Google Scholar] [CrossRef]
- de Souza, R.W.; Silva, D.S.; Passos, L.A.; Roder, M.; Santana, M.C.; Pinheiro, P.R.; de Albuquerque, V.H.C. Computer-assisted Parkinson’s disease diagnosis using fuzzy optimum-path forest and Restricted Boltzmann Machines. Comput. Biol. Med. 2021, 131, 104260. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Medikonda, J.; Namboothiri, P.K.; Natarajan, M. Parkinson’s Disease Stage Classification with Gait Analysis Using Machine Learning Techniques and SMOTE-Based Approach for Class Imbalance Problem. In Proceedings of the 2022 International Conference on Distributed Computing, VLSI, Electrical Circuits and Robotics (DISCOVER), Shivamogga, India, 14–15 October 2022; pp. 277–281. [Google Scholar]
- Oktay, A.B.; Kocer, A. Differential diagnosis of Parkinson and essential tremor with convolutional LSTM networks. Biomed. Signal Process. Control. 2020, 56, 101683. [Google Scholar] [CrossRef]
- Wang, M.; Ge, W.; Apthorp, D.; Suominen, H. Robust feature engineering for Parkinson disease diagnosis: New machine learning techniques. JMIR Biomed. Eng. 2020, 5, e13611. [Google Scholar] [CrossRef]
- Ramdhani, R.A.; Khojandi, A.; Shylo, O.; Kopell, B.H. Optimizing clinical assessments in Parkinson’s disease through the use of wearable sensors and data driven modeling. Front. Comput. Neurosci. 2018, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Belić, M.; Bobić, V.; Badža, M.; Šolaja, N.; Đurić-Jovičić, M.; Kostić, V. Artificial intelligence for assisting diagnostics and assessment of Parkinson’s disease—A review. Clin. Neurol. Neurosurg. 2019, 184, 105442. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Kim, S.L.; Lyoo, C.H.; Lee, M.S. Kinematic analysis in patients with Parkinson’s disease and SWEDD. J. Park. Dis. 2014, 4, 421–430. [Google Scholar] [CrossRef]
- Erro, R.; Schneider, S.A.; Stamelou, M.; Quinn, N.P.; Bhatia, K.P. What do patients with scans without evidence of dopaminergic deficit (SWEDD) have? New evidence and continuing controversies. J. Neurol. Neurosurg. Psychiatry 2016, 87, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Chou, K.L. Diagnosis and Differential Diagnosis of Parkinson Disease; UpToDate: Waltham, MA, USA, 2017. [Google Scholar]
- Kwon, D.Y.; Kwon, Y.; Kim, J.W. Quantitative analysis of finger and forearm movements in patients with off state early stage Parkinson’s disease and scans without evidence of dopaminergic deficit (SWEDD). Park. Relat. Disord. 2018, 57, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, L.; Perez, C.J.; Martin, J.; Campos-Roca, Y. A two-stage variable selection and classification approach for Parkinson’s disease detection by using voice recording replications. Comput. Methods Programs Biomed. 2017, 142, 147–156. [Google Scholar] [CrossRef]
- Che, C.; Xiao, C.; Liang, J.; Jin, B.; Zho, J.; Wang, F. An RNN Architecture with Dynamic Temporal Matching for Personalized Predictions of Parkinson’s Disease. In Proceedings of the 2017 SIAM International Conference on Data Mining, Houston, TX, USA, 27–29 April 2017; Society for Industrial and Applied Mathematics: Philadelphia, PA, USA; pp. 198–206. [Google Scholar]
- Kuresan, H.; Samiappan, D.; Jeevan, A.; Gupta, S. A Performance Study of ML Models and Neural Networks for Detection of Parkinson Disease using Dysarthria Symptoms. Eur. J. Mol. Clin. Med. 2021, 8, 2021. [Google Scholar]
- Afonso, L.C.; Rosa, G.H.; Pereira, C.R.; Weber, S.A.; Hook, C.; Albuquerque VH, C.; Papa, J.P. A recurrence plot-based approach for Parkinson’s disease identification. Future Gener. Comput. Syst. 2019, 94, 282–292. [Google Scholar] [CrossRef]
- Haller, S.; Badoud, S.; Nguyen, D.; Garibotto, V.; Lovblad, K.O.; Burkhard, P.R. Individual detection of patients with Parkinson disease using support vector machine analysis of diffusion tensor imaging data: Initial results. Am. J. Neuroradiol. 2012, 33, 2123–2128. [Google Scholar] [CrossRef] [PubMed]
- Prince, J.; De Vos, M. A Deep Learning Framework for the Remote Detection of Parkinson’s Disease Using Smart-Phone Sensor Data. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 3144–3147. [Google Scholar]
- Hoehn, M.; Yahr, M. Parkinsonism: Onset, progression, and mortality. Neurology 1967, 17, 427. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Du, J.; Li, Z.; Jiang, X.; Miller, T.; Wang, F.; Zheng, W.J.; Roberts, K. Deep representation learning of patient data from Electronic Health Records (EHR): A systematic review. J. Biomed. Inform. 2021, 115, 103671. [Google Scholar] [CrossRef] [PubMed]
- Swarna, S.R.; Kumar, A.; Dixit, P.; Sairam, T.V.M. Parkinson’s Disease Prediction Using Adaptive Quantum Computing. In Proceedings of the 2021 Third International Conference on Intelligent Communication Technologies and Virtual Mobile Networks (ICICV), Tirunelveli, India, 4–6 February 2021; pp. 1396–1401. [Google Scholar]
- Bengio, Y. Practical Recommendations for Gradient-Based Training of Deep Architectures. Neural Networks: Tricks of the Trade; Springer: Berlin/Heidelberg, Germany, 2012; pp. 437–478. [Google Scholar]
- Le, Q.; Ngiam, J.; Coates, A.; Lahiri, A.; Prochnow, B.; Ng, A. On Optimization Methods for Deep Learning. In Proceedings of the 28th International Conference on Machine Learning (ICML-11), Bellevue, DC, USA, 28 June–2 July 2011; pp. 265–272. [Google Scholar]
- Severson, K.A.; Chahine, L.M.; Smolensky, L.A.; Dhuliawala, M.; Frasier, M.; Ng, K.; Ghosh, S.; Hu, J. Discovery of Parkinson’s disease states and disease progression modelling: A longitudinal data study using machine learning. Lancet Digit. Health 2021, 3, e555–e564. [Google Scholar] [CrossRef]
- Deng, L.; Yu, D. Deep learning: Methods and applications. Found. Trends Signal Process. 2014, 7, 197–387. [Google Scholar] [CrossRef]
- Hinton, G. A practical guide to training restricted Boltzmann machines. Momentum 2010, 9, 926. [Google Scholar]
- Little, M.A.; McSharry, P.E.; Hunter, E.J.; Spielman, J.; Ramig, L. Suitability of Dysphonia Measurements for Telemonitoring of Parkinson Disease. IEEE Trans. Biomed. Eng. 2008, 56, 1015–1022. [Google Scholar] [CrossRef]
- Nilashi, M.; Ahmadi, H.; Sheikhtaheri, A.; Naemi, R.; Alotaibi, R.; Alarood, A.A.; Munshi, A.; Rashid, T.; Zhao, J. Remote tracking of Parkinson’s disease progression using ensembles of deep belief network and self-organizing map. Expert Syst. Appl. 2020, 159, 113562. [Google Scholar] [CrossRef]
- Hinton, G.E.; Osindero, S.; Teh, Y.-W. A fast learning algorithm for deep belief nets. Neural Comput. 2006, 18, 1527–1554. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Correa, J.C.; Arias-Vergara, T.; Orozco-Arroyave, J.R.; Eskofier, B.; Klucken, J.; Nöth, E. Multimodal assessment of Parkinson’s disease: A deep learning approach. IEEE J. Biomed. Health Inform. 2018, 23, 1618–1630. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.B.; Lee, W.W.; Kim, A.; Lee, H.J.; Park, H.Y.; Jeon, H.S.; Kim, S.K.; Jeon, B.; Park, K.S. Wrist sensor-based tremor severity quantification in Parkinson’s disease using convolutional neural network. Comput. Biol. Med. 2018, 95, 140–146. [Google Scholar] [CrossRef]
- Goodfellow, I.; Bengio, Y.; Courville, A. Deep Learning; MIT Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Kaur, S.; Aggarwal, H.; Rani, R. Diagnosis of Parkinson’s disease using deep CNN with transfer learning and data augmentation. Multimed. Tools Appl. 2021, 80, 10113–10139. [Google Scholar] [CrossRef]
- Kilicarslan, S.; Celik, M.; Sahin, S. Hybrid models based on genetic algorithm and deep learning algorithms for nutritional anemia disease classification. Biomed. Signal Process. Control 2021, 63, 102231. [Google Scholar] [CrossRef]
- Vásquez-Correa, J.; Orozco-Arroyave, J.R.; Nöth, E. Convolutional Neural Network to Model Articulation Impairments in Patients with Parkinson’s Disease. In Proceedings of the INTERSPEECH, Stockholm, Sweden, 20–24 August 2017; pp. 314–318. [Google Scholar]
- Hosny, M.; Zhu, M.; Gao, W.; Fu, Y. A novel deep LSTM network for artifacts detection in microelectrode recordings. Biocybern. Biomed. Eng. 2020, 40, 1052–1063. [Google Scholar] [CrossRef]
- Folador, J.P.; Andrade, A.O. Deep Learning Framework Used in Parkinson’s Disease Analysis. In Proceedings of the XI Simpósio de Engenharia Biomédica, Minas Gerais, Brazil, 20–24 August 2018. [Google Scholar]
- Passos, L.A.; Papa, J.P. A metaheuristic-driven approach to fine-tune deep Boltzmann machines. Appl. Soft Comput. 2020, 97, 105717. [Google Scholar] [CrossRef]
- Pereira, C.A.; Rodrigues, F.L.; Ruginsk, S.G.; Zanotto, C.Z.; Rodrigues, J.A.; Duarte, D.A.; Costa-Neto, C.M.; Resstel, L.B.; Carneiro, F.S.; Tostes, R.C. Chronic treatment with fluoxetine modulates vascular adrenergic responses by inhibition of pre-and post-synaptic mechanisms. Eur. J. Pharmacol. 2017, 800, 70–80. [Google Scholar] [CrossRef]
- Felsberg, M., Heyden, A., Krüger, N., Eds.; Computer Analysis of Images and Patterns. In Proceedings of the 17th International Conference, CAIP 2017, Ystad, Sweden, 22–24 August 2017; Proceedings, Part II. Springer: Berlin/Heidelberg, Germany, 2017; Volume 10425. [Google Scholar]
- Souriau, R.; Vigneron, V.; Lerbet, J.; Chen, H. Boltzmann Machines for Signals Decomposition. Application to Parkinson’s Disease Control. In Proceedings of the XXVIIème Colloque Francophone de Traitement du Signal et des Images (GRETSI 2019), Lille, France, 26–29 August 2019. [Google Scholar]
- Watts, J.; Khojandi, A.; Vasudevan, R.; Ramdhani, R. Optimizing Individualized Treatment Planning for Parkinson’s Disease Using Deep Reinforcement Learning. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Virtual, 20–24 July 2020; pp. 5406–5409. [Google Scholar]
- Grogan, J.P.; Tsivos, D.; Smith, L.; Knight, B.E.; Bogacz, R.; Whone, A.; Coulthard, E.J. Effects of dopamine on reinforcement learning and consolidation in Parkinson’s disease. Elife 2017, 6, e26801. [Google Scholar] [CrossRef]
- Gokul, S.; Sivachitra, M.; Vijayachitra, S. Parkinson’s Disease Prediction Using Machine Learning Approaches. In Proceedings of the 2013 Fifth International Conference on Advanced Computing (ICoAC), Chennai, India, 18–20 December 2013; pp. 246–252. [Google Scholar]
- Lipton, Z.C.; Kale, D.C.; Elkan, C.; Wetzel, R. Learning to diagnose with LSTM recurrent neural networks. arXiv 2015, arXiv:1511.03677. [Google Scholar]
- Senthilarumugam Veilukandammal, M.; Nilakanta, S.; Ganapathysubramanian, B.; Anantharam, V.; Kanthasamy, A.; Willette, A. Big Data and Parkinson’s Disease: Exploration, Analyses, and Data Challenges. In Proceedings of the 51st Hawaii International Conference on System Sciences, Hilton Waikoloa Village, HI, USA, 3–6 January 2018. [Google Scholar]
- Hallett, M. Parkinson’s disease tremor: Pathophysiology. Park. Relat. Disord. 2012, 18, S85–S86. [Google Scholar] [CrossRef] [PubMed]
- Faghri, F.; Hashemi, S.H.; Leonard, H.; Scholz, S.W.; Campbell, R.H.; Nalls, M.A.; Singleton, A.B. Predicting onset, progression, and clinical subtypes of Parkinson disease using machine learning. bioRxiv 2018, 338913. [Google Scholar] [CrossRef]
- Devarajan, M.; Ravi, L. Intelligent cyber-physical system for an efficient detection of Parkinson disease using fog computing. Multimed. Tools Appl. 2019, 78, 32695–32719. [Google Scholar] [CrossRef]
- Canning, C.G.; Allen, N.E.; Nackaerts, E.; Paul, S.S.; Nieuwboer, A.; Gilat, M. Virtual reality in research and rehabilitation of gait and balance in Parkinson disease. Nat. Rev. Neurol. 2020, 16, 409–425. [Google Scholar] [CrossRef]
- Tăuţan, A.M.; Ionescu, B.; Santarnecchi, E. Artificial intelligence in neurodegenerative diseases: A review of available tools with a focus on machine learning techniques. Artif. Intell. Med. 2021, 117, 102081. [Google Scholar] [CrossRef]
- Pasluosta, C.F.; Gassner, H.; Winkler, J.; Klucken, J.; Eskofier, B.M. An emerging era in the management of Parkinson’s disease: Wearable technologies and the internet of things. IEEE J. Biomed. Health Inform. 2015, 19, 1873–1881. [Google Scholar] [CrossRef]
- Vlamos, P.; Harms, D.R. Can Detection and Prediction Models for Alzheimer’s Disease Be Applied to Prodromal Parkinson’s Disease Using Explainable Artificial Intelligence? A Brief Report on Digital Neuro Signatures; European Commission: Brussels, Belgium, 2022. [Google Scholar]
- Singh, G.; Samavedham, L. Unsupervised learning based feature extraction for differential diagnosis of neurodegenerative diseases: A case study on early-stage diagnosis of Parkinson disease. J. Neurosci. Methods 2015, 256, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Sun, H.; Wang, T.; Tang, M.; Bohnen, N.I.; Müller, M.L.T.M.; Herman, T.; Giladi, N.; Kalinin, A.; Spino, C.; et al. Model-based and model-free machine learning techniques for diagnostic prediction and classification of clinical outcomes in Parkinson’s disease. Sci. Rep. 2018, 8, 7129. [Google Scholar] [CrossRef] [PubMed]
- Perju-Dumbrava, L.; Barsan, M.; Leucuta, D.C.; Popa, L.C.; Pop, C.; Tohanean, N.; Popa, S.L. Artificial intelligence applications and robotic systems in Parkinson’s disease. Exp. Ther. Med. 2022, 23, 153. [Google Scholar] [CrossRef]
- Geerse, D.J.; Roerdink, M.; Marinus, J.; Van Hilten, J.J. Assessing Walking Adaptability in Parkinson’s Disease: The Interactive Walkway. Front. Neurol. 2018, 9, 1096. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, S.J.; Langbehn, D.R.; Leavitt, B.R.; Roos, R.A.; Durr, A.; Craufurd, D.; Kennard, C.; Hicks, S.L.; Fox, N.C.; Scahill, R.I.; et al. Biological and clinical manifestations of Huntington’s disease in the longitudinal TRACK-HD study: Cross-sectional analysis of baseline data. Lancet Neurol. 2009, 8, 791–801. [Google Scholar] [CrossRef]
- Marek, K.; Jennings, D.; Lasch, S.; Siderowf, A.; Tanner, C.; Simuni, T.; Parkinson Progression Marker Initiative. The Parkinson progression marker initiative (PPMI). Prog. Neurobiol. 2011, 95, 629–635. [Google Scholar] [CrossRef]
- Paulsen, J.S.; Langbehn, D.R.; Stout, J.C.; Aylward, E.; Ross, C.A.; Nance, M.; Guttman, M.; Johnson, S.; MacDonald, M.; Beglinger, L.J.; et al. Detection of Huntington’s disease decades before diagnosis: The Predict-HD study. J. Neurol. Neurosurg. Psychiatry 2008, 79, 874–880. [Google Scholar] [CrossRef] [PubMed]
- LaMontagne, P.J.; Benzinger, T.L.; Morris, J.C.; Keefe, S.; Hornbeck, R.; Xiong, C.; Grant, E.; Hassenstab, J.; Moulder, K.; Vlassenko, A.G.; et al. OASIS-3: Longitudinal neuroimaging, clinical, and cognitive dataset for normal aging and Alzheimer disease. MedRxiv 2019. [Google Scholar] [CrossRef]
- Frenkel-Toledo, S.; Giladi, N.; Peretz, C.; Herman, T.; Gruendlinger, L.; Hausdorff, J.M. Effect of gait speed on gait rhythmicity in Parkinson’s disease: Variability of stride time and swing time respond differently. J. Neuroeng. Rehabil. 2005, 2, 1–7. [Google Scholar] [CrossRef]
- Taleb, C.; Khachab, M.; Mokbel, C.; Likforman-Sulem, L. Feature Selection for an Improved Parkinson’s Disease Identification Based on Handwriting. In Proceedings of the 2017 1st International Workshop on Arabic Script Analysis and Recognition (ASAR), Nancy, France, 3–5 April 2017; pp. 52–56. [Google Scholar]
- Jack, C.R., Jr.; Bernstein, M.A.; Fox, N.C.; Thompson, P.; Alexander, G.; Harvey, D.; Weiner, M.W. The Alzheimer’s disease neuroimaging initiative (ADNI): MRI methods. J. Magn. Reson. Imaging 2008, 27, 685–691. [Google Scholar] [CrossRef]
- López-de-Ipiña, K.; Alonso, J.-B.; Travieso, C.M.; Solé-Casals, J.; Egiraun, H.; Faundez-Zanuy, M.; Ezeiza, A.; Barroso, N.; Ecay-Torres, M.; Martinez-Lage, P.; et al. On the selection of non-invasive methods based on speech analysis oriented to automatic Alzheimer disease diagnosis. Sensors 2013, 13, 6730–6745. [Google Scholar] [CrossRef]
- Katsiaris, P.T.; Artemiadis, P.K.; Kyriakopoulos, K.J. Relating Postural Synergies to Low-D Muscular Activations: Towards Bio-Inspired Control of Robotic Hands. In Proceedings of the 2012 IEEE 12th International Conference on Bioinformatics & Bioengineering (BIBE), Larnaca, Cyprus, 11–13 November 2012; pp. 245–250. [Google Scholar]
- Ali, L.; Zhu, C.; Zhang, Z.; Liu, Y. Automated detection of Parkinson’s disease based on multiple types of sustained phonations using linear discriminant analysis and genetically optimized neural network. IEEE J. Transl. Eng. Health Med. 2019, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Julka, A.; Jain, S.; Aggarwal, T.; Khanna, A.; Arunkumar, N.; de Albuquerque, V.H.C. Optimized cuttlefish algorithm for diagnosis of Parkinson’s disease. Cogn. Syst. Res. 2018, 52, 36–48. [Google Scholar] [CrossRef]
- Cai, Z.; Gu, J.; Chen, H.L. A new hybrid intelligent framework for predicting Parkinson’s disease. IEEE Access 2017, 5, 17188–17200. [Google Scholar] [CrossRef]
- Bhosale, M.P.G.; Patil, S. Classification of EMG signals using wavelet transform and hybrid classifier for Parkinson’s disease detection. Int. J. Eng. Res. Technol. 2012, 2, 106–112. [Google Scholar]
- Rana, B.; Juneja, A.; Saxena, M.; Gudwani, S.; Kumaran, S.S.; Agrawal, R.K.; Behari, M. Regions-of-interest based automated diagnosis of Parkinson’s disease using T1-weighted MRI. Expert Syst. Appl. 2015, 42, 4506–4516. [Google Scholar] [CrossRef]
- Babu, G.S.; Suresh, S. Parkinson’s disease prediction using gene expression–A projection-based learning meta-cognitive neural classifier approach. Expert Syst. Appl. 2013, 40, 1519–1529. [Google Scholar] [CrossRef]
- Gazda, M.; Hireš, M.; Drotár, P. Multiple-fine-tuned convolutional neural networks for Parkinson’s disease diagnosis from offline handwriting. IEEE Trans. Syst. Man Cybern. Syst. 2021, 52, 78–89. [Google Scholar] [CrossRef]
- Zeng, W.; Liu, F.; Wang, Q.; Wang, Y.; Ma, L.; Zhang, Y. Parkinson’s disease classification using gait analysis via deterministic learning. Neurosci. Lett. 2016, 633, 268–278. [Google Scholar] [CrossRef]
- Alharthi, A.S.; Casson, A.J.; Ozanyan, K.B. Gait spatiotemporal signal analysis for Parkinson’s disease detection and severity rating. IEEE Sens. J. 2020, 21, 1838–1848. [Google Scholar] [CrossRef]
- Kamran, I.; Naz, S.; Razzak, I.; Imran, M. Handwriting dynamics assessment using deep neural network for early identification of Parkinson’s disease. Future Gener. Comput. Syst. 2021, 117, 234–244. [Google Scholar] [CrossRef]
- Peker, M.; Sen, B.; Delen, D. Computer-aided diagnosis of Parkinson’s disease using complex-valued neural networks and mRMR feature selection algorithm. J. Healthc. Eng. 2015, 6, 281–302. [Google Scholar] [CrossRef] [PubMed]
- Avci, D.; Dogantekin, A. An expert diagnosis system for Parkinson disease based on genetic algorithm-wavelet kernel-extreme learning machine. Park. Dis. 2016, 2016, 5264743. [Google Scholar] [CrossRef] [PubMed]
- Yurdakul, O.C.; Subathra MS, P.; George, S.T. Detection of Parkinson’s disease from gait using neighborhood representation local binary patterns. Biomed. Signal Process. Control 2020, 62, 102070. [Google Scholar] [CrossRef]
- Hirschauer, T.J.; Adeli, H.; Buford, J.A. Computer-aided diagnosis of Parkinson’s disease using enhanced probabilistic neural network. J. Med. Syst. 2015, 39, 179. [Google Scholar] [CrossRef]
- Oh, S.L.; Hagiwara, Y.; Raghavendra, U.; Yuvaraj, R.; Arunkumar, N.; Murugappan, M.; Acharya, U.R. A deep learning approach for Parkinson’s disease diagnosis from EEG signals. Neural Comput. Appl. 2020, 32, 10927–10933. [Google Scholar] [CrossRef]
- Shen, T.; Jiang, J.; Lin, W.; Ge, J.; Wu, P.; Zhou, Y.; Zuo, C.; Wang, J.; Yan, Z.; Shi, K. Use of overlapping group LASSO sparse deep belief network to discriminate Parkinson’s disease and normal control. Front. Neurosci. 2019, 13, 396. [Google Scholar] [CrossRef] [PubMed]
- Parkinson’s Foundation. Stages of Parkinson’s. 2023. Available online: https://www.parkinson.org/understanding-parkinsons/what-is-parkinsons/stages (accessed on 31 January 2023).
- 5 Stages of Parkinson’s Disease. 2023. Available online: https://www.healthline.com/health/parkinsons/stages (accessed on 31 January 2023).
- Prashanth, R.; Roy, S.D.; Mandal, P.K.; Ghosh, S. High-accuracy detection of early Parkinson’s disease through multimodal features and machine learning. Int. J. Med. Inform. 2016, 90, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Sakar, B.E.; Isenkul, M.E.; Sakar, C.O.; Sertbas, A.; Gurgen, F.; Delil, S.; Apaydin, H.; Kursun, O. Collection and analysis of a Parkinson speech dataset with multiple types of sound recordings. IEEE J. Biomed. Health Inform. 2013, 17, 828–834. [Google Scholar] [CrossRef]
- Sathiya, T.; Reenadevi, R.; Sathiyabhama, B. Random Forest Classifier based detection of Parkinson’s disease. Ann. Rom. Soc. Cell Biol. 2021, 25, 2980–2987. [Google Scholar]
- Arasteh, E.; Mahdizadeh, A.; Mirian, M.S.; Lee, S.; McKeown, M.J. Deep transfer learning for Parkinson’s disease monitoring by image-based representation of resting-state EEG using directional connectivity. Algorithms 2022, 15, 5. [Google Scholar] [CrossRef]
- Basnin, N.; Sumi, T.A.; Hossain, M.S.; Andersson, K. Early Detection of Parkinson’s Disease from Micrographic Static Hand Drawings. In Proceedings of the Brain Informatics: 14th International Conference, BI 2021, Virtual Event, 17–19 September 2021; Proceedings 14. Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 433–447. [Google Scholar]
- Taleb, C.; Likforman-Sulem, L.; Mokbel, C.; Khachab, M. Detection of Parkinson’s disease from handwriting using deep learning: A comparative study. Evol. Intell. 2020, 1–12. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, X.; Wang, J.; Yu, C.; Gao, W. Fedhealth: A federated transfer learning framework for wearable healthcare. IEEE Intell. Syst. 2020, 35, 83–93. [Google Scholar] [CrossRef]
- Zheng, X.; Shah, S.B.H.; Ren, X.; Li, F.; Nawaf, L.; Chakraborty, C.; Fayaz, M. Mobile edge computing enabled efficient communication based on federated learning in internet of medical things. Wirel. Commun. Mob. Comput. 2021, 2021, 4410894. [Google Scholar] [CrossRef]
- Dipro, S.H.; Islam, M.; Nahian, M.; Al, A.; Azad, M.S. A Federated Learning Approach for Detecting Parkinson’s Disease through Privacy Preserving by Blockchain. Ph.D. Thesis, Brac University, Dhaka, Bangladesh, 2022. [Google Scholar]





| Acronym | Definition |
|---|---|
| A3C | Asynchronous Advantage Actor-Critic |
| Acc | Accuracy |
| ADAM | A Stochastic Optimization Variant |
| AD | Alzheimer’s Disease |
| AE | Auto Encoder |
| ANN | Artificial Neural Network |
| BFS | Base Feature Selection |
| CNN | Convolutional Neural Network |
| DCNN | Deep Convolution Neural Network |
| DBN | Deep Belief Network |
| DTW | Dynamic Time Warping |
| DNN | Deep Neural Network |
| DRL | Deep Reinforcement Learning |
| EHR | Electronic Health Record |
| ELM | Extreme Learning Machine |
| ELEP | English Language Empowerment Programme |
| FD | Future Directions |
| FNS | Fuzzy Neural System |
| FC-RBF | Fully Complex-Valued Radial Basis Function Networks |
| FoG | Freezing of Gait |
| GA | Genetic Algorithm |
| GRU | Gated Recurrent Unit |
| HC | Health Control |
| HD | Huntington’s Disease |
| ICDs | Impulse Control Disorders |
| IH | Idiopathic Hyposmia |
| LR | Logistic Regression |
| LSTM | Long Short-Term Memory |
| LSVM | Lagrangian Support Vector Machines |
| McFCRBF | Meta-cognitive fully complex-valued RBF network |
| MRI | Magnetic Resonance Imaging |
| ML | Machine Learning |
| MSE | Mean Square Error |
| OC | Open Challenges |
| OPF | Optimum Path Forest |
| PD-MCI | PD–Mild Cognitive Impairment |
| PD | Parkinson Disease |
| PET | Positron Emission Tomography |
| PPMI | Parkinson’s progression markers initiative |
| PCA | Principal Component Analysis |
| RBM | Restricted Boltzmann Machine |
| RL | Reinforcement Learning |
| RNN | Recurrent Neural Network |
| RBF | Radial Basis Function |
| SAE | Stacked Autoencoder |
| SVM | Super Vector Machine |
| SVD | Singular Value Decomposition |
| TCN | Temporal Convolution Networks |
| TFR | Time-Frequency Representation |
| VGFR | Spectrogram Detector and Voice Impairment Classifier (DEEP LEARNING MODEL) |
| VEGF | Vascular Endothelial Growth Factor |
| VGRF | Vertical ground reaction force |
| Reference | Summary | Shortcomings of the Reviews | ML | DL | OC | FD |
|---|---|---|---|---|---|---|
| Our paper | This research provides a thorough analysis of methods based on AI for PD diagnosis. Different computational-based methodologies for PD prediction are also briefly described. | - | H | H | H | H |
| [7] | The use of smartphones and tablets to track the individual at home appears to be the most viable path toward understanding PD, according to this report. It also discusses how e-health research kits are continually being improved. | The majority of works utilize signal or graphics information, necessitating some type of AI-supported decision-making system that needs further improvement. | H | N | H | H |
| [8] | This study’s main finding was how frequently CNN was used to diagnose Parkinson’s. On the other hand, DNN is applied more often to identify neurogenerative illnesses. | High-dimensional CNNs, such as 2D and 3D-CNN, that would have given reliable findings for big and multimodal neuroimages, have not been deployed. | N | H | H | H |
| [9] | The risk factors, pathophysiology, and personality characteristics in patients with PD with ICD are the main topics of this review. According to the results, both extrinsic and intrinsic factors play an important role in how behavioral difficulties arise. | Additional prospective studies with bigger sample sizes are required to identify the risk factors causing behavioral alterations in PD patients with ICD. | N | N | N | H |
| [10] | According to this survey’s findings, 90% of patients with PD have a vocal impairment. Using speech datasets, several studies can be conducted to automate the diagnosis of PD. | It does not include extreme machine learning and genetic algorithms which can be incredibly useful for PD detection | H | N | H | L |
| [11] | Information from 91 studies that investigated the use of neural nets, primarily DL algorithms, for the early identification of Parkinson’s disease was collated for this review. The information covered voltage sensor data, biological voice data, and pictures for both PD and HC subjects. | Many different types of disorders can cause PD, each with its own set of symptoms. Therefore, from a clinical standpoint, they have overlooked classifying disorders. | H | H | M | H |
| [12] | This review’s primary goal was to identify existing ML-based work to diagnose PD using handwriting patterns, voice characteristics, and gait datasets. It also sought to identify the most effective method for diagnosing the disease with a high rate of accuracy. | Existence of a dataset imbalance in the study. | H | H | M | L |
| [13] | They address how ML can help with earlier detection, the interpretation of medical imaging, the discovery and development of new treatments, and much more in this review. | Due to data constraints, the majority of ML pipelines in practice begin with meticulous data curation, which takes time and professional assistance. | H | H | H | M |
| [14] | This study aims to investigate some information and the status of sensor-based methods for the identification of PD. It also addresses ensemble methods for integrating sensor-based data to create ML models for customized risk prediction. | They do not discuss dimensionality reduction algorithms in ensemble techniques, which would allow the application of several classification models on data spaces for better disease classification. | H | M | H | M |
| [15] | They did a thorough analysis of 217 research papers that discussed the use of different ML techniques and DNN designs to diagnose PD. They also carefully looked through and examined the researcher’s architectural plans. | The discussion about the recent technology is very limited. | H | H | H | M |
| S. No. | Stages—Parkinson’s Disease | Symptoms—Parkinson’s Disease | Patient’s Appearance | Impact on the Patient |
|---|---|---|---|---|
| 1 | Stage 1—Only one half of the patient’s body is affected | Mild tremor and rigidity, slight changes in facial expressions, little challenges in posture, balance, and walking. |  | Does not affect the daily activities and life style of the patient. |
| 2 | Stage 2—Full patient’s body becomes affected; however, the patient is still able to balance himself/herself. Affects the midline of the patient’s body; namely, neck and trunk. | Challenges in walking and balancing. Pitiable posture, stiffness, tremors, and trembling may be more noticeable. Noticeable changes in facial expressions and sometimes difficulties in speaking. |  | Daily tasks of the patient become more challenging and time consuming. |
| 3 | Stage 3—Impaired balance, but the patient remains independent | Loss of balance, reduced reflexes, tremor, rigidity, slowness of movement, falls, and dizziness. Freezing and muscle cramps. |  | Daily tasks of the patient become significantly impaired; however, the patient completes basic daily activities at a slow pace. |
| 4 | Stage 4—Walking and standing with external assistance | Substantial decrease in the movement and reaction times of the patient. |  | Patient requires external assistance for daily activities and independent living is not possible. |
| 5 | Stage 5—Debilitating stage | Stiffness in the legs, unable to stand or walk. Freezing upon standing, confusion, loss of smell, hallucinations, delusions, constipation, poor reasoning and memory. Loss of body weight, disturbances during sleep, problems in eyesight |  | Patient is bedridden or confined to a wheel chair. |
| Reference Number | Year | Dataset Used | Availability | Dataset Size | Details about the Dataset | Data Type |
|---|---|---|---|---|---|---|
| [155] | 2009 | Track HD | Open Dataset | 366 individuals | Genetic information and HD detection were connected | physiological, intellectual, quantitative motor, oculomotor, chromosomal, and psychiatric evaluations |
| [156] | 2011 | PPMI | Open Dataset | 64 early patients,196 HC, and 65 REM patients | PD Biological markers | medical record, biological material, and pictures of the brain |
| [157] | 2008 | Predict HD | Proprietary Database | 438 pre-HD patients | Genetic information and HD identification were connected | MRI, smell recognition, verbal learning/memory task, tapping test, genetic information, and cognitive assessment |
| [72] | 2014 | PaHaW | Open Dataset | 37 PD, 38 HC individuals | Archimedean spirals and writing for PD | Altitude, x-y dimensions, tilt, height, and the state of the in-air and on-air surface |
| [158] | 2019 | OASIS | Open Dataset | 1098 individuals | Identification of AD | CT, PET (Positron Emission Tomography) |
| [159] | 2005 | Gait in Parkinson’s disease | Open Dataset | 93 PD and 73 HC patients | Step in PD | recordings of force sensors |
| [160] | 2017 | PDMultiMC | Proprietary Database | 16 PD and 16 HC individuals | Written words, spoken words, and eye tracking in PD | Settings for digital tablets and speech |
| [161] | 2008 | ADNI | Open Dataset | ADNI-GO: 200 early 400 MCI, and 200 AD patients | identification of AD and pre-AD; tracking the condition’s development | Biomarkers, medical, chromosomal, MRI, and PET |
| [162] | 2013 | AZTIAHO | proprietary database | 50 HC and 20 AD patients | Biological markers of AD in voice | Speech Database |
| [163] | 2012 | NTUA | Open Dataset | There were 78 people, 55 of whom had PD, and 23 HC patients | Hand gestures in PD | Testing using MRI and Dopamine Transporter Scan scans |
| Reference | Machine Learning Approaches Used | Dataset | Model is Pre-Trained | Feature Extraction Approach | Limitations | Performance Evaluation Metrics |
|---|---|---|---|---|---|---|
| [164] | Neural Network | Voice Database | Yes | Linear Discriminant Analysis | The testing database did not include any healthy classes, which shows that the data is unbalanced. Information about feature extraction was lacking. | Acc = 0.95 |
| [165] | K-nearest neighbor and Decision Tree | Speech, audio, and hand PD database | Yes | Improved cuttlefish algorithm | Unable to merge the models of HandPD and Voice Datasets. | Acc = 0.92 |
| [72] | SVM with RBF kernel | Handwriting Dataset for PD | No | NCP Method | They chose to only concentrate on PD and the HC group in this investigation. Various illnesses also need to be examined. | Acc = 0.81 specificity = 0.809 sensitivity = 0.84 |
| [166] | Super vector machines | Sound Database | Yes | Bacterial Foraging Optimization | The surrounding environment of a bacterium has a great impact on the search capabilities of a BFO algorithm. Additionally, parallel computing techniques could increase computational efficiency which was not used. | Acc = 0.975 |
| [167] | SVM-MLP | EEG database | No | Constant Fourier Transform | It only offers a solution for data that is linearly segregated. | Acc = 0.1 |
| [168] | PBL-McRBFN + RFE | MIR BRAIN IMAGES | Yes | Voxel-Based Morphometry | A decision model’s performance degrades due to the high dimensionality of MRI data and the scarcity of samples. | Acc = 0.87 |
| [110] | Bayesian approach | Acoustic characteristics are taken from duplicate recordings | Yes | Gibbs sampling method | The dependency nature of the data being mostly ignored, voice recording replications have not typically been addressed for PD discrimination. | Acc = 0.86 |
| [169] | PBL-McRBFN | ParkDB database. | Yes | ICA | - | Acc = 0.95 |
| Reference | Learning Model | Dataset | Selected Features | Main Contributions | Limitations | Performance Evaluation Metrics |
|---|---|---|---|---|---|---|
| [170] | Convolutional Neural network | PaHaW dataset, HandPD dataset | Handwriting images | Presented an effective method for identifying handwriting degradation brought using static photographs of handwriting samples. With the NewHandPD dataset, this accuracy is the greatest ever obtained. | To validate this method, additional datasets and various network designs must be examined. | Acc = 0.94 |
| [171] | Radial Basis Function Networks | 93 PD; 73 HC | Gait features | GRF, a kinetic gait feature, can be used to distinguish between individuals with PD and HCs. As sensing devices and gait data analysis methods progress, the proposed method, which used GRF sensors, can be easily utilized in the clinical prediction of PD. | The test of the suggested approach’s generalizability is constrained by the limited size of the current database. | Acc = 0.96 |
| [172] | Convolutional Neural network | 20 PD; 20HC | Ground reaction force | The LRP research reveals that bodily balance, where increasing degrees of the disease hinder patients’ ability to walk without being at risk of falling, is a significant factor in diagnosing PD. | Lack of a plan for individualized longitudinal tracking to find the intensity of PD progression. | Acc = 0.83 |
| [173] | Convolutional Neural network | NewHandPD dataset | CNN-Based Features | Using an end-to-end deep transfer learning technique, they were able to transfer already acquired knowledge onto the realm of handwriting samples with positive results. | There was an absence of a dataset of difficult tasks with other clinical factors that will help in not just identifying PD early but also figuring out how severe it is and how levodopa and other medications affect it. | Acc = 0.99 |
| [174] | complex-valued artificial neural network | 23 PD; 8 HC (Little, 2007) | (mRMR) attribute selection algorithm | The primary innovation in the research design is the implementation of a hybrid method, mRMR + CVANN, which combined a powerful classifier with an efficient feature selection method. | The program’s data rate must be decreased and its efficiency must be raised to increase usability. | Acc = 0.98 |
| [175] | Extreme learning machine | 23 PD; 8 HC (Little, 2007), | 22 biomedical voice measurements | The recommended GA-WK-ELM PD diagnosis process has several benefits including the ability to generalize, the ability to find the best wavelet kernel with the ideal w, x, and y parameter combinations, and the direct use of feature vectors. | - | Acc = 0.97 |
| [176] | Artificial Neural network | 93 PD; 73 HC (public) | Statistical features | In this study, the suggested strategy restricts the number of alternative symbols per data point so that the frame of encoding is fairly close to the center pixel. | The NR-LBP method approaches the candidate codes as numeric values, taking their maximum, median, average, or other quantitative metrics. | Acc = 0.98 |
| [177] | Enhanced probabilistic neural network (EPNN) back propagation | 189 PD; 415 HC (PPMI) | motor, non-motor, and neuroimaging features | This study shows how combining motor and non-motor information might enhance multiclass classification. | - | Acc = 0.98 |
| [178] | 13-layer 1D-CNN | 20 PD; 20 HC (private) | end-to-end EEG signals | This study is the first to identify PD using EEG data using a thirteen-layer CNN architecture Despite the small number of participants, they were able to achieve high accuracy. | The created model should be tested in the future on a sizable subject population to detect PD in its initial stages. | Acc = 0.88 |
| [179] | Deep Belief Network | 125 PD; 225 HC (private) | laconic representation of PET images | The GLS-DBN model accurately classifies patients into diagnostic groups and provides a measurable biomarker that can spot early PD with minimum image analysis. | The learning rate was calculated by trial and error, and the network structure’s parameter values were refined by numerous tests, increasing the algorithm’s temporal complexity | Acc = 0.90 |
| References | Challenges in Computational ML Models | Challenges in Computational DL Models | Challenges in Integrating PD Diagnosis Data | Challenges in Precision Medicine and Identification of Personalized Treatment | Data Isolation Challenges | Data Management Challenges | Data Sparseness Challenges |
|---|---|---|---|---|---|---|---|
| [50] | × | ✔ | ✔ | ✔ | × | ✔ | ✔ |
| [145] | ✔ | × | ✔ | ✔ | ✔ | ✔ | ✔ |
| [95] | ✔ | × | ✔ | ✔ | ✔ | ✔ | ✔ |
| [146] | × | ✔ | × | × | × | ✔ | ✔ |
| [8] | × | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| [64] | × | ✔ | ✔ | ✔ | × | ✔ | × |
| [4] | × | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| [147] | × | × | ✔ | ✔ | × | ✔ | × |
| [148] | ✔ | ✔ | × | ✔ | × | ✔ | ✔ |
| [7] | ✔ | × | × | ✔ | × | × | × |
| S. No. | Name of the Smart Phone Application | Mobile Operating System | Free/Paid | App Description and Features | Users | Utility |
|---|---|---|---|---|---|---|
| 1 | Neurology Now | Android | Free | Official publication of the American Academy of Neurology | Health care specialists | ideal for PD. |
| 2 | Speech Too | iOS | Free | Voice volume training | Patients | ideal for PD. |
| 3 | Parkinson’s Disease | Android | Free | PD details | Health care specialists | Details about PD |
| 4 | Parkinson’s Toolkit | iOS/Android | Free | Clinical practice recommendations for treating PD | Health care specialists | Details about PD |
| 5 | PD Headline News | iOS | Free | Literature on PD | Health care specialists + patients | Details about PD |
| 6 | MDS UPDRS | iOS | 5.99 | MSD-UPDRS scale | Health care specialists | Evaluation |
| 7 | Fox Insight App | Android | Free | Movement, tremor, and sleep tracking | Patients | Evaluation t+ diagnosis |
| 8 | Prognosis | Windows Phone | Free | tests to evaluate one’s speech, upper limbs, rest, and gait | Health care specialists | Evaluation |
| 9 | ListenMee App | Android | 121 | Using cues to enhance gait | Patients | diagnosis |
| 10 | Parkinson Exercises | iOS/Android/Windows Phone | 4.22 | Videos of exercises for PD patients | Patients | diagnosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dixit, S.; Bohre, K.; Singh, Y.; Himeur, Y.; Mansoor, W.; Atalla, S.; Srinivasan, K. A Comprehensive Review on AI-Enabled Models for Parkinson’s Disease Diagnosis. Electronics 2023, 12, 783. https://doi.org/10.3390/electronics12040783
Dixit S, Bohre K, Singh Y, Himeur Y, Mansoor W, Atalla S, Srinivasan K. A Comprehensive Review on AI-Enabled Models for Parkinson’s Disease Diagnosis. Electronics. 2023; 12(4):783. https://doi.org/10.3390/electronics12040783
Chicago/Turabian StyleDixit, Shriniket, Khitij Bohre, Yashbir Singh, Yassine Himeur, Wathiq Mansoor, Shadi Atalla, and Kathiravan Srinivasan. 2023. "A Comprehensive Review on AI-Enabled Models for Parkinson’s Disease Diagnosis" Electronics 12, no. 4: 783. https://doi.org/10.3390/electronics12040783
APA StyleDixit, S., Bohre, K., Singh, Y., Himeur, Y., Mansoor, W., Atalla, S., & Srinivasan, K. (2023). A Comprehensive Review on AI-Enabled Models for Parkinson’s Disease Diagnosis. Electronics, 12(4), 783. https://doi.org/10.3390/electronics12040783







