Li-Ion Battery Cathode Recycling: An Emerging Response to Growing Metal Demand and Accumulating Battery Waste
Abstract
1. Introduction
1.1. Components of LIBs
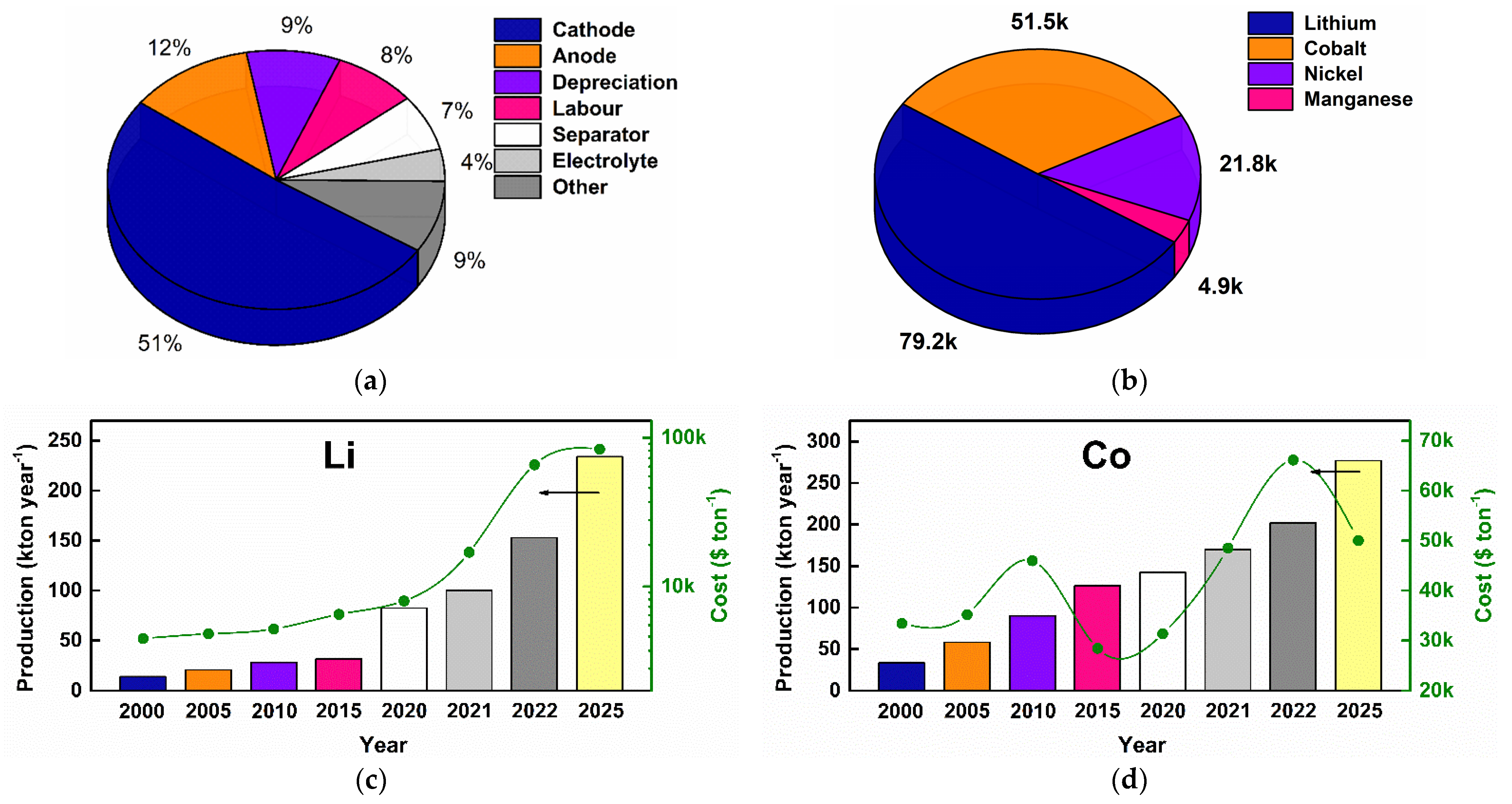
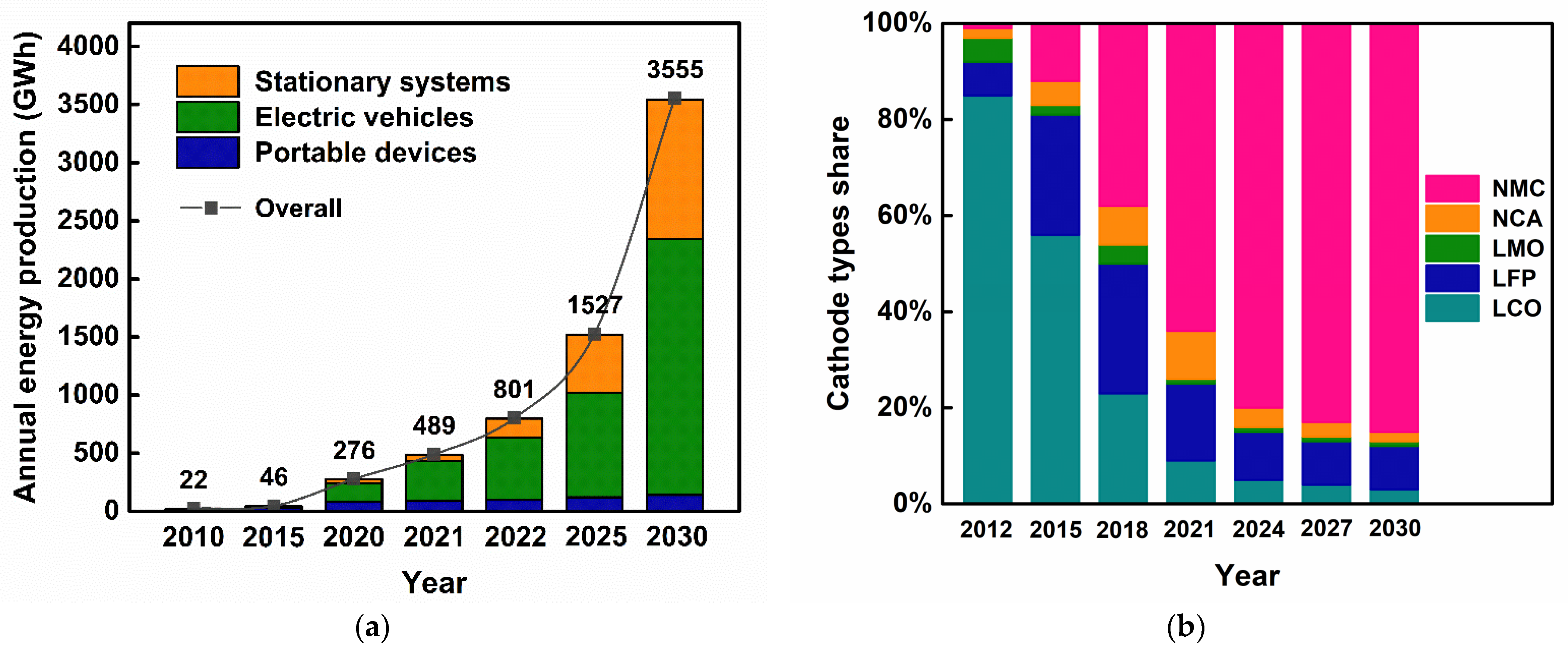
1.2. LIBs: Production, Cost, and Environmental Impact
1.3. Established LIBs Recycling Processes
2. Technology of Recycling Li-ion Battery Cathodes
2.1. Preparation of Spent LIB Cathodes for Recycling
2.1.1. Discharging
2.1.2. Manual Disassembly
2.1.3. Crushing, Comminution, and Separation
2.2. Types of Cathode Recycling Processes
2.2.1. Pyrometallurgy
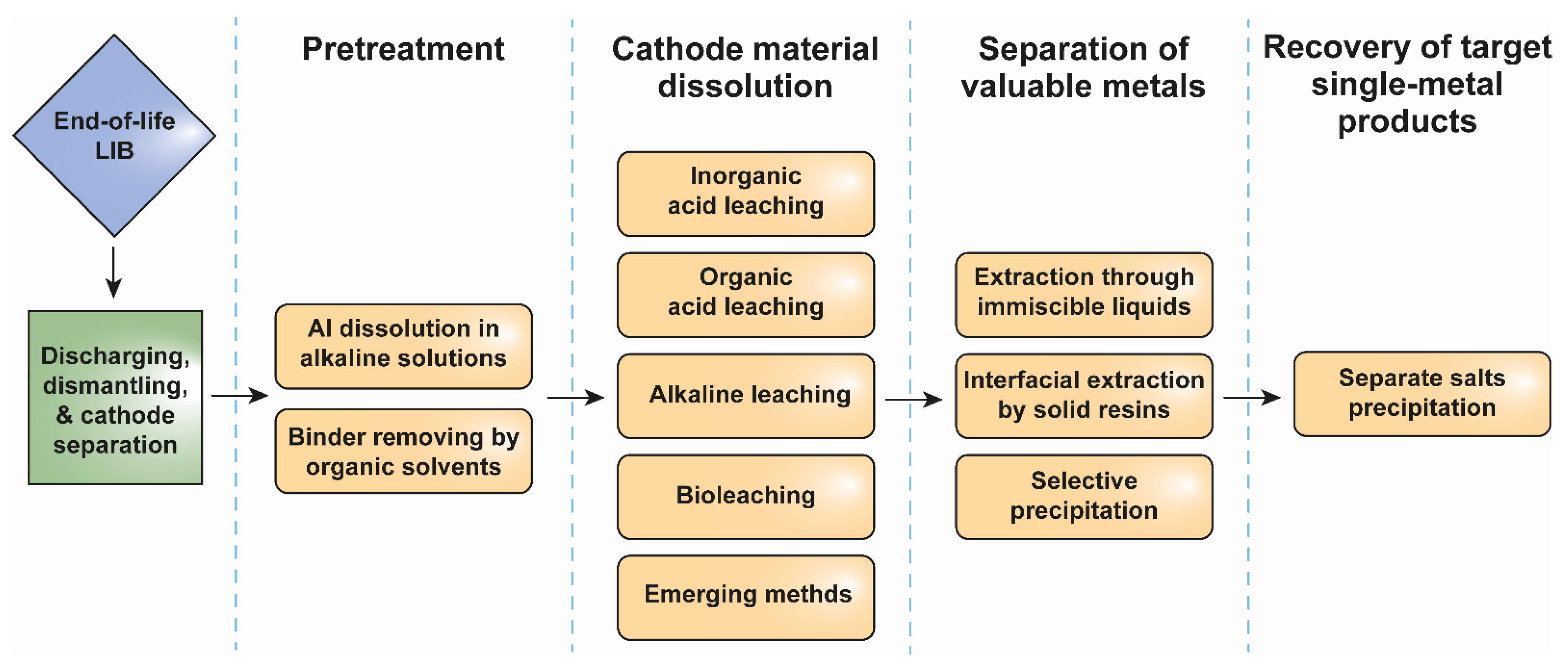
2.2.2. Hydrometallurgy
Active Material Detaching and Binder Removing
Leaching the Active Cathode Material
Separation of Valuable Metals
2.2.3. Emerging Recycling Techniques
Mechanochemical Pretreatment
Electrochemical Method
Ionic Liquids
Deep Eutectic Solvents
Supercritical Fluids
2.3. Methods of Cathode Direct Restoration
2.3.1. Resynthesis
2.3.2. Regeneration
3. Discussion and Perspective
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whittingham, M.S. The Role of Ternary Phases in Cathode Reactions. J. Electrochem. Soc. 1976, 123, 315–320. [Google Scholar] [CrossRef]
- Whittingham, M.S. Electrical Energy Storage and Intercalation Chemistry. Science 1976, 192, 1126–1127. [Google Scholar] [CrossRef] [PubMed]
- Ratnakumar, B.V.; Nagasubramanian, G.; Stefano, S.D.; Bankston, C.P. Kinetics of Intercalation of Lithium into NbSe3 and TiS2 Cathodes. J. Electrochem. Soc. 1992, 139, 1513–1521. [Google Scholar] [CrossRef]
- Kumagai, N.; Tanno, K.; Nakajima, T.; Watanabe, N. Structural Changes of Nb2O5 and V2O5 as Rechargeable Cathodes for Lithium Battery. Electrochim. Acta 1983, 28, 17–22. [Google Scholar] [CrossRef]
- Mizushima, K.; Jones, P.C.; Wiseman, P.J.; Goodenough, J.B. LixCoO2 (0 < x < −1): A new cathode material for batteries of high energy density. Mater. Res. Bull. 1980, 15, 783–789. [Google Scholar] [CrossRef]
- Banov, B.; Bourilkov, J.; Mladenov, M. Cobalt Stabilized Layered Lithium-Nickel Oxides, Cathodes in Lithium Rechargeable Cells. J. Power Sources 1995, 54, 268–270. [Google Scholar] [CrossRef]
- Ammundsen, B.; Paulsen, J. Novel Lithium-Ion Cathode Materials Based on Layered Manganese Oxides. Adv. Mater. 2001, 13, 943–956. [Google Scholar] [CrossRef]
- Kraytsberg, A.; Ein-Eli, Y.; Kraytsberg, A.; Ein-Eli, Y. Higher, Stronger, Better... A Review of 5 Volt Cathode Materials for Advanced Lithium-Ion Batteries. Adv. Energy Mater. 2012, 2, 922–939. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Dallas, J.; Casanova, S.; Pelech, T.; Bournival, G.; Saydam, S.; Canbulat, I. Towards a Low-Carbon Society: A Review of Lithium Resource Availability, Challenges and Innovations in Mining, Extraction and Recycling, and Future Perspectives. Miner. Eng. 2021, 163, 106743. [Google Scholar] [CrossRef]
- Verma, S.; Dwivedi, G.; Verma, P. Life Cycle Assessment of Electric Vehicles in Comparison to Combustion Engine Vehicles: A Review. Mater. Today Proc. 2021, 49, 217–222. [Google Scholar] [CrossRef]
- Burchart-Korol, D.; Jursova, S.; Folęga, P.; Korol, J.; Pustejovska, P.; Blaut, A. Environmental Life Cycle Assessment of Electric Vehicles in Poland and the Czech Republic. J. Clean. Prod. 2018, 202, 476–487. [Google Scholar] [CrossRef]
- Luong, J.H.T.; Tran, C.; Ton-That, D. A Paradox over Electric Vehicles, Mining of Lithium for Car Batteries. Energies 2022, 15, 7997. [Google Scholar] [CrossRef]
- Pražanová, A.; Knap, V.; Stroe, D.I. Literature Review, Recycling of Lithium-Ion Batteries from Electric Vehicles, Part II: Environmental and Economic Perspective. Energies 2022, 15, 7356. [Google Scholar] [CrossRef]
- Bejigo, K.S.; Natarajan, S.; Bhunia, K.; Elumalai, V.; Kim, S.J. Recycling of Value-Added Products from Spent Lithium-Ion Batteries for Oxygen Reduction and Methanol Oxidation Reactions. J. Clean. Prod. 2023, 384, 135520. [Google Scholar] [CrossRef]
- Sahu, M.; Hajra, S.; Jadhav, S.; Panigrahi, B.K.; Dubal, D.; Kim, H.J. Bio-Waste Composites for Cost-Effective Self-Powered Breathing Patterns Monitoring: An Insight into Energy Harvesting and Storage Properties. Sustain. Mater. Technol. 2022, 32, e00396. [Google Scholar] [CrossRef]
- Goyal, M.R.; Eilu, E. Digital Media and Wireless Communications in Developing Nations; Apple Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Hu, Y.; Yu, Y.; Huang, K.; Wang, L. Development Tendency and Future Response about the Recycling Methods of Spent Lithium-Ion Batteries Based on Bibliometrics Analysis. J. Energy Storage 2020, 27, 101111. [Google Scholar] [CrossRef]
- Lithium-Ion Battery Market Size & Share|Industry Report, 2021-2030|MarketsandMarketsTM. Available online: https://www.marketsandmarkets.com/Market-Reports/lithium-ion-battery-market-49714593.html (accessed on 30 June 2022).
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling Lithium-Ion Batteries from Electric Vehicles. Nature 2019, 575, 75. [Google Scholar] [CrossRef]
- Fan, E.; Li, L.; Wang, Z.; Lin, J.; Huang, Y.; Yao, Y.; Chen, R.; Wu, F. Sustainable Recycling Technology for Li-Ion Batteries and Beyond: Challenges and Future Prospects. Chem. Rev. 2020, 120, 7020. [Google Scholar] [CrossRef] [PubMed]
- Eurostat Statistics. Eurostat. Available online: https://ec.europa.eu/eurostat/databrowser/view/env_waspb/default/table?lang=en (accessed on 30 June 2022).
- Yanamandra, K.; Pinisetty, D.; Daoud, A.; Gupta, N. Recycling of Li-Ion and Lead Acid Batteries: A Review. J. Indian Inst. Sci. 2022, 102, 281–295. [Google Scholar] [CrossRef]
- Garole, D.J.; Hossain, R.; Garole, V.J.; Sahajwalla, V.; Nerkar, J.; Dubal, D.P. Recycle, Recover and Repurpose Strategy of Spent Li-Ion Batteries and Catalysts: Current Status and Future Opportunities. ChemSusChem 2020, 13, 3079–3100. [Google Scholar] [CrossRef]
- Or, T.; Gourley, S.W.D.; Kaliyappan, K.; Yu, A.; Chen, Z. Recycling of Mixed Cathode Lithium-Ion Batteries for Electric Vehicles: Current Status and Future Outlook. Carbon Energy 2020, 2, 6–43. [Google Scholar] [CrossRef]
- Perez, I.D.; Botelho, A.B., Jr.; Aliprandini, P.; Espinosa, D.C.R. Copper Recovery from Nickel Laterite with High-Iron Content: A Continuous Process from Mining Waste. Can. J. Chem. Eng. 2020, 98, 957–968. [Google Scholar] [CrossRef]
- Piątek, J.; Afyon, S.; Budnyak, T.M.; Budnyk, S.; Sipponen, M.H.; Slabon, A. Sustainable Li-Ion Batteries: Chemistry and Recycling. Adv. Energy Mater. 2021, 11, 3456. [Google Scholar] [CrossRef]
- Wang, Y.; An, N.; Wen, L.; Wang, L.; Jiang, X.; Hou, F.; Yin, Y.; Liang, J. Recent Progress on the Recycling Technology of Li-Ion Batteries. J. Energy Chem. 2020, 55, 391–419. [Google Scholar] [CrossRef]
- Kim, S.; Bang, J.; Yoo, J.; Shin, Y.; Bae, J.; Jeong, J.; Kim, K.; Dong, P.; Kwon, K. A Comprehensive Review on the Pretreatment Process in Lithium-Ion Battery Recycling. J. Clean. Prod. 2021, 294, 126329. [Google Scholar] [CrossRef]
- Baum, Z.J.; Bird, R.E.; Yu, X.; Ma, J. Lithium-Ion Battery Recycling─Overview of Techniques and Trends. ACS Energy Lett. 2022, 7, 712–719. [Google Scholar] [CrossRef]
- Miranda, D.; Gören, A.; Costa, C.M.; Silva, M.M.; Almeida, A.M.; Lanceros-Méndez, S. Theoretical Simulation of the Optimal Relation between Active Material, Binder and Conductive Additive for Lithium-Ion Battery Cathodes. Energy 2019, 172, 68–78. [Google Scholar] [CrossRef]
- Lee, Y.K. The Effect of Active Material, Conductive Additives, and Binder in a Cathode Composite Electrode on Battery Performance. Energies 2019, 12, 658. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, A.; Lee, J.Y. Cycle Life Improvement of LiMn2O4 Cathode in Rechargeable Lithium Batteries. J. Power Sources 1998, 74, 228–233. [Google Scholar] [CrossRef]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A Review of Recent Developments in Membrane Separators for Rechargeable Lithium-Ion Batteries; Royal Society of Chemistry: London, UK, 2014; Volume 7. [Google Scholar]
- Choudhury, S.; Tu, Z.; Nijamudheen, A.; Zachman, M.J.; Stalin, S.; Deng, Y.; Zhao, Q.; Vu, D.; Kourkoutis, L.F.; Mendoza-Cortes, J.L.; et al. Stabilizing Polymer Electrolytes in High-Voltage Lithium Batteries. Nat. Commun. 2019, 10, 3091. [Google Scholar] [CrossRef]
- Borah, R.; Hughson, F.R.; Johnston, J.; Nann, T. On Battery Materials and Methods; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; Volume 6. [Google Scholar]
- Li, J.; Kiggans, J.; Wood, D.L.; Rulison, C.; Daniel, C.; Kiggans, J.; Daniel, C.; Wood, D.L. Superior Performance of LiFePO4 Aqueous Dispersions via Corona Treatment and Surface Energy Optimization. J. Electrochem. Soc. 2012, 159, A1152–A1157. [Google Scholar] [CrossRef]
- Barbosa, J.C.; Dias, J.P.; Lanceros-Méndez, S.; Costa, C.M. Recent Advances in Poly(Vinylidene Fluoride) and Its Copolymers for Lithium-Ion Battery Separators. Membranes 2018, 8, 45. [Google Scholar] [CrossRef]
- Zeng, F.; Xu, R.; Ye, L.; Xiong, B.; Kang, J.; Xiang, M.; Li, L.; Sheng, X.; Hao, Z. Effects of Heat Setting on the Morphology and Performance of Polypropylene Separator for Lithium Ion Batteries. Ind. Eng. Chem. Res. 2019, 58, 2217–2224. [Google Scholar] [CrossRef]
- Costa, C.M.; Gomez Ribelles, J.L.; Lanceros-Méndez, S.; Appetecchi, G.B.; Scrosati, B. Poly(Vinylidene Fluoride)-Based, Co-Polymer Separator Electrolyte Membranes for Lithium-Ion Battery Systems. J. Power Sources 2014, 245, 779–786. [Google Scholar] [CrossRef]
- Goncąlves, R.; Lizundia, E.; Silva, M.M.; Costa, C.M.; Lanceros-Méndez, S. Mesoporous Cellulose Nanocrystal Membranes as Battery Separators for Environmentally Safer Lithium-Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 3749–3761. [Google Scholar] [CrossRef]
- Dai, J.; Yang, C.; Wang, C.; Pastel, G.; Hu, L. Interface Engineering for Garnet-Based Solid-State Lithium-Metal Batteries: Materials, Structures, and Characterization. Adv. Mater. 2018, 30, 1802068. [Google Scholar] [CrossRef]
- Chang, P.; Mei, H.; Zhou, S.; Dassios, K.G.; Cheng, L. 3D Printed Electrochemical Energy Storage Devices; Royal Society of Chemistry: London, UK, 2019; Volume 7, pp. 4230–4258. [Google Scholar]
- Chagnes, A. Chapter 5-Lithium Battery Technologies: Electrolytes BT-Lithium Process Chemistry. In Lithium Process Chemistry; IntechOpen: London, UK, 2015; pp. 167–189. ISBN 978-0-12-801417-2. [Google Scholar]
- Zeng, X.; Li, J.; Singh, N. Recycling of Spent Lithium-Ion Battery: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1129. [Google Scholar] [CrossRef]
- USGS National Minerals Information Center. U.S. Geological Survey 2022 Final List of Critical Minerals; U.S. Geological Survey; USGS National Minerals Information Center: Reston, VA, USA, 2022. [Google Scholar]
- Kavanagh, L.; Keohane, J.; Cabellos, G.G.; Lloyd, A.; Cleary, J. Global Lithium Sources-Industrial Use and Future in the Electric Vehicle Industry: A Review. Resources 2018, 7, 57. [Google Scholar] [CrossRef]
- Pehlken, A.; Albach, S.; Vogt, T. Is There a Resource Constraint Related to Lithium Ion Batteries in Cars? Int. J. Life Cycle Assess. 2017, 22, 40–53. [Google Scholar] [CrossRef]
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and Cost of Materials for Lithium-Based Rechargeable Automotive Batteries; Nature Publishing Group: Berlin, Germany, 2018; Volume 3, pp. 267–278. [Google Scholar]
- Slack, J.F.; Kimball, B.E.; Shedd, K.B. Cobalt; No. 1802-F; US Geological Survey: Reston, VA, USA, 2017. [Google Scholar]
- EC Critical Raw Materials. Critical Raw Materials; European Commission: Luxembourg, 2021. [Google Scholar]
- Sonter, L.J.; Ali, S.H.; Watson, J.E.M. Mining and Biodiversity: Key Issues and Research Needs in Conservation Science. Proc. R. Soc. B Biol. Sci. 2018, 285, 20181926. [Google Scholar] [CrossRef] [PubMed]
- King, S.; Boxall, N.J. Lithium Battery Recycling in Australia: Defining the Status and Identifying Opportunities for the Development of a New Industry. J. Clean. Prod. 2019, 215, 1279–1287. [Google Scholar] [CrossRef]
- Khatibi, H.; Hassan, E.; Frisone, D.; Amiriyan, M.; Farahati, R.; Farhad, S. Recycling and Reusing Copper and Aluminum Current-Collectors from Spent Lithium-Ion Batteries. Energies 2022, 15, 9069. [Google Scholar] [CrossRef]
- IEA. Global EV Outlook 2020: Technology Report; International Energy Agency: Paris, France, 2020. [Google Scholar]
- Global Light Duty EV Sales to Rise to 26.8 Mil by 2030: Platts Analytics|S&P Global Commodity Insights. Available online: https://www.spglobal.com/commodityinsights/en/market-insights/latest-news/energy-transition/021622-global-light-duty-ev-sales-to-rise-to-268-mil-by-2030-platts-analytics#article0 (accessed on 30 June 2022).
- Zubi, G.; Dufo-López, R.; Carvalho, M.; Pasaoglu, G. The Lithium-Ion Battery: State of the Art and Future Perspectives. Renew. Sustain. Energy Rev. 2018, 89, 292–308. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Fan, E.; Xue, Q.; Bian, Y.; Wu, F.; Chen, R. Toward Sustainable and Systematic Recycling of Spent Rechargeable Batteries. Chem. Soc. Rev. 2018, 47, 7239–7302. [Google Scholar] [CrossRef]
- Al-Thyabat, S.; Nakamura, T.; Shibata, E.; Iizuka, A. Adaptation of Minerals Processing Operations for Lithium-Ion (LiBs) and Nickel Metal Hydride (NiMH) Batteries Recycling: Critical Review. Miner. Eng. 2013, 45, 4. [Google Scholar] [CrossRef]
- Velázquez-Martínez, O.; Valio, J.; Santasalo-Aarnio, A.; Reuter, M.; Serna-Guerrero, R. A Critical Review of Lithium-Ion Battery Recycling Processes from a Circular Economy Perspective. Batteries 2019, 5, 68. [Google Scholar] [CrossRef]
- Clark, J.H.; Farmer, T.J.; Herrero-Davila, L.; Sherwood, J. Circular Economy Design Considerations for Research and Process Development in the Chemical Sciences. Green Chem. 2016, 18, 3914–3934. [Google Scholar] [CrossRef]
- Heelan, J.; Gratz, E.; Zheng, Z.; Wang, Q.; Chen, M.; Apelian, D.; Wang, Y. Current and Prospective Li-Ion Battery Recycling and Recovery Processes. JOM 2016, 68, 2632–2638. [Google Scholar] [CrossRef]
- Wills, B.A.; Napier-Munn, T.J. Mineral Processing Technology; Elsevier Science & Technology Books: Amsterdam, The Netherlands, 2006; pp. 267–352. [Google Scholar]
- Lv, W.; Wang, Z.; Cao, H.; Sun, Y.; Zhang, Y.; Sun, Z. A Critical Review and Analysis on the Recycling of Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 1504–1521. [Google Scholar] [CrossRef]
- Gaines, L.L.; Dunn, J.B. Lithium-Ion Battery Environmental Impacts. In Lithium-Ion Batteries: Advances and Applications; Elsevier B.V. Center for Transportation Research, Argonne National Laboratory: Argonne, IL, USA, 2014; ISBN 9780444595133. [Google Scholar]
- McLaughlin, W.; Adams, T.S. Li Reclamation Process. US5888463A, 30 March 1999. [Google Scholar]
- Cardarelli, F.; Dube, J. Method for Rrecycling Spent Llithium Metal Polymer Rechargeable Batteries and Related Materials. US7192564B2, 20 March 2007. [Google Scholar]
- Tedjar, F.; Foudraz, J.C. Method for the Mixed Recycling of Lithium-Based Anode Batteries and Cells. US7820317B2, 23 August 2007. [Google Scholar]
- Georgi-Maschler, T.; Friedrich, B.; Weyhe, R.; Heegn, H.; Rutz, M. Development of a Recycling Process for Li-Ion Batteries. J. Power Sources 2012, 207, 173–182. [Google Scholar] [CrossRef]
- Vezzini, A. Manufacturers, Materials and Recycling Technologies. In Lithium-Ion Batteries: Advances and Applications; Elsevier: Biel, Switzerland, 2014; pp. 529–551. ISBN 9780444595133. [Google Scholar]
- Cheret, D.; Santen, S. Battery Recycling. U.S. Patent US7169206B2, 30 January 2007. [Google Scholar]
- Sayilgan, E.; Kukrer, T.; Civelekoglu, G.; Ferella, F.; Akcil, A.; Veglio, F.; Kitis, M. A Review of Technologies for the Recovery of Metals from Spent Alkaline and Zinc-Carbon Batteries. Hydrometallurgy 2009, 97, 158–166. [Google Scholar] [CrossRef]
- Bernardes, A.M.; Espinosa, D.C.R.R.; Tenório, J.A.S.S. Recycling of Batteries: A Review of Current Processes and Technologies. J. Power Sources 2004, 130, 291–298. [Google Scholar] [CrossRef]
- Rombach, E.; Friedrich, B. Recycling of Rare Metals. In Handbook of Recycling: State-of-the-Art for Practitioners, Analysts, and Scientists; Elsevier Inc.: Aachen, Germany, 2014; pp. 125–150. ISBN 9780123965066/9780123964595. [Google Scholar]
- Worrel, E.; Reuter, M.A. Handbook of Recycling; Newnes: Oxford, UK, 2014; pp. 85–94. [Google Scholar]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Extraction of Lithium from Primary and Secondary Sources by Pre-Treatment, Leaching and Separation: A Comprehensive Review. Hydrometallurgy 2014, 150, 192. [Google Scholar] [CrossRef]
- Zhang, N.; Deng, T.; Zhang, S.; Wang, C.; Chen, L.; Wang, C.; Fan, X.; Zhang, N.; Zhang, S.; Chen, L.; et al. Critical Review on Low-Temperature Li-Ion/Metal Batteries. Adv. Mater. 2022, 34, 2107899. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Yuan, N.; Ding, J.; Qin, S.; Razal, J.M.; Wang, X.; Ge, S.; Gogotsi, Y. Extending the low temperature operational limit of Li-ion battery to −80 C. Energy Storage Mater. 2019, 23, 383–389. [Google Scholar] [CrossRef]
- Collins, G.A.; Geaney, H.; Ryan, K.M. Alternative anodes for low temperature lithium-ion batteries. J. Mater. Chem. A 2021, 9, 14172–14213. [Google Scholar] [CrossRef]
- Wandt, J.; Freiberg, A.; Thomas, R.; Gorlin, Y.; Siebel, A.; Jung, R.; Gasteiger, H.A.; Tromp, M. Transition Metal Dissolution and Deposition in Li-Ion Batteries Investigated by Operando X-Ray Absorption Spectroscopy. J. Mater. Chem. A Mater. 2016, 4, 18300–18305. [Google Scholar] [CrossRef]
- Wu, X.L.; Guo, Y.G.; Su, J.; Xiong, J.W.; Zhang, Y.L.; Wan, L.J. Carbon-Nanotube-Decorated Nano-LiFePO4@C Cathode Material with Superior High-Rate and Low-Temperature Performances for Lithium-Ion Batteries. Adv. Energy Mater. 2013, 3, 1155–1160. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Y.; Zhao, C.; Yang, X.; Jiang, Q. Effects of Carbon Coating and Metal Ions Doping on Low Temperature Electrochemical Properties of LiFePO4 Cathode Material. Electrochim. Acta 2012, 83, 341–347. [Google Scholar] [CrossRef]
- Xu, C.; Li, J.; Feng, X.; Zhao, J.; Tang, C.; Ji, B.; Hu, J.; Cao, C.; Zhu, Y.; Butt, F.K. The Improved Performance of Spinel LiMn2O4 Cathode with Micro-Nanostructured Sphere-Interconnected-Tube Morphology and Surface Orientation at Extreme Conditions for Lithium-Ion Batteries. Electrochim. Acta 2020, 358, 136901. [Google Scholar] [CrossRef]
- Zhang, D.; Tan, C.; Ou, T.; Zhang, S.; Li, L.; Ji, X. Constructing Advanced Electrode Materials for Low-Temperature Lithium-Ion Batteries: A Review. Energy Rep. 2022, 8, 4525–4534. [Google Scholar] [CrossRef]
- Wen, J.; Hou, X.; Yao, M.; Chen, J.; Tian, M.; Feng, E.; Huang, G.; Xu, S. Tuning of Vanadium Valence for Value-Added Recycling and Utilization of Vanadium from Spent Selective Catalytic Reduction Catalysts. J. Clean. Prod. 2023, 390, 136151. [Google Scholar] [CrossRef]
- Marshall, J.; Gastol, D.; Sommerville, R.; Middleton, B.; Goodship, V.; Kendrick, E. Disassembly of Li Ion Cells—Characterization and Safety Considerations of a Recycling Scheme. Metals 2020, 10, 773. [Google Scholar] [CrossRef]
- Xiao, J.; Guo, J.; Zhan, L.; Xu, Z. A Cleaner Approach to the Discharge Process of Spent Lithium Ion Batteries in Different Solutions. J. Clean. Prod. 2020, 255, 120064. [Google Scholar] [CrossRef]
- Wang, J.; Chen, M.; Chen, H.; Luo, T.; Xu, Z. Leaching Study of Spent Li-Ion Batteries. Procedia Environ. Sci. 2012, 16, 443–450. [Google Scholar] [CrossRef]
- Ojanen, S.; Lundström, M.; Santasalo-Aarnio, A.; Serna-Guerrero, R. Challenging the Concept of Electrochemical Discharge Using Salt Solutions for Lithium-Ion Batteries Recycling. Waste Manag. 2018, 76, 242–249. [Google Scholar] [CrossRef]
- Yao, L.P.; Zeng, Q.; Qi, T.; Li, J. An Environmentally Friendly Discharge Technology to Pretreat Spent Lithium-Ion Batteries. J. Clean. Prod. 2020, 245, 118820. [Google Scholar] [CrossRef]
- Shaw-Stewart, J.; Alvarez-Reguera, A.; Greszta, A.; Marco, J.; Masood, M.; Sommerville, R.; Kendrick, E. Aqueous Solution Discharge of Cylindrical Lithium-Ion Cells. Sustain. Mater. Technol. 2019, 22, e00110. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Xu, Z. Generation and Detection of Metal Ions and Volatile Organic Compounds (VOCs) Emissions from the Pretreatment Processes for Recycling Spent Lithium-Ion Batteries. Waste Manag. 2016, 52, 221–227. [Google Scholar] [CrossRef]
- Cui, J.; Forssberg, E. Mechanical Recycling of Waste Electric and Electronic Equipment: A Review. J. Hazard. Mater. 2003, 99, 243–263. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zheng, P.; Yang, T.; Sturges, R.; Ellis, M.W.; Li, Z. Disassembly Automation for Recycling End-of-Life Lithium-Ion Pouch Cells. JOM 2019, 71, 4457–4464. [Google Scholar] [CrossRef]
- Herrmann, C.; Raatz, A.; Mennenga, M.; Schmitt, J.; Andrew, S. Assessment of Automation Potentials for the Disassembly of Automotive Lithium Ion Battery Systems. In Leveraging Technology for a Sustainable World—Proceedings of the 19th CIRP Conference on Life Cycle Engineering, Berkeley, CA, USA, 23–25 May 2012; Dornfeld, D.A., Linke, B.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 149–154. [Google Scholar]
- Granata, G.; Pagnanelli, F.; Moscardini, E.; Takacova, Z.; Havlik, T.; Toro, L. Simultaneous Recycling of Nickel Metal Hydride, Lithium Ion and Primary Lithium Batteries: Accomplishment of European Guidelines by Optimizing Mechanical Pre-Treatment and Solvent Extraction Operations. J. Power Sources 2012, 212, 205–211. [Google Scholar] [CrossRef]
- Zhang, T.; He, Y.; Ge, L.; Fu, R.; Zhang, X.; Huang, Y. Characteristics of Wet and Dry Crushing Methods in the Recycling Process of Spent Lithium-Ion Batteries. J. Power Sources 2013, 240, 766–771. [Google Scholar] [CrossRef]
- Guan, J.; Li, Y.; Guo, Y.; Su, R.; Gao, G.; Song, H.; Yuan, H.; Liang, B.; Guo, Z. Mechanochemical Process Enhanced Cobalt and Lithium Recycling from Wasted Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2017, 5, 1026–1032. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Bai, X.; Wang, S.; Yang, D.; Fu, Y.; He, Y. Separation of the Cathode Materials from the Al Foil in Spent Lithium-Ion Batteries by Cryogenic Grinding. Waste Manag. 2019, 91, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Pinegar, H.; Smith, Y.R. Recycling of End-of-Life Lithium Ion Batteries, Part I: Commercial Processes. J. Sustain. Metall. 2019, 5, 402–416. [Google Scholar] [CrossRef]
- Zhang, T.; He, Y.; Wang, F.; Ge, L.; Zhu, X.; Li, H. Chemical and Process Mineralogical Characterizations of Spent Lithium-Ion Batteries: An Approach by Multi-Analytical Techniques. Waste Manag. 2014, 34, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Gratz, E.; Sa, Q.; Apelian, D.; Wang, Y. A Closed Loop Process for Recycling Spent Lithium Ion Batteries. J. Power Sources 2014, 262, 255–262. [Google Scholar] [CrossRef]
- Wang, X.; Gaustad, G.; Babbitt, C.W. Targeting High Value Metals in Lithium-Ion Battery Recycling via Shredding and Size-Based Separation. Waste Manag. 2016, 51, 204–213. [Google Scholar] [CrossRef]
- He, Y.; Zhang, T.; Wang, F.; Zhang, G.; Zhang, W.; Wang, J. Recovery of LiCoO2 and Graphite from Spent Lithium-Ion Batteries by Fenton Reagent-Assisted Flotation. J. Clean. Prod. 2017, 143, 319–325. [Google Scholar] [CrossRef]
- Shin, H.; Zhan, R.; Dhindsa, K.S.; Pan, L.; Han, T. Electrochemical Performance of Recycled Cathode Active Materials Using Froth Flotation-Based Separation Process. J. Electrochem. Soc. 2020, 167, 6280. [Google Scholar] [CrossRef]
- Zhao, C.; Zhong, X. Reverse Flotation Process for the Recovery of Pyrolytic LiFePO4. Colloids Surf. A Physicochem. Eng. Asp. 2020, 596, 124741. [Google Scholar] [CrossRef]
- Yu, J.; He, Y.; Li, H.; Xie, W.; Zhang, T. Effect of the Secondary Product of Semi-Solid Phase Fenton on the Flotability of Electrode Material from Spent Lithium-Ion Battery. Powder Technol. 2017, 315, 139–146. [Google Scholar] [CrossRef]
- Zhang, G.; He, Y.; Feng, Y.; Wang, H.; Zhu, X. Pyrolysis-Ultrasonic-Assisted Flotation Technology for Recovering Graphite and LiCoO2 from Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 10896–10904. [Google Scholar] [CrossRef]
- Silveira, A.V.M.; Santana, M.P.; Tanabe, E.H.; Bertuol, D.A. Recovery of Valuable Materials from Spent Lithium Ion Batteries Using Electrostatic Separation. Int. J. Miner. Process. 2017, 169, 91–98. [Google Scholar] [CrossRef]
- Bi, H.; Zhu, H.; Zu, L.; Bai, Y.; Gao, S.; Gao, Y. A New Model of Trajectory in Eddy Current Separation for Recovering Spent Lithium Iron Phosphate Batteries. Waste Manag. 2019, 100, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Marinos, D.; Mishra, B. An Approach to Processing of Lithium-Ion Batteries for the Zero-Waste Recovery of Materials. J. Sustain. Metall. 2015, 1, 263–274. [Google Scholar] [CrossRef]
- Zhang, G.; He, Y.; Wang, H.; Feng, Y.; Xie, W.; Zhu, X. Removal of Organics by Pyrolysis for Enhancing Liberation and Flotation Behavior of Electrode Materials Derived from Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2020, 8, 2205–2214. [Google Scholar] [CrossRef]
- Li, J.; Shi, P.; Wang, Z.; Chen, Y.; Chang, C.-C. A Combined Recovery Process of Metals in Spent Lithium-Ion Batteries. Chemosphere 2009, 77, 1132–1136. [Google Scholar] [CrossRef]
- Nife, A.B.S.; Landskrona, S.; Melin, A.L.; Svensson, V.H. Process for the Recovery of Metals from the Scrap from Nickel-cadmium Electric Storage Batteries. U.S. Patent 4,401,463, 30 August 1983. [Google Scholar]
- Fouad, O.A.; Farghaly, F.I.; Bahgat, M. A Novel Approach for Synthesis of Nanocrystalline γ-LiAlO2 from Spent Lithium-Ion Batteries. J. Anal. Appl. Pyrolysis 2007, 78, 65–69. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, G.; Xu, S.; He, Y.; Liu, X. Thermal Treatment Process for the Recovery of Valuable Metals from Spent Lithium-Ion Batteries. Hydrometallurgy 2016, 165, 390. [Google Scholar] [CrossRef]
- Zhang, G.; He, Y.; Wang, H.; Feng, Y.; Xie, W.; Zhu, X. Application of Mechanical Crushing Combined with Pyrolysis-Enhanced Flotation Technology to Recover Graphite and LiCoO2 from Spent Lithium-Ion Batteries. J. Clean. Prod. 2019, 231, 1418–1427. [Google Scholar] [CrossRef]
- Diaz, F.; Wang, Y.; Moorthy, T.; Friedrich, B. Degradation Mechanism of Nickel-Cobalt-Aluminum (NCA) Cathode Material from Spent Lithium-Ion Batteries in Microwave-Assisted Pyrolysis. Metals 2018, 8, 565. [Google Scholar] [CrossRef]
- Wang, M.; Tan, Q.; Liu, L.; Li, J. Efficient Separation of Aluminum Foil and Cathode Materials from Spent Lithium-Ion Batteries Using a Low-Temperature Molten Salt. ACS Sustain. Chem. Eng. 2019, 7, 8287–8294. [Google Scholar] [CrossRef]
- Xiao, J.; Li, J.; Xu, Z. Recycling Metals from Lithium Ion Battery by Mechanical Separation and Vacuum Metallurgy. J. Hazard. Mater. 2017, 338, 124. [Google Scholar] [CrossRef]
- Sun, L.; Qiu, K. Vacuum Pyrolysis and Hydrometallurgical Process for the Recovery of Valuable Metals from Spent Lithium-Ion Batteries. J. Hazard. Mater. 2011, 194, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Contestabile, M.; Panero, S.; Scrosati, B. Laboratory-Scale Lithium-Ion Battery Recycling Process. J. Power Sources 2001, 92, 65–69. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Hu, Q.-Y.; Li, X.-H.; Wang, Z.-X.; Guo, H.-J. Recycle and Synthesis of LiCoO2 from Incisors Bound of Li-Ion Batteries. Trans. Nonferrous Met. Soc. China 2006, 16, 956–959. [Google Scholar] [CrossRef]
- Yang, L.; Xi, G.; Xi, Y. Recovery of Co, Mn, Ni, and Li from Spent Lithium Ion Batteries for the Preparation of LiNixCoyMnzO2 Cathode Materials. Ceram. Int. 2015, 41, 11498–11503. [Google Scholar] [CrossRef]
- He, L.-P.; Sun, S.-Y.; Song, X.-F.; Yu, J.-G. Recovery of Cathode Materials and Al from Spent Lithium-Ion Batteries by Ultrasonic Cleaning. Waste Manag. 2015, 46, 523. [Google Scholar] [CrossRef]
- Song, D.; Wang, X.; Zhou, E.; Hou, P.; Guo, F.; Zhang, L. Recovery and Heat Treatment of the Li(Ni1/3Co1/3Mn1/3)O2 Cathode Scrap Material for Lithium Ion Battery. J. Power Sources 2013, 232, 348. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, Y.; Cao, H.; Nawaz, F.; Zhang, Y. A Novel Process for Recycling and Resynthesizing LiNi1/3Co1/3Mn1/3O2 from the Cathode Scraps Intended for Lithium-Ion Batteries. Waste Manag. 2014, 34, 1715–1724. [Google Scholar] [CrossRef]
- Nan, J.; Han, D.; Zuo, X. Recovery of Metal Values from Spent Lithium-Ion Batteries with Chemical Deposition and Solvent Extraction. J. Power Sources 2005, 152, 278–284. [Google Scholar] [CrossRef]
- Zhang, P.; Yokoyama, T.; Itabashi, O.; Suzuki, T.M.; Inoue, K. Hydrometallurgical Process for Recovery of Metal Values from Spent Lithium-Ion Secondary Batteries. Hydrometallurgy 1998, 47, 259. [Google Scholar] [CrossRef]
- Joulié, M.; Laucournet, R.; Billy, E. Hydrometallurgical Process for the Recovery of High Value Metals from Spent Lithium Nickel Cobalt Aluminum Oxide Based Lithium-Ion Batteries. J. Power Sources 2014, 247, 551–555. [Google Scholar] [CrossRef]
- Lv, W.; Wang, Z.; Cao, H.; Zheng, X.; Jin, W.; Zhang, Y.; Sun, Z. A Sustainable Process for Metal Recycling from Spent Lithium-Ion Batteries Using Ammonium Chloride. Waste Manag. 2018, 79, 545–553. [Google Scholar] [CrossRef]
- Granata, G.; Moscardini, E.; Pagnanelli, F.; Trabucco, F.; Toro, L. Product Recovery from Li-Ion Battery Wastes Coming from an Industrial Pre-Treatment Plant: Lab Scale Tests and Process Simulations. J. Power Sources 2012, 206, 393. [Google Scholar] [CrossRef]
- Sayilgan, E.; Kukrer, T.; Yigit, N.O.; Civelekoglu, G.; Kitis, M. Acidic Leaching and Precipitation of Zinc and Manganese from Spent Battery Powders Using Various Reductants. J. Hazard. Mater. 2010, 173, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Nayaka, G.P.; Pai, K.V.; Manjanna, J.; Keny, S.J. Use of Mild Organic Acid Reagents to Recover the Co and Li from Spent Li-Ion Batteries. Waste Manag. 2016, 51, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Recovery of Valuable Metals from Cathodic Active Material of Spent Lithium Ion Batteries: Leaching and Kinetic Aspects. Waste Manag. 2015, 45, 306–313. [Google Scholar] [CrossRef]
- Zhuang, L.; Sun, C.; Zhou, T.; Li, H.; Dai, A. Recovery of Valuable Metals from LiNi0.5Co0.2Mn0.3O2 Cathode Materials of Spent Li-Ion Batteries Using Mild Mixed Acid as Leachant. Waste Manag. 2019, 85, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Vieceli, N.; Nogueira, C.A.; Guimarães, C.; Pereira, M.F.C.; Durão, F.O.; Margarido, F. Hydrometallurgical Recycling of Lithium-Ion Batteries by Reductive Leaching with Sodium Metabisulphite. Waste Manag. 2018, 71, 350–361. [Google Scholar] [CrossRef]
- Rocchetti, L.; Vegliò, F.; Kopacek, B.; Beolchini, F. Environmental Impact Assessment of Hydrometallurgical Processes for Metal Recovery from WEEE Residues Using a Portable Prototype Plant. Environ. Sci. Technol. 2013, 47, 1581–1588. [Google Scholar] [CrossRef]
- Li, L.; Ge, J.; Wu, F.; Chen, R.; Chen, S.; Wu, B. Recovery of Cobalt and Lithium from Spent Lithium Ion Batteries Using Organic Citric Acid as Leachant. J. Hazard. Mater. 2010, 176, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Golmohammadzadeh, R.; Faraji, F.; Rashchi, F. Recovery of Lithium and Cobalt from Spent Lithium Ion Batteries (LIBs) Using Organic Acids as Leaching Reagents: A Review. Resour. Conserv. Recycl. 2018, 136, 418–435. [Google Scholar] [CrossRef]
- Shih, Y.J.; Chien, S.K.; Jhang, S.R.; Lin, Y.C. Chemical Leaching, Precipitation and Solvent Extraction for Sequential Separation of Valuable Metals in Cathode Material of Spent Lithium Ion Batteries. J. Taiwan. Inst. Chem. Eng. 2019, 100, 151–159. [Google Scholar] [CrossRef]
- Yao, X.; Xu, Z.; Yao, Z.; Cheng, W.; Gao, H.; Zhao, Q.; Li, J.; Zhou, A. Oxalate Co-Precipitation Synthesis of LiNi0.6Co0.2Mn0.2O2 for Low-Cost and High-Energy Lithium-Ion Batteries. Mater. Today Commun. 2019, 19, 262–270. [Google Scholar] [CrossRef]
- Zhang, X.; Bian, Y.; Xu, S.; Fan, E.; Xue, Q.; Guan, Y.; Wu, F.; Li, L.; Chen, R. Innovative Application of Acid Leaching to Regenerate Li(Ni1/3Co1/3Mn1/3)O2 Cathodes from Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 5959–5968. [Google Scholar] [CrossRef]
- Sun, L.; Qiu, K. Organic Oxalate as Leachant and Precipitant for the Recovery of Valuable Metals from Spent Lithium-Ion Batteries. Waste Manag. 2012, 32, 1575–1582. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, X.; Zheng, X.; Lin, X.; Cao, H.; Zhang, Y.; Sun, Z. Lithium Carbonate Recovery from Cathode Scrap of Spent Lithium-Ion Battery: A Closed-Loop Process. Environ. Sci. Technol. 2017, 51, 1662–1669. [Google Scholar] [CrossRef]
- Li, L.; Bian, Y.; Zhang, X.; Xue, Q.; Fan, E.; Wu, F.; Chen, R. Economical Recycling Process for Spent Lithium-Ion Batteries and Macro- and Micro-Scale Mechanistic Study. J. Power Sources 2018, 377, 70–79. [Google Scholar] [CrossRef]
- Li, L.; Dunn, J.B.; Zhang, X.X.; Gaines, L.; Chen, R.J.; Wu, F.; Amine, K. Recovery of Metals from Spent Lithium-Ion Batteries with Organic Acids as Leaching Reagents and Environmental Assessment. J. Power Sources 2013, 233, 180. [Google Scholar] [CrossRef]
- Nayaka, G.P.; Pai, K.V.; Santhosh, G.; Manjanna, J. Recovery of Cobalt as Cobalt Oxalate from Spent Lithium Ion Batteries by Using Glycine as Leaching Agent. J. Environ. Chem. Eng. 2016, 4, 2378. [Google Scholar] [CrossRef]
- He, L.P.; Sun, S.Y.; Yu, J.G. Performance of LiNi1/3Co1/3Mn1/3O2 Prepared from Spent Lithium-Ion Batteries by a Carbonate Co-Precipitation Method. Ceram. Int. 2018, 44, 351–357. [Google Scholar] [CrossRef]
- Chen, X.; Fan, B.; Xu, L.; Zhou, T.; Kong, J. An Atom-Economic Process for the Recovery of High Value-Added Metals from Spent Lithium-Ion Batteries. J. Cleaner Prod. 2016, 112, 3562. [Google Scholar] [CrossRef]
- Pant, D.; Dolker, T. Green and Facile Method for the Recovery of Spent Lithium Nickel Manganese Cobalt Oxide (NMC) Based Lithium Ion Batteries. Waste Manag. 2017, 60, 689–695. [Google Scholar] [CrossRef]
- Meng, X.; Han, K.N. Principles and Applications of Ammonia Leaching of Metals—A Review. Miner. Process. Extr. Metall. Rev. 1996, 16, 23–61. [Google Scholar] [CrossRef]
- Zuniga, M.; Parada, L.F.; Asselin, E. Leaching of a Limonitic Laterite in Ammoniacal Solutions with Metallic Iron. Hydrometallurgy 2010, 104, 260–267. [Google Scholar] [CrossRef]
- Zheng, X.; Gao, W.; Zhang, X.; He, M.; Lin, X.; Cao, H.; Zhang, Y.; Sun, Z. Spent Lithium-Ion Battery Recycling—Reductive Ammonia Leaching of Metals from Cathode Scrap by Sodium Sulphite. Waste Manag. 2017, 60, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Meng, K.; Cao, Y.; Zhang, B.; Ou, X.; Li, D.M.; Zhang, J.F.; Ji, X. Comparison of the Ammoniacal Leaching Behavior of Layered LiNixCoyMn1-x-yO2 (x = 1/3, 0.5, 0.8) Cathode Materials. ACS Sustain. Chem. Eng. 2019, 7, 7750–7759. [Google Scholar] [CrossRef]
- Wang, H.; Huang, K.; Zhang, Y.; Chen, X.; Jin, W.; Zheng, S.; Zhang, Y.; Li, P. Recovery of Lithium, Nickel, and Cobalt from Spent Lithium-Ion Battery Powders by Selective Ammonia Leaching and an Adsorption Separation System. ACS Sustain. Chem. Eng. 2017, 5, 11489–11495. [Google Scholar] [CrossRef]
- Botelho, A.B., Jr.; Stopic, S.; Friedrich, B.; Tenório, J.A.; Espinosa, D.C. Cobalt Recovery from Li-Ion Battery Recycling: A Critical Review. Metals 2021, 11, 1999. [Google Scholar] [CrossRef]
- Bahaloo-Horeh, N.; Mousavi, S.M. Enhanced Recovery of Valuable Metals from Spent Lithium-Ion Batteries through Optimization of Organic Acids Produced by Aspergillus Niger. Waste Manag. 2017, 60, 666. [Google Scholar] [CrossRef]
- Mishra, D.; Kim, D.J.; Ralph, D.E.; Ahn, J.G.; Rhee, Y.H. Bioleaching of Metals from Spent Lithium Ion Secondary Batteries Using Acidithiobacillus Ferrooxidans. Waste Manag. 2008, 28, 333. [Google Scholar] [CrossRef]
- Niu, Z.; Zou, Y.; Xin, B.; Chen, S.; Liu, C.; Li, Y. Process Controls for Improving Bioleaching Performance of Both Li and Co from Spent Lithium Ion Batteries at High Pulp Density and Its Thermodynamics and Kinetics Exploration. Chemosphere 2014, 109, 92. [Google Scholar] [CrossRef]
- Xin, Y.; Guo, X.; Chen, S.; Wang, J.; Wu, F.; Xin, B. Bioleaching of Valuable Metals Li, Co, Ni and Mn from Spent Electric Vehicle Li-Ion Batteries for the Purpose of Recovery. J. Clean. Prod. 2016, 116, 249. [Google Scholar] [CrossRef]
- Takahashi, V.C.I.; Botelho, A.B., Jr.; Espinosa, D.C.R.; Tenório, J.A.S. Enhancing Cobalt Recovery from Li-Ion Batteries Using Grinding Treatment Prior to the Leaching and Solvent Extraction Process. J. Environ. Chem. Eng. 2020, 8, 103801. [Google Scholar] [CrossRef]
- Dolker, T.; Pant, D. Chemical-Biological Hybrid Systems for the Metal Recovery from Waste Lithium Ion Battery. J. Environ. Manag. 2019, 248, 109270. [Google Scholar] [CrossRef]
- Zeng, G.; Deng, X.; Luo, S.; Luo, X.; Zou, J. A Copper-Catalyzed Bioleaching Process for Enhancement of Cobalt Dissolution from Spent Lithium-Ion Batteries. J. Hazard. Mater. 2012, 199, 164. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Luo, S.; Deng, X.; Li, L.; Au, C. Influence of Silver Ions on Bioleaching of Cobalt from Spent Lithium Batteries. Miner. Eng. 2013, 49, 40. [Google Scholar] [CrossRef]
- Lee, C.K.; Rhee, K.I.K.-I. Preparation of LiCoO2 from Spent Lithium-Ion Batteries. J. Power Sources 2002, 109, 17–21. [Google Scholar] [CrossRef]
- Sattar, R.; Ilyas, S.; Bhatti, H.N.; Ghaffar, A. Resource Recovery of Critically-Rare Metals by Hydrometallurgical Recycling of Spent Lithium Ion Batteries. Sep. Purif. Technol. 2019, 209, 725–733. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Hydrometallurgical Processing of Spent Lithium Ion Batteries (LIBs) in the Presence of a Reducing Agent with Emphasis on Kinetics of Leaching. Chem. Eng. J. 2015, 281, 418. [Google Scholar] [CrossRef]
- Alvial-Hein, G.; Mahandra, H.; Ghahreman, A. Separation and Recovery of Cobalt and Nickel from End of Life Products via Solvent Extraction Technique: A Review. J. Clean. Prod. 2021, 297, 126592. [Google Scholar] [CrossRef]
- Mansur, M.B.; Guimarães, A.S.; Petraniková, M. An Overview on the Recovery of Cobalt from End-of-Life Lithium Ion Batteries. Miner. Process. Extr. Metall. Rev. 2022, 43, 489–509. [Google Scholar] [CrossRef]
- Guimarães, A.S.; Silva, M.D.F.D.; Resende, G.P.D.S.; Santos, I.D.D.; Mansur, M.B. Solvent Extraction of Metals from a Brazilian Nickel Lateritic Liquor with D2EHPA and Cyanex 272. Braz. J. Chem. Eng. 2022, 1–8. [Google Scholar] [CrossRef]
- Voropanova, L.A.; Pukhova, V.P. Extraction of Copper, Cobalt and Nickel Ions from Aqueous Solutions by Extractant Cyanex 272. J. Min. Inst. 2018, 233, 498–505. [Google Scholar] [CrossRef]
- Tsakiridis, P.E.; Agatzini-Leonardou, S. Solvent Extraction of Aluminium in the Presence of Cobalt, Nickel and Magnesium from Sulphate Solutions by Cyanex 272. Hydrometallurgy 2005, 80, 90–97. [Google Scholar] [CrossRef]
- Ichlas, Z.T.; Ibana, D.C. Process Development for the Direct Solvent Extraction of Nickel and Cobalt from Nitrate Solution: Aluminum, Cobalt, and Nickel Separation Using Cyanex 272. Int. J. Miner. Metall. Mater. 2017, 24, 37–46. [Google Scholar] [CrossRef]
- Nayl, A.A.; Hamed, M.M.; Rizk, S.E. Selective Extraction and Separation of Metal Values from Leach Liquor of Mixed Spent Li-Ion Batteries. J. Taiwan Inst. Chem. Eng. 2015, 55, 119–125. [Google Scholar] [CrossRef]
- Swain, B.; Jeong, J.; Lee, J.-C.; Lee, G.-H.; Sohn, J.-S. Hydrometallurgical Process for Recovery of Cobalt from Waste Cathodic Active Material Generated during Manufacturing of Lithium Ion Batteries. J. Power Sources 2007, 167, 536–544. [Google Scholar] [CrossRef]
- Jha, A.K.; Jha, M.K.; Kumari, A.; Sahu, S.K.; Kumar, V.; Pandey, B.D. Selective Separation and Recovery of Cobalt from Leach Liquor of Discarded Li-Ion Batteries Using Thiophosphinic Extractant. Sep. Purif. Technol. 2013, 104, 160–166. [Google Scholar] [CrossRef]
- Dorella, G.; Mansur, M.B. A Study of the Separation of Cobalt from Spent Li-Ion Battery Residues. J. Power Sources 2007, 170, 210–215. [Google Scholar] [CrossRef]
- Wang, F.; Sun, R.; Xu, J.; Chen, Z.; Kang, M. Recovery of Cobalt from Spent Lithium Ion Batteries Using Sulphuric Acid Leaching Followed by Solid–Liquid Separation and Solvent Extraction. RSC Adv. 2016, 6, 85303. [Google Scholar] [CrossRef]
- Vieceli, N.; Reinhardt, N.; Ekberg, C.; Petranikova, M. Optimization of Manganese Recovery from a Solution Based on Lithium-Ion Batteries by Solvent Extraction with D2ehpa. Metals 2021, 11, 54. [Google Scholar] [CrossRef]
- Keller, A.; Hlawitschka, M.W.; Bart, H.-J. Manganese Recycling of Spent Lithium Ion Batteries via Solvent Extraction. Sep. Purif. Technol. 2021, 275, 119166. [Google Scholar] [CrossRef]
- Dhiman, S.; Gupta, B. Partition Studies on Cobalt and Recycling of Valuable Metals from Waste Li-Ion Batteries via Solvent Extraction and Chemical Precipitation. J. Clean. Prod. 2019, 225, 820–832. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, T.; Kong, J.; Fang, H.; Chen, Y. Separation and Recovery of Metal Values from Leach Liquor of Waste Lithium Nickel Cobalt Manganese Oxide Based Cathodes. Sep. Purif. Technol. 2015, 141, 76. [Google Scholar] [CrossRef]
- Yoshimi, T.; Narusako, M.; Nakamura, Y. Method of Separating/Recovering Current Collector and Positive Electrode Active Material from Positive Electrode Member for Lithium Ion Battery. Metals 2013, 11, 1999. [Google Scholar]
- Abdou, T.R.; Botelho, A.B., Jr.; Espinosa, D.C.R.; Tenório, J.A.S. Recycling of Polymeric Composites from Industrial Waste by Pyrolysis: Deep Evaluation for Carbon Fibers Reuse. Waste Manag. 2021, 120, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zainol, Z.; Nicol, M.J. Ion-Exchange Equilibria of Ni2+, Co2+, Mn2+ and Mg2+ with Iminodiacetic Acid Chelating Resin Amberlite IRC 748. Hydrometallurgy 2009, 99, 175–180. [Google Scholar] [CrossRef]
- Mendes, F.D.; Martins, A.H. Selective Nickel and Cobalt Uptake from Pressure Sulfuric Acid Leach Solutions Using Column Resin Sorption. Int. J. Miner. Process. 2005, 77, 53–63. [Google Scholar] [CrossRef]
- Wołowicz, A.; Hubicki, Z. The Use of the Chelating Resin of a New Generation Lewatit MonoPlus TP-220 with the Bis-Picolylamine Functional Groups in the Removal of Selected Metal Ions from Acidic Solutions. Chem. Eng. J. 2012, 197, 493–508. [Google Scholar] [CrossRef]
- Littlejohn, P.; Vaughan, J. Recovery of Nickel and Cobalt from Laterite Leach Tailings through Resin-in-Pulp Scavenging and Selective Ammoniacal Elution. Miner. Eng. 2013, 54, 14–20. [Google Scholar] [CrossRef]
- Botelho, A.B., Jr.; Dreisinger, D.B.; Espinosa, D.C.R.; Tenório, J.A.S. Pre-Reducing Process Kinetics to Recover Metals from Nickel Leach Waste Using Chelating Resins. Int. J. Chem. Eng. 2018, 2018, 9161323. [Google Scholar] [CrossRef]
- Strauss, M.L.; Diaz, L.A.; McNally, J.; Klaehn, J.; Lister, T.E. Separation of Cobalt, Nickel, and Manganese in Leach Solutions of Waste Lithium-Ion Batteries Using Dowex M4195 Ion Exchange Resin. Hydrometallurgy 2021, 206, 105757. [Google Scholar] [CrossRef]
- Virolainen, S.; Wesselborg, T.; Kaukinen, A.; Sainio, T. Removal of Iron, Aluminium, Manganese and Copper from Leach Solutions of Lithium-Ion Battery Waste Using Ion Exchange. Hydrometallurgy 2021, 202, 105602. [Google Scholar] [CrossRef]
- Albrecht, T.W.J.; Addai-Mensah, J.; Fornasiero, D. Effect of PH Concentration and Temperature on Copper and Zinc Hydroxide Formation/Precipitation in Solution. In Proceedings of the CHEMECA 2011 “Engineering a Better World”, Sidney, Australia, 18–21 September 2011; Engineers Australia: Barton, Australia, 2011; pp. 1–4. [Google Scholar]
- Hidmi, L.; Edwards, M. Role of Temperature and PH in Cu(OH)2 Solubility. Environ. Sci. Technol. 1999, 33, 2607–2610. [Google Scholar] [CrossRef]
- Jandová, J.; Dvořák, P.; Vu, H.N. Processing of Zinnwaldite Waste to Obtain Li2CO3. Hydrometallurgy 2010, 103, 12–18. [Google Scholar] [CrossRef]
- Provazi, K.; Campos, B.A.; Espinosa, D.C.R.; Tenório, J.A.S. Metal Separation from Mixed Types of Batteries Using Selective Precipitation and Liquid-Liquid Extraction Techniques. Waste Manag. 2011, 31, 59–64. [Google Scholar] [CrossRef]
- Chen, L.; Tang, X.; Zhang, Y.; Li, L.; Zeng, Z.; Zhang, Y. Process for the Recovery of Cobalt Oxalate from Spent Lithium-Ion Batteries. Hydrometallurgy 2011, 108, 80–86. [Google Scholar] [CrossRef]
- Suzuki, T.; Nakamura, T.; Inoue, Y.; Niinae, M.; Shibata, J. A Hydrometallurgical Process for the Separation of Aluminum, Cobalt, Copper and Lithium in Acidic Sulfate Media. Sep. Purif. Technol. 2012, 98, 396–401. [Google Scholar] [CrossRef]
- Kang, J.; Senanayake, G.; Sohn, J.; Shin, S.M. Recovery of Cobalt Sulfate from Spent Lithium Ion Batteries by Reductive Leaching and Solvent Extraction with Cyanex 272. Hydrometallurgy 2010, 100, 168–171. [Google Scholar] [CrossRef]
- Nayl, A.A.; Elkhashab, R.A.; Badawy, S.M.; El-Khateeb, M.A. Acid Leaching of Mixed Spent Li-Ion Batteries. Arab. J. Chem. 2017, 10, S3632–S3639. [Google Scholar] [CrossRef]
- Cai, G.; Fung, K.Y.; Ng, K.M.; Wibowo, C. Process Development for the Recycle of Spent Lithium Ion Batteries by Chemical Precipitation. Ind. Eng. Chem. Res. 2014, 53, 18245–18259. [Google Scholar] [CrossRef]
- He, S.; Xiang, W.; He, W.; Yu, F.; Liu, Z. Recovery of Spent LiCoO2 Cathode Material: Thermodynamic Analysis and Experiments for Precipitation and Separation of Elements. Chem. Eng. J. 2022, 429, 132371. [Google Scholar] [CrossRef]
- Choubey, P.K.; Dinkar, O.S.; Panda, R.; Kumari, A.; Jha, M.K.; Pathak, D.D. Selective Extraction and Separation of Li, Co and Mn from Leach Liquor of Discarded Lithium Ion Batteries (LIBs). Waste Manag. 2021, 121, 452–457. [Google Scholar] [CrossRef]
- Wang, R.C.; Lin, Y.C.; Wu, S.H. A Novel Recovery Process of Metal Values from the Cathode Active Materials of the Lithium-Ion Secondary Batteries. Hydrometallurgy 2009, 99, 194–201. [Google Scholar] [CrossRef]
- Dash, S.; Patel, S.; Mishra, B.K. Oxidation by Permanganate: Synthetic and Mechanistic Aspects. Tetrahedron 2009, 65, 707–739. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, C.Y. Manganese Metallurgy Review. Part II: Manganese Separation and Recovery from Solution. Hydrometallurgy 2007, 89, 160–177. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, H.; Cao, H.; Wang, Z.; Liu, C.; Sun, Y.; Zhao, H.; Zhang, Y.; Sun, Z. Direct Preparation of Efficient Catalyst for Oxygen Evolution Reaction and High-Purity Li2CO3 from Spent LiNi0.5Mn0.3Co0.2O2 Batteries. J. Clean. Prod. 2019, 236, 117576. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, J.; Zhang, W.; Chen, Y.; Wang, C. Efficient and Economical Recovery of Lithium, Cobalt, Nickel, Manganese from Cathode Scrap of Spent Lithium-Ion Batteries. J. Clean. Prod. 2018, 204, 437–446. [Google Scholar] [CrossRef]
- Liu, P.; Xiao, L.; Chen, Y.; Tang, Y.; Wu, J.; Chen, H. Recovering Valuable Metals from LiNixCoyMn1-x-yO2 Cathode Materials of Spent Lithium Ion Batteries via a Combination of Reduction Roasting and Stepwise Leaching. J. Alloys Compd. 2019, 783, 743–752. [Google Scholar] [CrossRef]
- Saeki, S.; Lee, J.; Zhang, Q.; Saito, F. Co-Grinding LiCoO2 with PVC and Water Leaching of Metal Chlorides Formed in Ground Product. Int. J. Miner. Process. 2004, 74, S373. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, C.; Zhang, F. An Environmental Benign Process for Cobalt and Lithium Recovery from Spent Lithium-Ion Batteries by Mechanochemical Approach. Waste Manag. 2016, 51, 239. [Google Scholar] [CrossRef]
- Lain, M.J. Recycling of Lithium Ion Cells and Batteries. J. Power Sources 2001, 97, 736–738. [Google Scholar] [CrossRef]
- Myoung, J.; Jung, Y.; Lee, J.; Tak, Y. Cobalt Oxide Preparation from Waste LiCoO2 by Electrochemical-Hydrothermal Method. J. Power Sources 2002, 112, 639–642. [Google Scholar] [CrossRef]
- Freitas, M.B.J.G.; Garcia, E.M. Electrochemical Recycling of Cobalt from Cathodes of Spent Lithium-Ion Batteries. J. Power Sources 2007, 171, 953–959. [Google Scholar] [CrossRef]
- Freitas, M.B.J.G.; Garcia, E.M.; Celante, V.G. Electrochemical and Structural Characterization of Cobalt Recycled from Cathodes of Spent Li-Ion Batteries. J. Appl. Electrochem. 2009, 39, 601–607. [Google Scholar] [CrossRef]
- Freitas, M.B.J.G.; Celante, V.G.; Pietre, M.K. Electrochemical Recovery of Cobalt and Copper from Spent Li-Ion Batteries as Multilayer Deposits. J. Power Sources 2010, 195, 3309–3315. [Google Scholar] [CrossRef]
- Se, B. Commercial Applications of Ionic Liquids—An Overview; Shiflett, M.B., Ed.; Green Chemistry and Sustainable Technology; Springer: Cham, Switzerland, 2008; ISBN 978-3-030-35244-8. [Google Scholar]
- Osada, I.; de Vries, H.; Scrosati, B.; Passerini, S. Ionic-Liquid-Based Polymer Electrolytes for Battery Applications. Angew. Chem. Int. Ed. 2016, 55, 500–513. [Google Scholar] [CrossRef]
- Paiva, A.P.; Nogueira, C.A. Ionic Liquids in the Extraction and Recycling of Critical Metals from Urban Mines. Waste Biomass Valorization 2021, 12, 1725–1747. [Google Scholar] [CrossRef]
- Łukomska, A.; Wiśniewska, A.; Dąbrowski, Z.; Kolasa, D.; Luchcińska, S.; Domańska, U. Separation of Cobalt, Lithium and Nickel from the “Black Mass” of Waste Li-Ion Batteries by Ionic Liquids, DESs and Organophosphorous-Based Acids Extraction. J. Mol. Liq. 2021, 343, 117694. [Google Scholar] [CrossRef]
- Morizono, H.; Oshima, T.; Baba, Y. Extraction Mechanism of Cobalt(II) Using an Alkylated Histidine Extractant in an Ionic Liquid System. Solvent Extr. Res. Dev. 2011, 18, 181–186. [Google Scholar] [CrossRef]
- Xu, L.; Chen, C.; Fu, M.-L. Separation of Cobalt and Lithium from Spent Lithium-Ion Battery Leach Liquors by Ionic Liquid Extraction Using Cyphos IL-101. Hydrometallurgy 2020, 197, 105439. [Google Scholar] [CrossRef]
- Zante, G.; Masmoudi, A.; Barillon, R.; Trébouet, D.; Boltoeva, M. Separation of Lithium, Cobalt and Nickel from Spent Lithium-Ion Batteries Using TBP and Imidazolium-Based Ionic Liquids. J. Ind. Eng. Chem. 2020, 82, 269–277. [Google Scholar] [CrossRef]
- Zante, G.; Braun, A.; Masmoudi, A.; Barillon, R.; Trébouet, D.; Boltoeva, M. Solvent Extraction Fractionation of Manganese, Cobalt, Nickel and Lithium Using Ionic Liquids and Deep Eutectic Solvents. Miner. Eng. 2020, 156, 106512. [Google Scholar] [CrossRef]
- Othman, E.A.; van der Ham, A.G.J.; Miedema, H.; Kersten, S.R.A. Recovery of Metals from Spent Lithium-Ion Batteries Using Ionic Liquid [P8888][Oleate]. Sep. Purif. Technol. 2020, 252, 117435. [Google Scholar] [CrossRef]
- Singh Mr, M.B.; Kumar Mr, V.S.; Chaudhary, M.; Singh, P. A Mini Review on Synthesis, Properties and Applications of Deep Eutectic Solvents. J. Indian Chem. Soc. 2021, 98, 100210. [Google Scholar] [CrossRef]
- Tran, M.K.; Rodrigues, M.-T.F.; Kato, K.; Babu, G.; Ajayan, P.M. Deep Eutectic Solvents for Cathode Recycling of Li-Ion Batteries. Nat. Energy 2019, 4, 339–345. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Z.; Lu, Z.; Xu, Z. A Novel Method for Screening Deep Eutectic Solvent to Recycle the Cathode of Li-Ion Batteries. Green Chem. 2020, 22, 4473–4482. [Google Scholar] [CrossRef]
- Wang, M.; Tan, Q.; Liu, L.; Li, J. A Low-Toxicity and High-Efficiency Deep Eutectic Solvent for the Separation of Aluminum Foil and Cathode Materials from Spent Lithium-Ion Batteries. J. Hazard. Mater. 2019, 380, 120846. [Google Scholar] [CrossRef]
- Roldán-Ruiz, M.J.; Ferrer, M.L.; Gutiérrez, M.C.; del Monte, F. Highly Efficient P-Toluenesulfonic Acid-Based Deep-Eutectic Solvents for Cathode Recycling of Li-Ion Batteries. ACS Sustain. Chem. Eng. 2020, 8, 5437–5445. [Google Scholar] [CrossRef]
- Chen, L.; Chao, Y.; Li, X.; Zhou, G.; Lu, Q.; Hua, M.; Li, H.; Ni, X.; Wu, P.; Zhu, W. Engineering a Tandem Leaching System for the Highly Selective Recycling of Valuable Metals from Spent Li-Ion Batteries. Green Chem. 2021, 23, 2177–2184. [Google Scholar] [CrossRef]
- Schiavi, P.G.; Altimari, P.; Branchi, M.; Zanoni, R.; Simonetti, G.; Navarra, M.A.; Pagnanelli, F. Selective Recovery of Cobalt from Mixed Lithium Ion Battery Wastes Using Deep Eutectic Solvent. Chem. Eng. J. 2021, 417, 129249. [Google Scholar] [CrossRef]
- Bertuol, D.A.; Machado, C.M.; Silva, M.L.; Calgaro, C.O.; Dotto, G.L.; Tanabe, E.H. Recovery of Cobalt from Spent Lithium-Ion Batteries Using Supercritical Carbon Dioxide Extraction. Waste Manag. 2016, 51, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Schuster, J.; Petranikova, M.; Ebin, B. Innovative Recycling of Organic Binders from Electric Vehicle Lithium-Ion Batteries by Supercritical Carbon Dioxide Extraction. Resour. Conserv. Recycl. 2021, 172, 105666. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, F. Innovative Leaching of Cobalt and Lithium from Spent Lithium-Ion Batteries and Simultaneous Dechlorination of Polyvinyl Chloride in Subcritical Water. J. Hazard. Mater. 2016, 316, 19. [Google Scholar] [CrossRef]
- Bertuol, D.A.; Amado, F.R.; Cruz, E.D.; Tanabe, E.H. Metal Recovery Using Supercritical Carbon Dioxide. In Green Sustainable Process for Chemical and Environmental Engineering and Science: Supercritical Carbon Dioxide as Green Solvent; Elsevier: Santa Maria, Brazil, 2019; pp. 85–103. ISBN 9780128173886/9780128173893. [Google Scholar]
- Sa, Q.; Gratz, E.; He, M.; Lu, W.; Apelian, D.; Wang, Y. Synthesis of High Performance LiNi1/3Mn1/3Co1/3O2 from Lithium Ion Battery Recovery Stream. J. Power Sources 2015, 282, 140. [Google Scholar] [CrossRef]
- Yao, L.; Yao, H.; Xi, G.; Feng, Y. Recycling and Synthesis of LiNi1/3Co1/3Mn1/3O2 from Waste Lithium Ion Batteries Using d,l-Malic Acid. RSC Adv. 2016, 6, 17947–17954. [Google Scholar] [CrossRef]
- Hu, C.-Y.; Guo, J.; Du, Y.; Xu, H.-H.; He, Y.-H. Effects of Synthesis Conditions on Layered Li[Ni1/3Co1/3Mn1/3]O2 Positive-Electrode via Hydroxide Co-Precipitation Method for Lithium-Ion Batteries. Trans. Nonferrous Met. Soc. China 2011, 21, 114–120. [Google Scholar] [CrossRef]
- Zou, H.; Gratz, E.; Apelian, D.; Wang, Y. A Novel Method to Recycle Mixed Cathode Materials for Lithium Ion Batteries. Green Chem. 2013, 15, 1183. [Google Scholar] [CrossRef]
- Senćanski, J.; Bajuk-Bogdanović, D.; Majstorović, D.; Tchernychova, E.; Papan, J.; Vujković, M. The Synthesis of Li(Co-Mn-Ni)O2 Cathode Material from Spent-Li Ion Batteries and the Proof of Its Functionality in Aqueous Lithium and Sodium Electrolytic Solutions. J. Power Sources 2017, 342, 690–703. [Google Scholar] [CrossRef]
- Ohzuku, T.; Makimura, Y. Layered Lithium Insertion Material of LiNi1/2Mn1/2O2: A Possible Alternative to LiCoO2 for Advanced Lithium-Ion Batteries. Chem. Lett. 2001, 30, 744–745. [Google Scholar] [CrossRef]
- Zheng, Z.; Chen, M.; Wang, Q.; Zhang, Y.; Ma, X.; Shen, C.; Xu, D.; Liu, J.; Liu, Y.; Gionet, P.; et al. High Performance Cathode Recovery from Different Electric Vehicle Recycling Streams. ACS Sustain. Chem. Eng. 2018, 6, 13977–13982. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, X.; Yin, C.; Li, J. Regeneration of LiNi0.5Co0.2Mn0.3O2 Cathode Material from Spent Lithium-Ion Batteries. Electrochim. Acta 2018, 291, 142–150. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, G.; Liu, F.; Yue, X.; Chen, Z. Resolving the Compositional and Structural Defects of Degraded LiNixCoyMnzO2 Particles to Directly Regenerate High-Performance Lithium-Ion Battery Cathodes. ACS Energy Lett. 2018, 3, 1683–1692. [Google Scholar] [CrossRef]
- Li, J.; Hu, L.; Zhou, H.; Wang, L.; Zhai, B.; Yang, S.; Meng, P.; Hu, R. Regenerating of LiNi0.5Co0.2Mn0.3O2 Cathode Materials from Spent Lithium-Ion Batteries. J. Mater. Sci. Mater. Electron. 2018, 29, 17661–17669. [Google Scholar] [CrossRef]
- Kim, D.S.; Sohn, J.S.; Lee, C.K.; Lee, J.H.; Han, K.S.; Lee, Y.I. Simultaneous Separation and Renovation of Lithium Cobalt Oxide from the Cathode of Spent Lithium Ion Rechargeable Batteries. J. Power Sources 2004, 132, 145–149. [Google Scholar] [CrossRef]
- Meng, X.; Hao, J.; Cao, H.; Lin, X.; Ning, P.; Zheng, X.; Chang, J.; Zhang, X.; Wang, B.; Sun, Z. Recycling of LiNi1/3Co1/3Mn1/3O2 Cathode Materials from Spent Lithium-Ion Batteries Using Mechanochemical Activation and Solid-State Sintering. Waste Manag. 2019, 84, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Ra, D.-i.; Han, K.S. Used Lithium Ion Rechargeable Battery Recycling Using Etoile-Rebatt Technology. J. Power Sources 2006, 163, 284–288. [Google Scholar] [CrossRef]
- Zhang, Z.; He, W.; Li, G.; Xia, J.; Hu, H.; Huang, J. Ultrasound-Assisted Hydrothermal Renovation of LiCoO2 from the Cathode of Spent Lithium-Ion Batteries. Int. J. Electrochem. Sci. 2014, 9, 3691–3700. [Google Scholar]
- Zhang, Z.; He, W.; Li, G.; Xia, J.; Hu, H.; Huang, J.; Zhang, S. Recovery of Lithium Cobalt Oxide Material from the Cathode of Spent Lithium-Ion Batteries. ECS Electrochem. Lett. 2014, 3, A58–A61. [Google Scholar] [CrossRef]
- Zhang, Z.; He, W.; Li, G.; Xia, J.; Hu, H.; Huang, J. Renovation of LiCoO2 Crystal Structure from Spent Lithium Ion Batteries by Ultrasonic Hydrothermal Reaction. Res. Chem. Intermed. 2015, 41, 3367–3373. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Chen, R.; Zhao, T.; Lu, J.; Wu, F.; Amine, K. Synthesis and Electrochemical Performance of Cathode Material Li1.2Co0.13Ni0.13Mn0.54O2 from Spent Lithium-Ion Batteries. J. Power Sources 2014, 249, 28. [Google Scholar] [CrossRef]




| Cathode Material | Leaching Solution Configuration | T, °C | Time, h | Efficiency, % | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Li | Co | Ni | Mn | |||||
| Inorganic acid leaching | ||||||||
| LiCoO2 | 4 M HCl; Solid:Liquid ratio, g/L (S/L) = 10 | 80 | 1 | 99 | 99 | - | - | [128] |
| LiCoO2 | 1 M HNO3 + 1.7 vol.% H2O2; S/L = 20 | 75 | 1 | 95 | 95 | - | - | [165] |
| LiNi0.1Mn0.1Co0.3O2 | 1 M H2SO4; S/L = 50 | 95 | 6 | 93 | 66 | 96 | 50 | [134] |
| LiNi0.5Mn0.3Co0.2O2 | 0.2 M H3PO4 + 0.4 M citric acid; S/L = 20 | 90 | 0.5 | 100 | 92 | 93 | 92 | [135] |
| LiNixMnyCozO2 | 1.25 M H2SO4 + 0.1 M Na2S2O5; S/L = 100 | 60 | 0.5 | 96 | 94 | 89 | 85 | [136] |
| LiNixMnyCozO2 | 2 M H2SO4 + 1 vol.% H2O2; S/L = 25 | 90 | 1 | 98 | 100 | 88 | 89 | [140] |
| LiNixMnyCozO2 | 2 M H2SO4 + 4 vol.% H2O2; S/L = 50 | 50 | 2 | 99 | 98 | 98 | 98 | [166] |
| LiNixMnyCozO2 | 1 M H2SO4 + 0.075 M NaHSO3; S/L = 20 | 95 | 4 | 97 | 92 | 96 | 88 | [167] |
| Organic acid leaching | ||||||||
| LiCoO2 | 1 M maleic acid + 0.02 M ascorbic acid; S/L = 2 | 80 | 6 | 100 | 97 | - | - | [133] |
| LiCoO2 | 1.25 M citric acid + 1 vol.% H2O2; S/L = 20 | 90 | 0.5 | 100 | 90 | - | - | [138] |
| LiCoO2 | 1 M oxalic acid; S/L = 50 | 80 | 2 | 99 | 98 | - | - | [143] |
| LiNixMnyCozO2 | 2 M formic acid + 6 vol% H2O2; S/L = 50 | 60 | 0.5 | 98 | 100 | 100 | 100 | [144] |
| LiCoO2 | 1.5 M DL-malic acid + 2 vol% H2O2; S/L = 20 | 90 | 0.5 | 100 | 90 | - | - | [146] |
| LiCoO2 | 0.5 M glycine + 0.02 M ascorbic; S/L = 2 | 80 | 6 | 95 | 95 | - | - | [147] |
| LiNixMnyCozO2 | Citrus juice; S/L = 50 | 90 | 0.3 | 100 | 94 | 98 | 99 | [150] |
| Alkaline leaching | ||||||||
| LiNixMnyCozO2 | 4 M NH3 + 1.5 M (NH4)2SO4 + 0.5 M Na2SO3; S/L = 10 | 80 | 5 | 95 | 81 | 90 | 4 | [153] |
| LiNixMnyCozO2 | 4 M NH3 + 1.5 M NH4Cl + 0.5 M Na2SO3; S/L = 10 | 80 | 5 | 93 | 98 | 98 | <10 | [154] |
| LiNi0.5Mn0.3Co0.2O2 | 4 M NH3 + 1.5 M NH4HCO3 + 30 wt.% H2O2; S/L = 20 | 60 | 8 | 76 | 95 | 96 | - | [155] |
| Bioleaching | ||||||||
| LiNixMnyCozO2 | Aspergillus niger (produces citric, gluconic, malic, oxalic acids) | 30 | 336 | 100 | 64 | 54 | 77 | [157] |
| LiCoO2 | Acidithiobacillus ferrooxidans (H2SO4, Fe3+) | 30 | 480 | 10 | 55 | - | - | [158] |
| LiCoO2 | Alicyclobacillus + Sulfobacillus; S/L = 20 | 30 | 720 | 89 | 72 | - | - | [159] |
| LiNixMnyCozO2 | Acidithiobacillus + Leptospirillum; S/L = 20 | 30 | 168 | 95 | 96 | 96 | 95 | [160] |
| LiCoO2 | Lysinibacillus + 0.05 M citric acid; S/L = 5 | 37 | 720 | 25 | 98 | - | - | [162] |
| Ions Presented in a Leachate | Separation/Recovery Methodology | Extraction, % | Ref. | |||
|---|---|---|---|---|---|---|
| Li | Co | Ni | Mn | |||
| Solvent extraction | ||||||
| Li+, Co2+, SO42− | (1) Co extraction by Cyanex 272 at organic/aqueous ration (O/A) = 1, 25 °C, pH = 4. 4 times repeating; (2) Co stripping with 2 M H2SO4 at pH = 0–1. 4 times repeating; | - | 85 | - | - | [130] |
| Fe3+, Cu2+, Al3+, Li+, Ni2+, Mn2+, Co2+, SO42−, NH4+ | (1) Fe3+, Cu2+, Al3+ impurities extraction with 20% Acorga M5640 in kerosene at pH = 1.0–2.2; (2) Li+, Ni2+, Mn2+, Co2+ extraction by 0.04 M Na-Cyanex 272 in kerosene at O/A = 1, pH = 5, 25 °C. Scrub Ni and Li with Na2CO3 at pH = 9.0 and 12.0; (3) Strip Mn and Co with 0.1 M H2SO4 at O/A = 0.5 and precipitate MnCO3 at pH = 7.5 and Co(OH)2 at pH = 11.0; | 99.6 | 99.0 | 99.4 | 99.7 | [174] |
| Cu2+, Al3+, Li+, Ni2+, Mn2+, Co2+, SO42 | (1) D2EHPA in kerosene to extract Cu and Mn at O/A = 1, pH = 2.6–2.7, 25 °C; (2) PC-88A to separate Ni and Co at O/A = 1, pH = 4.25, 25 °C; (3) Stripping Co with 0.5 M oxalate; | - | 80 | - | - | [178] |
| Cu2+, Al3+, Li+, Ni2+, Mn2+, Co2+, Cl− | (1) Fe(OH)3 precipitation by NaOH at 95 °C and pH = 3.0–3.5; (2) MnO2 precipitation by 10% (NH4)2S2O8 at 70 °C and pH = 4; (3) Cu(OH)2 and Al(OH)3 precipitation at pH = 5.5; (4) 0.2 M Cyphos IL 102 extracted Co, and CoC2O4 was precipitated; (5) Ni separated from Li by NH3(aq.) and DMG at pH = 9; (6) Li2CO3 deposited by NaOH + Na2CO3 at 100 °C and pH = 11; | 99.6 | 98.6 | - | 99.9 | [181] |
| Li+, Ni2+, Mn2+, Co2+, citric | (1) Ni precipitated with 0.05 M DMG at pH = 6 and recycled as NiCl2 by further 1 M HCl treatment; (2) Co precipitated by (NH4)2C2O4 at pH = 6 and 55 °C; (3) Mn separated by 20 vol.% D2EHPA at O/A = 2, pH = 6; (4) Li recovered as Li3PO4 by 0.5 M Na3PO4; | 89 | 97 | 98 | 97 | [182] |
| Extraction by ion-exchange resins | ||||||
| Ni2+, Mn2+, Co2+, Mg2+, SO42− | Amberlite IRC 748 (carries iminodiacetate functional groups) at pH = 4–5 selectively extracts Ni and Co via automated titration column set-up; | - | 100 | 100 | - | [185] |
| Fe3+, Al3+, Mn2+, Ni2+, Co2+, Mg2+, SO42− | (1) Lewatit TP 220 separated Ni and Co; (2) 6.8 wt.% NH3(aq.) solution eluted Ni and Co; | - | 85 | 95 | - | [188] |
| Fe3+, Al3+, Zn2+, Li+, Ni2+, Mn2+, Co2+ | Using two-column set-up Dowex M4195 separated Ni at pH = 1.1 and Co at pH = 4.1; | - | 98.5 | 99.0 | - | [190] |
| Fe3+, Al3+, Cu2+, Li+, Ni2+, Mn2+, Co2+ | (1) Lewatit TP260 (contains aminomethylphosphonic groups) separated Li, Co, and Ni of battery grade purity; (2) Cu and Mn were eluted with 2 M H2SO4, Fe and Al—with 0.4 M K2C2O4; | 99.6 | 99.6 | 99.6 | - | [191] |
| Selective precipitation | ||||||
| Fe3+, Al3+, Cu2+, Li+, Co2+, Cl− | Subsequent addition of 40% NaOH until pH = 6 to remove impurities (Fe, Al, Cu); | - | 89 | - | - | [112] |
| Fe3+, Cu2+, Li+, Mn2+ Co2+, SO42− | (1) Removing Fe as sodium jarosite by 10 wt.% Na2SO4 at pH = 3, 95 °C for 2 h; (2) MnO2 deposition by (NH4)2S2O8 at pH = 4 and 70 °C; (3) Cu(OH)2 precipitated at pH = 5.5; (4) Co extraction from Li and Ni with 25 wt.% P507 in kerosene; (5) CoC2O4 precipitation by (NH4)2C2O4 at pH = 1.5; | 97 | 98 | 97 | 99 | [196] |
| Fe3+, Al3+, Cu2+, Li+, Ni2+, Mn2+, Co2+ | (1) Removing Fe, Al, and Cu by adding 4 M NaOH and 50 wt.% CaCO3 solution at pH = 6.5; (2) Extraction of Co with 0.5 M Cyanex 272 in kerosene at pH = 6.0, O/A = 2; (3) Stripping Co with 2 M H2SO4; | - | 92 | - | - | [198] |
| Al3+, Li+, Ni2+, Co2+ | (1) NaClO added to transit Co2+ to Co3+ (Co2O3), ClO-/Co2+ = 3, at pH = 3; (2) Ni(OH)2 deposited at pH = 11; (3) Ni(OH)2-contained precipitate washed with base to solubilize Al into Al(OH)4−; | - | 90 | 96 | - | [129] |
| Li+, Ni2+, Mn2+, Co2+, SO42− | (1) MnCO3 precipitation by saturated Na2CO3 at pH = 7.5 adjusted by 2.0 M NaOH; (2) NiCO3 precipitation by stirring for 1 h at 25 °C; (3) Co(OH)2 by saturated NaOH for 2 h at pH = 11–12; (4) Li2CO3 by Na2CO3 at pH = 12 for 1 h stirring; | 90 | 95 | 91 | 94 | [199] |
| Li+, Mn2+, Co2+, SO42− | (1) 1 M Na2S added to precipitate MnS and CoS; (2) MnS selective dissolution by proper amount of 0.05 M acetic acid; (3) Separation; Mn(OH)2 precipitation by 2 M NaOH; (4) Li3PO4 precipitation by 1 M Na3PO4; | - | 99 | - | 98 | [200] |
| Li+, Ni2+, Mn2+, Co2+, Cl− | (1) MnO2 recovery by KMnO4 (Mn2+/Mn7+ = 2) at pH = 2 and 40 °C; (2) Saturated NH3(aq.) solution is added to the leachate; (3) Ni(DMG)2 precipitation (DMG/[Ni(NH3)6]2+ = 2) at pH = 9 and 25 °C; then, can be recovered as Ni(OH)2 by 1 M NaOH at pH = 11; (4) Co(OH)3 recovery from [Co(NH3)6]3+ by 1 M NaOH at pH = 11; (5) Li2CO3 precipitation by Na2CO3 at 100 °C; | 80 | 97 | 97 | 98 | [203] |
| Cathode Material | Restoration Methodology | Performance of Restored Cathode | Ref. |
|---|---|---|---|
| Resynthesis | |||
| LiNi0.5Mn0.3Co0.2O2 | (1) Adjust the selected ratio of Ni, Mn, and Co; (2) Deposit hydroxides via NH3 and NaOH; (3) Co-grind the product with 3%-excess of Li2CO3; (4) Calcine at 500 °C for 5 h; then at 750–900 °C for 12 h; | 150 mAh g−1 at 0.1 C; 80% capacity retention after 100 cycles at 0.5 C; | [236] |
| LiNixMnyCozO2 | (1) Adjust Ni:Mn:Co ratio by nitrate precursors and pH to 8 by NH3(aq.); (2) After stirring at 80 °C calcine at 400 °C for 2 h and at 800 °C for 8 h; | 147 mAh g−1 at 0.5 C; 94% capacity retention after 100 cycles at 0.5 C; | [237] |
| LiNixMnyCozO2 | (1) Remove Fe3+ ions at pH = 3; (2) Adjust Ni:Mn:Co ratio by sulfate precursors; (3) Coprecipitate hydroxides at pH = 11; (4) Sintering with Li2CO3 at 900 °C for 15 h; | 130 mAh g−1 at 0.4 C; 82% capacity retention after 50 cycles at 0.4 C; | [239] |
| LiNixMnyCozO2 | (1) Adjust Ni:Mn:Co ratio by sulfate precursors; (2) Obtain a mixture of metal carbonates by NH3(aq.) and Na2CO3 at pH = 7.5 and 60 °C and holding for 12 h; (3) Calcine at 500 °C for 5 h; (4) Grind with Li2CO3 and sinter at 500 °C for 5 h and at 900 °C for 12 h; | 163 mAh g−1 at 0.1 C; 94% capacity retention after 50 cycles at 1 C; | [148] |
| LiNixMnyCozO2 | (1) Adjust metal ratios by acetate precursors; (2) Add NH3(aq.) at pH = 7 and obtain gel precursor at 80 °C; (3) Calcine at 450 °C for 5 h and sinter at 900 °C for 12 h; | 151 mAh g−1 at 0.2 C; 84% capacity retention after 150 cycles at 0.2 C; | [145] |
| Li1.2Ni0.13Mn0.54Co0.13O2 | (1) Adjust metal ratios by acetate precursors; (2) Precipitate metals by oxalic acid; (3) Heat hydrothermally at 200 °C for 8 h; (4) Calcine at 450 °C for 5 h and sinter 900 °C for 12 h; | 237 mAh g−1 at 0.5 C; 77% capacity retention after 50 cycles at 0.5 C; | [252] |
| Regeneration | |||
| LiCoO2 | Hydrothermally treated cathode in 5.0 M LiOH at 200 °C for 20 h; | 144 mAh g−1 at 0.2 C; 92% capacity retention after 40 cycles at 0.2 C; | [246] |
| LiNi0.5Co0.2Mn0.3O2 | (1) Cathode scraps heated at 400 °C for 6 h to burn out acetylene black; (2) Adjust metal ratio (5% Li excess) with lithium acetate; (3) Calcine at 500 °C for 5 h and at 900 °C for 12 h; | 164 mAh g−1 at 0.1 C; 89% capacity retention after 100 cycles at 1 C; | [243] |
| LiNi0.5Co0.2Mn0.3O2 | (1) Calcination at 550 °C for 4 h to remove binder and carbon black; (2) Regeneration by LiOH (14% excess); | 161 mAh g−1 at 0.1 C; 95% capacity retention after 50 cycles at 0.5 C; | [245] |
| LiNixCoyMnzO2 | (1) Separation of cathode active material from Al substrate, binder, and carbon by means of dimethyl carbonate and NMP; (2) Cathode is added to 4 M LiOH and autoclaved at 220 °C for 4 h; (3) Sintering with 5% Li2CO3 excess in O2 atmosphere at 850 °C for 4 h; | 157 mAh g−1 at 1 C; 79% capacity retention after 100 cycles at 1 C; | [244] |
| LiNi1/3Co1/3Mn1/3O2 | (1) Cathode material is separated from Al substrate, binder, and carbon by calcination; (2) Ball milling with Li2CO3 at Li/Me ratio of 1.2 and 500 rpm for 4 h; (3) Sintering at 800 °C for 10 h; | 165 mAh g−1 at 0.2 C; 80% capacity retention after 100 cycles at 0.2 C; | [247] |
| LiCoO2 | (1) Cathode material is immersed in 4 M LiOH+KOH solution; (2) Electrochemical dissolution and subsequent precipitation of LiCoO2 at 1 mA cm−2 and 40–100 °C; | 135 mAh g−1 at 0.2 C; 97% capacity retention after 50 cycles at 0.2 C; | [248] |
| LiCoO2 | (1) Spent LCO powder is placed with 2 M LiOH at S/L = 14; (2) Mixture is heated at 120 °C and sonicated at 1 kW for 10 h; | 131 mAh g−1 at 0.2 C; 98% capacity retention after 20 cycles at 0.2 C; | [252] |
| Method | Efficiency | Energy Consumption | Duration | Environmental Impact | Reuse of Reagents | Cost |
|---|---|---|---|---|---|---|
| Pyrometallurgy | ++++ | + | ++ | + | − | + |
| Inorganic acid leaching | ++++ | +++ | ++++ | ++ | ++ | ++ |
| Organic acid leaching | +++ | ++ | +++ | +++ | ++++ | +++ |
| Alkaline leaching | ++ | ++ | ++ | +++ | ++++ | +++ |
| Bioleaching | +++ | ++ | + | ++++ | + | ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhmetov, N.; Manakhov, A.; Al-Qasim, A.S. Li-Ion Battery Cathode Recycling: An Emerging Response to Growing Metal Demand and Accumulating Battery Waste. Electronics 2023, 12, 1152. https://doi.org/10.3390/electronics12051152
Akhmetov N, Manakhov A, Al-Qasim AS. Li-Ion Battery Cathode Recycling: An Emerging Response to Growing Metal Demand and Accumulating Battery Waste. Electronics. 2023; 12(5):1152. https://doi.org/10.3390/electronics12051152
Chicago/Turabian StyleAkhmetov, Nikita, Anton Manakhov, and Abdulaziz S. Al-Qasim. 2023. "Li-Ion Battery Cathode Recycling: An Emerging Response to Growing Metal Demand and Accumulating Battery Waste" Electronics 12, no. 5: 1152. https://doi.org/10.3390/electronics12051152
APA StyleAkhmetov, N., Manakhov, A., & Al-Qasim, A. S. (2023). Li-Ion Battery Cathode Recycling: An Emerging Response to Growing Metal Demand and Accumulating Battery Waste. Electronics, 12(5), 1152. https://doi.org/10.3390/electronics12051152






