From Bioimpedance to Volume Estimation: A Model for Edema Calculus in Human Legs
Abstract
:1. Introduction
2. Material and Methods
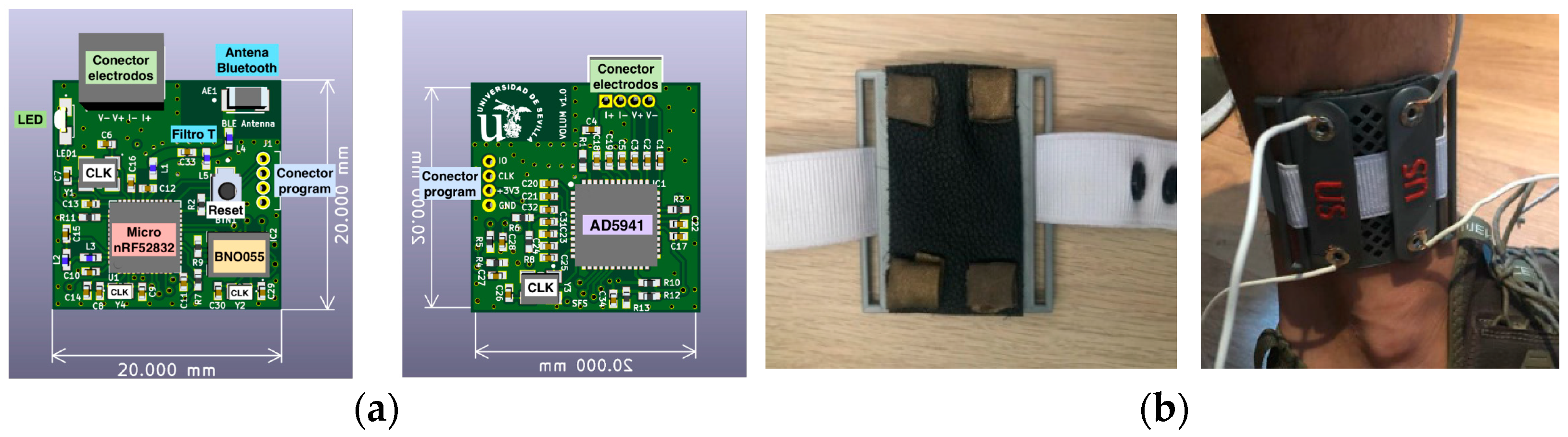
2.1. Wearable Bioimpedance System
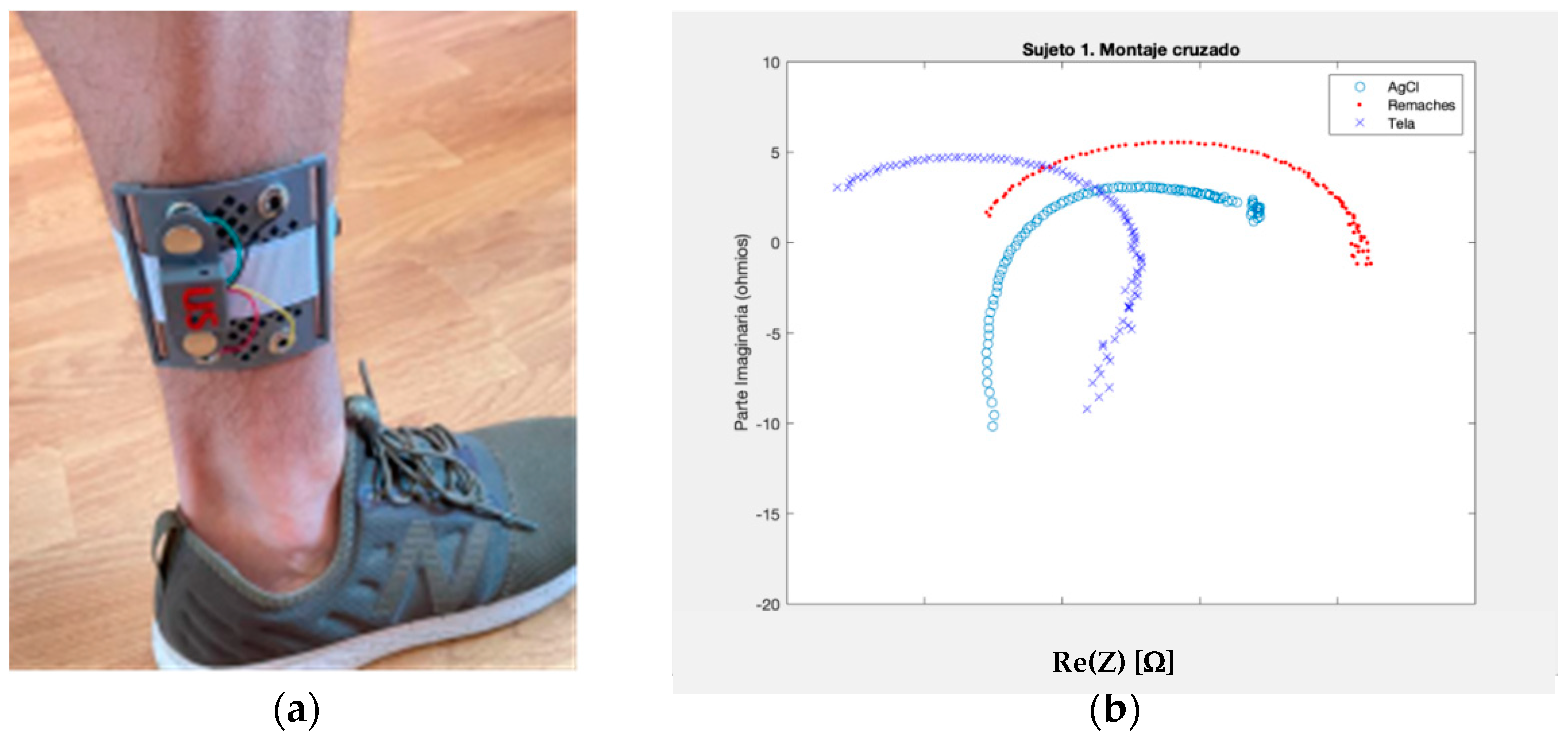
2.2. The Electrode Selection
2.3. The Wireless Communication
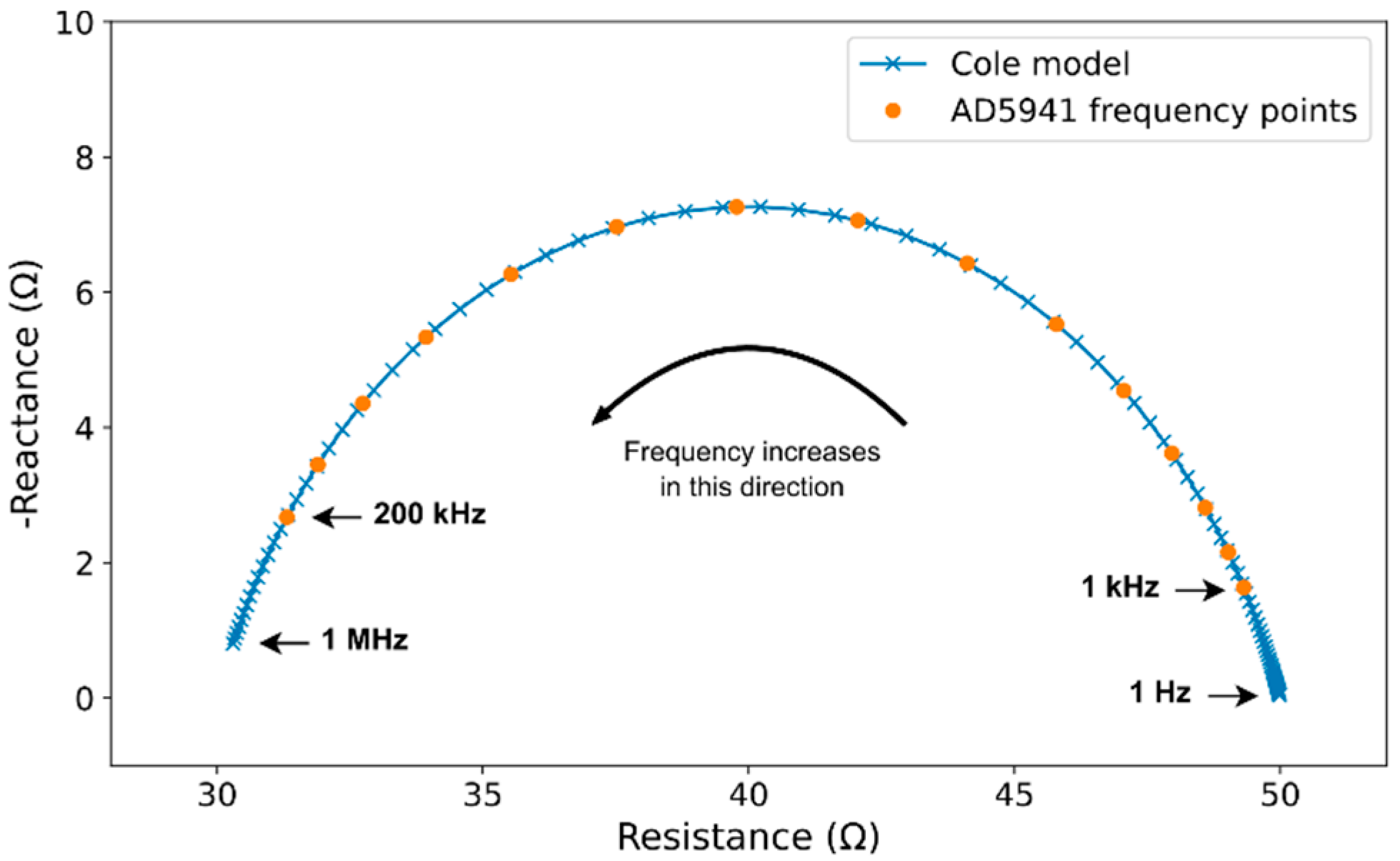
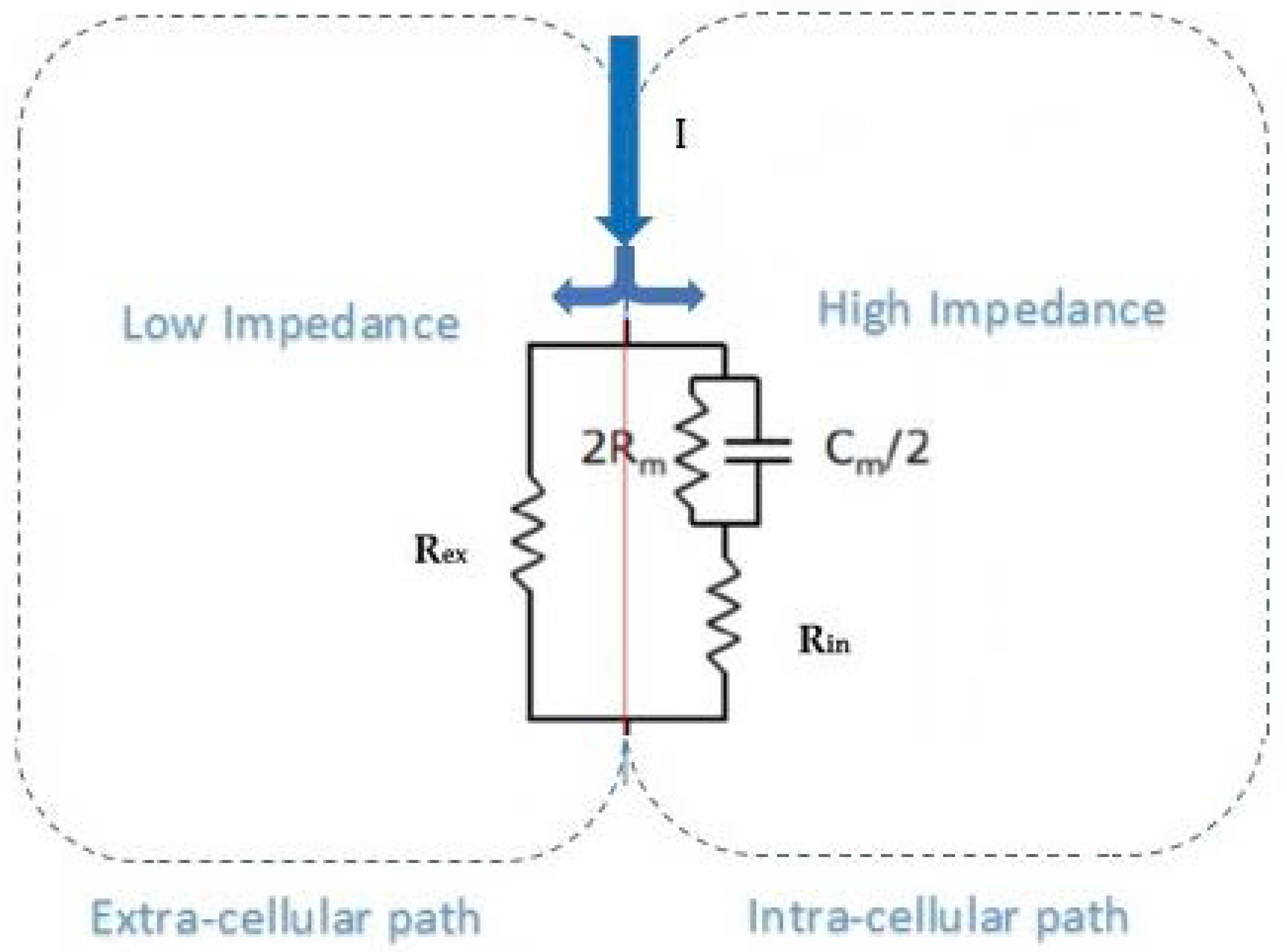
2.4. Bioimpedance Model
2.5. Pilot Test Design
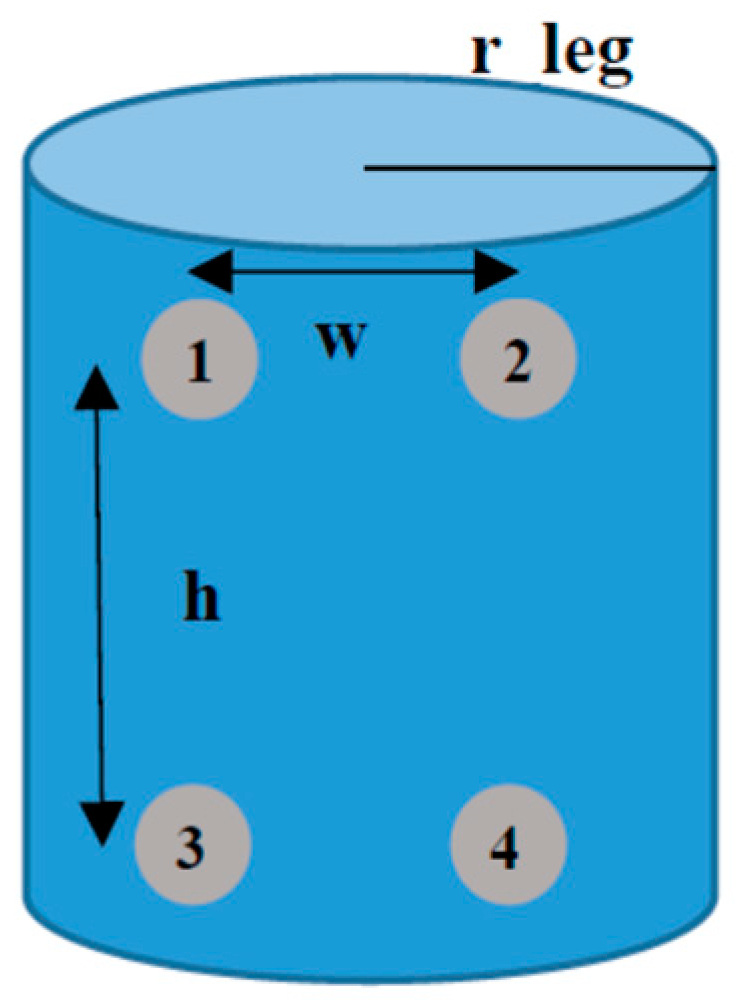
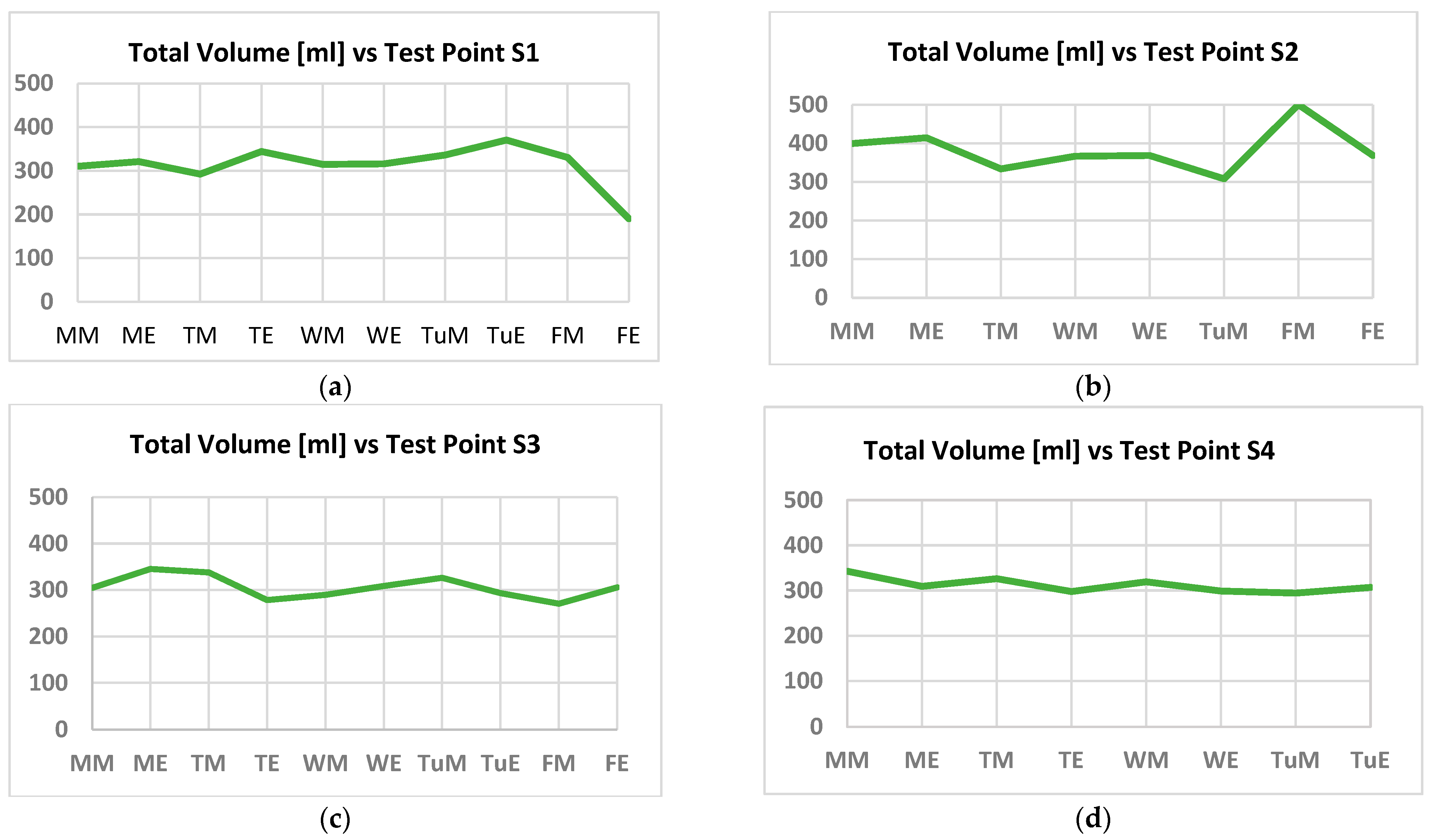
2.6. Leg Volume Calculus Procedure
- The total volume contained inside the leg ankle considered is VT. From geometrical view in Figure 2, a standard r_leg value can be considered for VT calculation. It was chosen to be 4 cm for the first evaluation. A height value of 6 cm is set. This volume is the contribution of the extracellular (Vex) and intracellular (Vin) contributions,VT = Vex + Vin
- When low frequencies (frequency approach to zero) are considered, the equivalent resistance of the volume under test in the leg is given by Rex. This is because all current lines of electric field travel through the extracellular liquid between electrodes 2 and 3, and none through intracellular paths due to the high impedance of cell membrane resistances and capacitances (Rm, Cm).
- Extracellular fluid is considered to be 1/3 of total body fluid in the human body. It can be considered a good approach that 37.5% of the volume in the human body is due to extracellular fluids.
- The calibration system is based on a basic cylinder of r_leg = 4 cm, and h = 6 cm. This volume contains an extracellular volume, Vex, due to extracellular liquids, mainly composed by water, and an intracellular volume, Vin, due to tissues and intracellular liquids. Our approach considers that increments in VT are due to increments in Vex, as a consequence of liquid accumulation, maintaining constant the Vin contribution. For this case: VT = 301.10 mL, and Vin = 188.44 mL for the calibration.
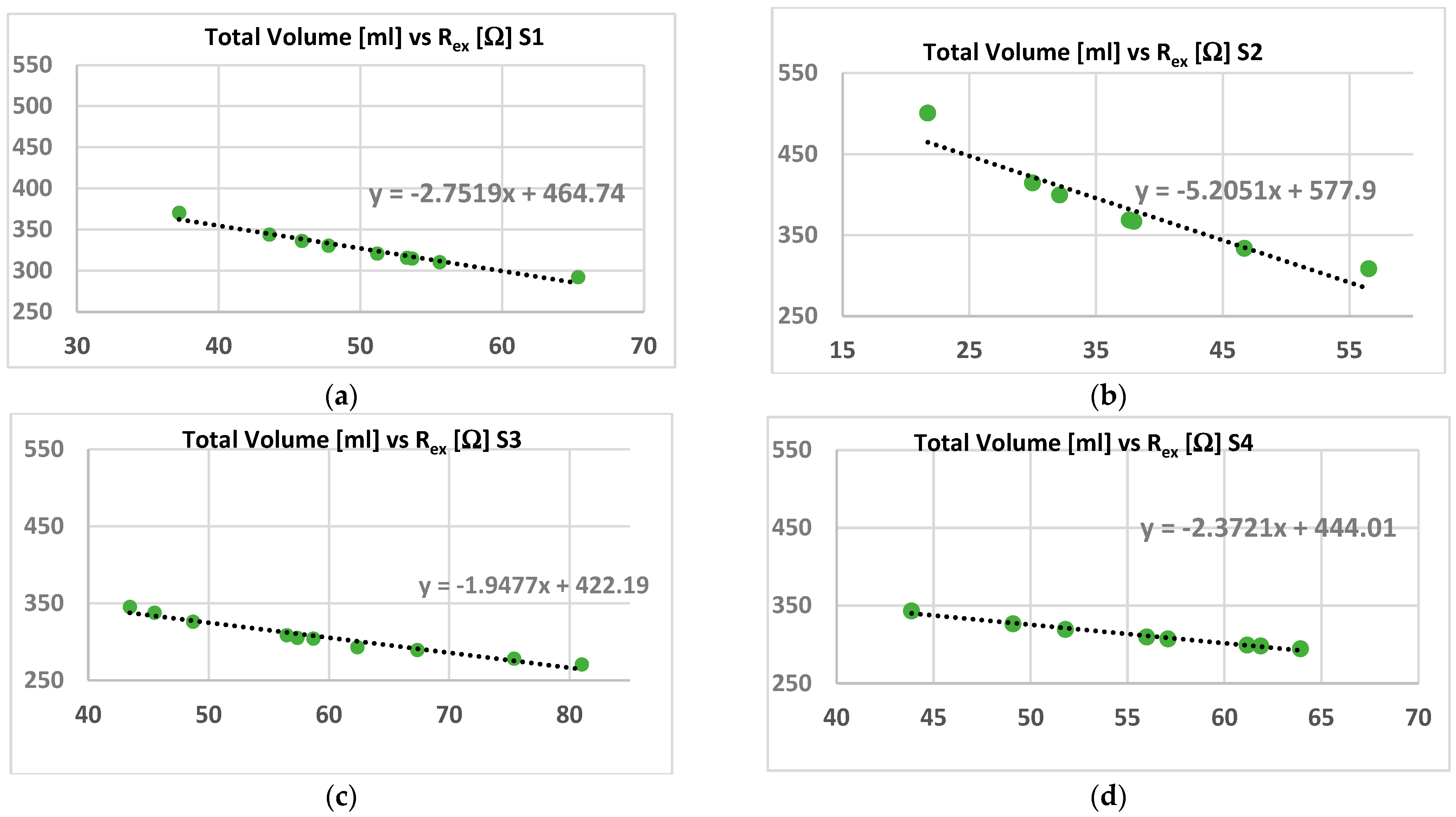
- It is considered the dependence between resistance and volume given by:where k is a constant that includes the conductivity parameter (σ) and the geometry factors [40]. The values of the extracellular volume due to fluids’ (water) variations can be estimated by knowing the extracellular resistance. This can be considered a relationship between Rex and Vex for low frequency conditions, being Vin constant in these cases.Rex = k/Vex
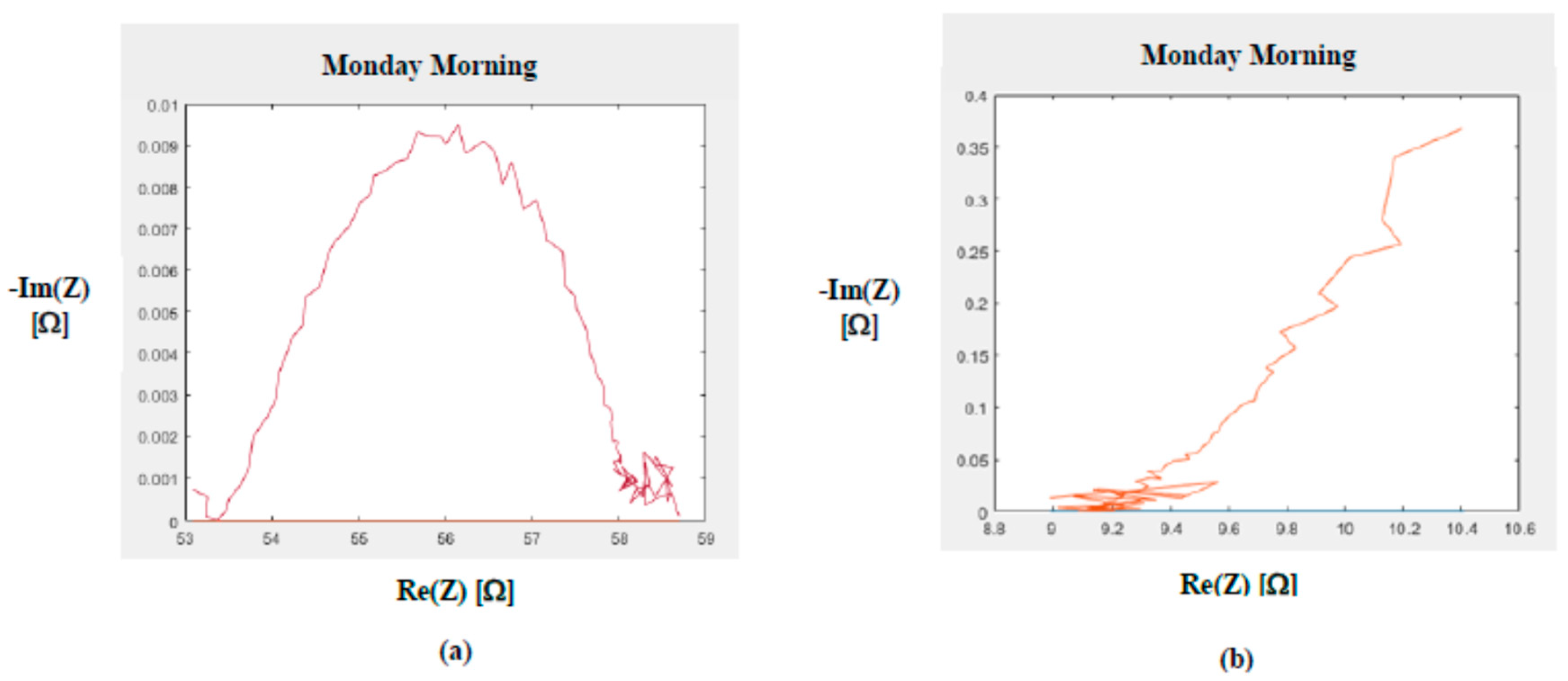
- The Rex and Rin resistances can be calculated from the Cole–Cole parameters as:Rex = R_0Rin || Rex = R_inf
3. Results
3.1. Healthy Patients
3.2. Heart Failure Patients
3.3. Continuous Time Test
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tanai, E.; Frantz, S. Pathophysiology of Heart Failure. Compr. Physiol. 2015, 6, 187–214. [Google Scholar] [CrossRef]
- McMurray, J.J.; Pfeffer, M.A. Heart failure. Lancet 2005, 365, 1877–1889. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E. Cardiovascular Medicine at the Turn of the Millennium: Triumphs, Concerns, and Opportunities. N. Engl. J. Med. 1997, 337, 1360–1369. [Google Scholar] [CrossRef]
- Dharmarajan, K.; Rich, M.W. Epidemiology, Pathophysiology, and Prognosis of Heart Failure in Older Adults. Heart Fail. Clin. 2017, 13, 417–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vos, T.; Allen, C.; Arora, M.; Barber, R.M.; Bhutta, Z.A.; Brown, A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef] [Green Version]
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef] [PubMed]
- Gerber, Y.; Weston, S.A.; Redfield, M.M.; Chamberlain, A.M.; Manemann, S.M.; Jiang, R.; Killian, J.M.; Roger, V.L. A Contemporary Appraisal of the Heart Failure Epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern. Med. 2015, 175, 996–1004. [Google Scholar] [CrossRef] [Green Version]
- Braunschweig, F.; Cowie, M.; Auricchio, A. What are the costs of heart failure? Europace 2011, 13 (Suppl. 1), ii13–ii17. [Google Scholar] [CrossRef]
- Stewart, S.; Macintyre, K.; Holec, D.J.; Capewellb, S.; McMurray, J.J.V. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur. J. Heart Fail. 2001, 3, 315–322. [Google Scholar] [CrossRef]
- Kurmani, S.; Squire, I. Acute Heart Failure: Definition, Classification and Epidemiology. Curr. Heart Fail. Rep. 2017, 14, 385–392. [Google Scholar] [CrossRef] [Green Version]
- Marti, C.N.; Georgiopoulou, V.V.; Kalogeropoulos, A.P. Acute heart failure: Patient characteristics and pathophysiology. Curr. Heart Fail. Rep. 2013, 10, 427–433. [Google Scholar] [CrossRef] [Green Version]
- Adamson, P.B. Pathophysiology of the transition from chronic compensated and acute decompensated heart failure: New insights from continuous monitoring devices. Curr. Heart Fail. Rep. 2009, 6, 287–292. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [Green Version]
- Klersy, C.; De Silvestri, A.; Gabutti, G.; Regoli, F.; Auricchio, A. A Meta-Analysis of Remote Monitoring of Heart Failure Patients. J. Am. Coll. Cardiol. 2009, 54, 1683–1694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitsiou, S.; Paré, G.; Jaana, M. Effects of Home Telemonitoring Interventions on Patients With Chronic Heart Failure: An Overview of Systematic Reviews. J. Med. Internet Res. 2015, 17, e63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inglis, S.C.; Clark, R.A.; Dierckx, R.; Prieto-Merino, D.; Cleland, J.G. Structured telephone support or non-invasive telemonitoring for patients with heart failure. In Cochrane Database of Systematic Reviews 2015; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef] [Green Version]
- Bashi, N.; Karunanithi, M.; Fatehi, F.; Ding, H.; Walters, D. Remote Monitoring of Patients With Heart Failure: An Overview of Systematic Reviews. J. Med. Internet Res. 2017, 19, e18. [Google Scholar] [CrossRef] [PubMed]
- Ali, O.; Hajduczok, A.G.; Boehmer, J.P. Remote Physiologic Monitoring for Heart Failure. Curr. Cardiol. Rep. 2020, 22, 68. [Google Scholar] [CrossRef] [PubMed]
- Santaguida, P.L.; Don-Wauchope, A.; Oremus, M.; McKelvie, R.; Ali, U.; Hill, S.A.; Balion, C.; Booth, R.A.; Brown, J.A.; Bustamam, A.; et al. BNP and NT-proBNP as prognostic markers in persons with acute decompensated heart failure: A systematic review. Heart Fail. Rev. 2014, 19, 453–470. [Google Scholar] [CrossRef]
- Rodríguez-Moyano, M.; Díaz, I.; Dionisio, N.; Xuexin, Z.; Avila-Medina, J.; Calderón-Sánchez, E.M.; Trebak, M.; Rosado, J.A.; Ordoñez, A.; Smani, T. Urotensin-II promotes vascular smooth muscle cell proliferation through store-operated calcium entry and EGFR transactivation. Cardiovasc. Res. 2013, 100, 297–306. [Google Scholar] [CrossRef]
- Grieco, P.; Gomez-Monterrey, I. Natural and synthetic peptides in the cardiovascular diseases: An update on diagnostic and therapeutic potentials. Arch. Biochem. Biophys. 2018, 662, 15–32. [Google Scholar] [CrossRef]
- Ouwerkerk, W.; Voors, A.A.; Zwinderman, A.H. Factors Influencing the Predictive Power of Models for Predicting Mortality and/or Heart Failure Hospitalization in Patients With Heart Failure. JACC Heart Fail. 2014, 2, 429–436. [Google Scholar] [CrossRef]
- De la Cámara, A.G.; GuerraVales, J.M.; Tapia, P.M.; Esteban, E.A.; del Pozo, S.V.F.; Sandubete, E.C.; Ortega, F.J.M.; Puerto, A.N.; Marín-León, I.; PR Group. Role of biological and non biological factors in congestive heart failure mortality: PREDICE-SCORE: A clinical prediction rule. Cardiol. J. 2012, 19, 578–585. [Google Scholar] [CrossRef] [Green Version]
- Showkat, I.; Khanday, F.A.; Beigh, M.R. A review of bio-impedance devices. Med. Biol. Eng. Comput. 2023, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Pérez, P.; Huertas, G.; Maldonado-Jacobi, A.; Martín, M.; Serrano, J.A.; Olmo, A.; Daza, P.; Yúfera, A. Sensing Cell-Culture Assays with Low-Cost Circuitry. Sci. Rep. 2018, 8, 8841. [Google Scholar] [CrossRef] [Green Version]
- Yufera, A.; Rueda, A.; Munoz, J.; Doldan, R.; Leger, G.; Rodriguez-Villegas, E. A tissue impedance measurement chip for myocardial ischemia detection. IEEE Trans. Circuits Syst. I Regul. Pap. 2005, 52, 2620–2628. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Squillace, G.; Smeets, C.; Vandecasteele, M.; Grieten, L.; de Francisco, R.; Van Hoof, C. Congestive heart failure patient monitoring using wearable Bio-impedance sensor technology. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Milan, Italy, 25–29 August 2015; pp. 438–441. [Google Scholar] [CrossRef]
- Grimnes, S.; Martinsen, Ø. Bioimpedance and Bioelectricity Basics 2014, 3rd ed.; Academic Press: Cambridge, MA, USA, 2014; ISBN 9780124114708. [Google Scholar]
- Hankinson, S.J.; Williams, C.H.; Ton, V.-K.; Gottlieb, S.S.; Hong, C.C. Should we overcome the resistance to bioelectrical impedance in heart failure? Expert Rev. Med. Devices 2020, 17, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hanzaee, F.F.; Jiang, D.; Bayford, R.H.; Demosthenous, A. Electrical Impedance Tomography for Biomedical Applications: Circuits and Systems Review. IEEE Open J. Circuits Syst. 2021, 2, 380–397. [Google Scholar] [CrossRef]
- Xu, J.; Hong, Z. Low Power Bio-Impedance Sensor Interfaces: Review and Electronics Design Methodology. IEEE Rev. Biomed. Eng. 2020, 15, 23–35. [Google Scholar] [CrossRef]
- Lin, Q.; Song, S.; Castro, I.D.; Jiang, H.; Konijnenburg, M.; van Wegberg, R.; Biswas, D.; Stanzione, S.; Sijbers, W.; Van Hoof, C.; et al. Wearable Multiple Modality Bio-Signal Recording and Processing on Chip: A Review. IEEE Sen. J. 2020, 21, 1108–1123. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Yasumura, K.; Nishida, H.; Inoue, H.; Furukawa, T.; Shinouchi, K.; Miura, H.; Miyazaki, K.; Hamano, G.; Koide, M.; et al. Quantitative Assessment of Fluid Accumulation Using Bioelectrical Impedance Analysis in Patients With Acute Decompensated Heart Failure. Circ. J. 2015, 79, 2616–2622. [Google Scholar] [CrossRef] [Green Version]
- Lyons, K.J.; Bischoff, M.K.; Fonarow, G.; Horwich, T.B. Noninvasive Bioelectrical Impedance for Predicting Clinical Outcomes in Outpatients With Heart Failure. Crit. Pathways Cardiol. A J. Evid. -Based Med. 2017, 16, 32–36. [Google Scholar] [CrossRef]
- Pittella, E.; Piuzzi, E.; Rizzuto, E.; Pisa, S.; Del Prete, Z. Metrological characterization of a combined bio-impedance plethysmograph and spectrometer. Measurement 2018, 120, 221–229. [Google Scholar] [CrossRef]
- Lindholm, D.; Fukaya, E.; Leeper, N.J.; Ingelsson, E. Bioimpedance and New-Onset Heart Failure: A Longitudinal Study of >500 000 Individuals From the General Population. J. Am. Heart Assoc. 2018, 7, e008970. [Google Scholar] [CrossRef] [Green Version]
- Zink, M.D.; König, F.; Weyer, S.; Willmes, K.; Leonhardt, S.; Marx, N.; Napp, A. Segmental Bioelectrical Impedance Spectroscopy to Monitor Fluid Status in Heart Failure. Sci. Rep. 2020, 10, 3577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannon, T.; Choi, J. Development of a Segmental Bioelectrical Impedance Spectroscopy Device for Body Composition Measurement. Sensors 2019, 19, 4825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mabrouk, S.; Hersek, S.; Jeong, H.K.; Whittingslow, D.; Ganti, V.G.; Wolkoff, P.; Inan, O.T. Robust Longitudinal Ankle Edema Assessment Using Wearable Bioimpedance Spectroscopy. IEEE Trans. Biomed. Eng. 2019, 67, 1019–1029. [Google Scholar] [CrossRef]
- Hoffer, E.C.; Meador, C.K.; Simpson, D.C. Correlation of whole-body impedance with total body water volume. J. Appl. Physiol. 1969, 27, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Hersek, S.; Toreyin, H.; Teague, C.N.; Millard-Stafford, M.L.; Jeong, H.-K.; Bavare, M.M.; Wolkoff, P.; Sawka, M.N.; Inan, O.T. Wearable Vector Electrical Bioimpedance System to Assess Knee Joint Health. IEEE Trans. Biomed. Eng. 2016, 64, 2353–2360. [Google Scholar] [CrossRef]
- Hafid, A.; Benouar, S.; Kedir-Talha, M.; Abtahi, F.; Attari, M.; Seoane, F. Full Impedance Cardiography Measurement Device Using Raspberry PI3 and System-on-Chip Biomedical Instrumentation Solutions. IEEE J. Biomed. Health Inform. 2017, 22, 1883–1894. [Google Scholar] [CrossRef]
- Leonov, V.; Lee, S.; Londergan, A.; Martin, R.A.; De Raedt, W.; Van Hoof, C. Bioimpedance Method for Human Body Hydration Assessment. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 6036–6039. [Google Scholar] [CrossRef]
- Medrano, J.; Yúfera, A. Real-Time Monitoring Prognostic Value of Volume with BI Test in Patients with Acute HF. (HEART-FAIL VOLUM); DTS19/00134 and DTS19/00137; Instituto Carlos III: Madrid, Spain, 2019. [Google Scholar]
- Said, T.; Varadan, V.V. Variation of Cole-Cole model parameters with the complex permittivity of biological tissues. In Proceedings of the IEEE MTT-S International Microwave Symposium Digest, Boston, MA, USA, 7–12 June 2009; pp. 1445–1448. [Google Scholar] [CrossRef]
- Ayllon, D.; Seoane, F.; Gil-Pita, R. Cole equation and parameter estimation from electrical bioimpedance spectroscopy measurements—A comparative study. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 3779–3782. [Google Scholar] [CrossRef]
- Puertas, M.; Gimenez, L.; Perez, A.; Scagliusi, S.F.; Perez, P.; Olmo, A.; Huertas, G.; Medrano, J.; Yufera, A. Modeling Edema Evolution With Electrical Bioimpedance: Application to Heart Failure Patients. In Proceedings of the 2021 XXXVI Conference on Design of Circuits and Integrated Systems (DCIS), Vila do Conde, Portugal, 24–26 November 2021; pp. 1–6. [Google Scholar] [CrossRef]
- Blanco-Almazan, D.; Groenendaal, W.; Catthoor, F.; Jane, R. Wearable Bioimpedance Measurement for Respiratory Monitoring During Inspiratory Loading. IEEE Access 2019, 7, 89487–89496. [Google Scholar] [CrossRef]
- Ferreira, A.G.; Fernandes, D.; Branco, S.; Monteiro, J.L.; Cabral, J.; Catarino, A.P.; Rocha, A.M. A smart wearable system for sudden infant death syndrome monitoring. In Proceedings of the 2016 IEEE International Conference on Industrial Technology (ICIT), Taipei, Taiwan, 14–17 March 2016; pp. 1920–1925. [Google Scholar]
- Corchia, L.; Monti, G.; Raheli, F.; Candelieri, G.; Tarricone, L. Dry Textile Electrodes for Wearable Bio-Impedance Analyzers. IEEE Sen. J. 2020, 20, 6139–6147. [Google Scholar] [CrossRef]
- Hong, S.; Lee, J.; Yoo, H.-J. Wearable lung-health monitoring system with electrical impedance tomography. In Proceedings of the 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC 2015), Milan, Italy, 25–29 August 2015; pp. 1707–1710. [Google Scholar] [CrossRef]
- Ferreira, J.; Pau, I.; Lindecrantz, K.; Seoane, F. A Handheld and Textile-Enabled Bioimpedance System for Ubiquitous Body Composition Analysis. An Initial Functional Validation. IEEE J. Biomed. Health Inform. 2016, 21, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Reichmuth, M.; Schurle, S.; Magno, M. A Non-invasive Wearable Bioimpedance System to Wirelessly Monitor Bladder Filling. In Proceedings of the Design, Automation & Test in Europe Conference & Exhibition, Grenoble, France, 9–13 March 2020; pp. 338–341. [Google Scholar] [CrossRef]
- Joutsen, A.S.; Kaappa, E.S.; Karinsalo, T.J.; Vanhala, J. Dry electrode sizes in recording ECG and heart rate in wearable applications. IFMBE Proc. 2017, 65, 735–738. [Google Scholar] [CrossRef]
- Gong, W.Y.; Lv, J.H.; Wang, Y.; Sha, H.; Zhao, S.; Ren, C.S. The Impedance Property of Electrode Used in Electrical Bio-Impedance Measurement. In Proceedings of the 3rd International Conference on Bioinformatics and Biomedical Engineering, Beijing, China, 11–13 June 2009; pp. 1–3. [Google Scholar] [CrossRef]
- Nescolarde, L.; Lukaski, H.; De Lorenzo, A.; De-Mateo-Silleras, B.; Redondo-Del-Río, M.P.; Camina-Martín, M.A. Different displacement of bioimpedance vector due to Ag/AgCl electrode effect. Eur. J. Clin. Nutr. 2016, 70, 1401–1407. [Google Scholar] [CrossRef] [Green Version]
- National Institutes of Health. Bioelectrical impedance analysis in body composition measurement. NIH Technical Assessment Statement 1994. Am. J. Clin. Nutr. 1996, 64, 524S–532S. [Google Scholar]
- Kusche, R.; Kaufmann, S.; Ryschka, M. Dry electrodes for bioimpedance measurements—Design, characterization and comparison. arXiv 2019, arXiv:1910.05033. [Google Scholar] [CrossRef] [Green Version]
- Marquez, J.C.; Seoane, F.; Valimaki, E.; Lindecrantz, K. Textile electrodes in electrical bioimpedance measurements—A comparison with conventional Ag/AgCl electrodes. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2009, 2009, 4816–4819. [Google Scholar] [CrossRef]
- Fernández-Scagliusi, S. Sistema para Medida de Volumen Basado en Espectroscopia de Bioimpedancia. Master’s Thesis, Departamento de Tecnología Electrónica, Universidad de Sevilla, Sevilla, Spain, 2020. [Google Scholar]
- Albaugh, M.; Argote, P.; Patel, N.; Patel, R.; Boppana, S.; Ocken, A.; Masquelin, A.; Hoilett, O.; Miller, D.; Drakopoulos, M. Predictive Wear Matter of Manufacture of Compression Legging System and Associated Uses Cross-Reference to Related Applications 2020. Patent WO2020180919A1, 10 September 2020. DW2020077. [Google Scholar]
- Chahine, T.; Aitken, S.; Straka, A.; Alizadeh-Meghrazi, M.; Myant Inc. Multi-Functional Tubular Worn Garment 2029. Patent WO2019134033A2, 11 July 2019. DW201955. [Google Scholar]
- Buyya, R.; Dastjerdi, A.V. Internet of Things: Principles and Paradigms; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–354. [Google Scholar] [CrossRef]
- Rodriguez-Villegas, E.; Iranmanesh, S.; Imtiaz, S.A. Wearable Medical Devices: High-Level System Design Considerations and Tradeoffs. IEEE Solid-State Circuits Mag. 2018, 10, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Bluetooth® Technology Website. Available online: https://www.bluetooth.com/ (accessed on 1 February 2023).
- Lin, Z.; Ye, F.; Qin, W.; Cao, X.; Wang, Y.; Hu, R.; Yan, R.; Qin, Y.; Yi, T.; Hong, Z. A low-power, wireless, real-time, wearable healthcare system. In Proceedings of the 2016 IEEE MTT-S International Wireless Symposium, IWS 2016, Shanghai, China, 14–16 March 2016. [Google Scholar] [CrossRef]
- Dementyev, A.; Hodges, S.; Taylor, S.; Smith, J. Power consumption analysis of Bluetooth Low Energy, ZigBee and ANT sensor nodes in a cyclic sleep scenario. In Proceedings of the 2013 IEEE International Wireless Symposium, IWS 2013, Beijing, China, 14–18 April 2013. [Google Scholar] [CrossRef]

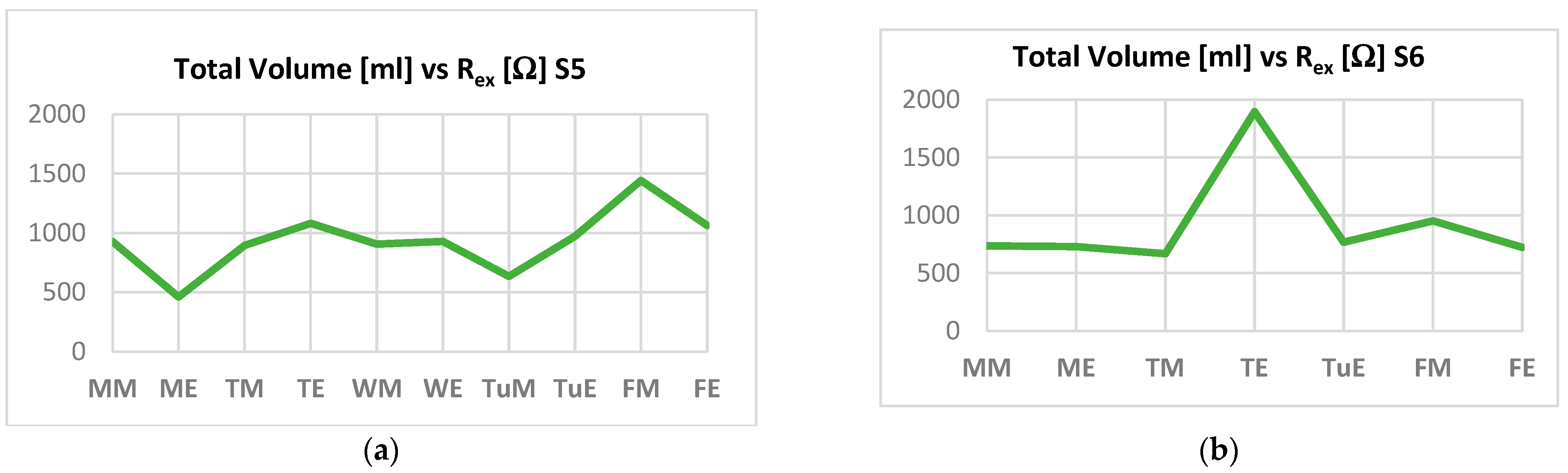

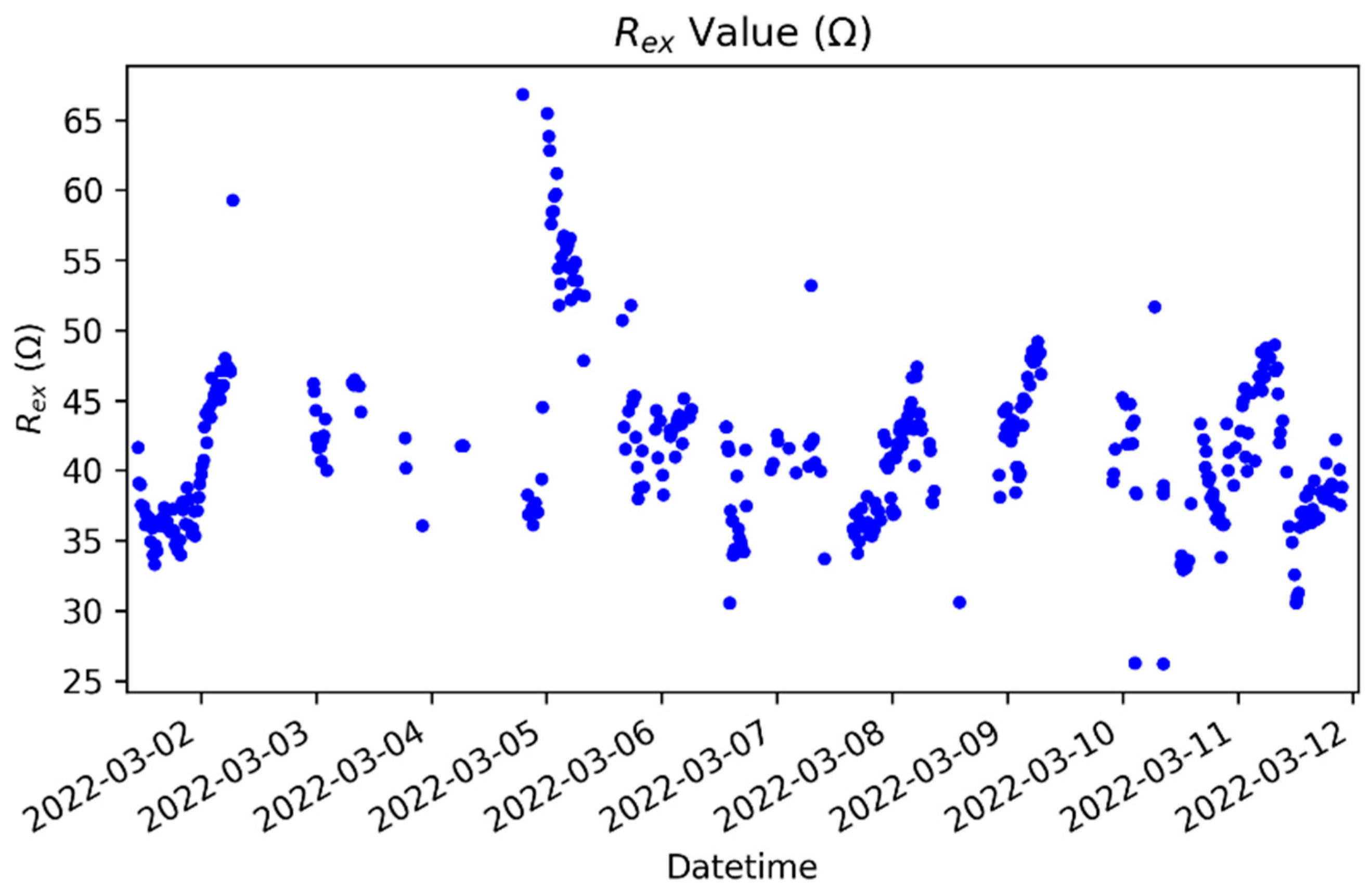
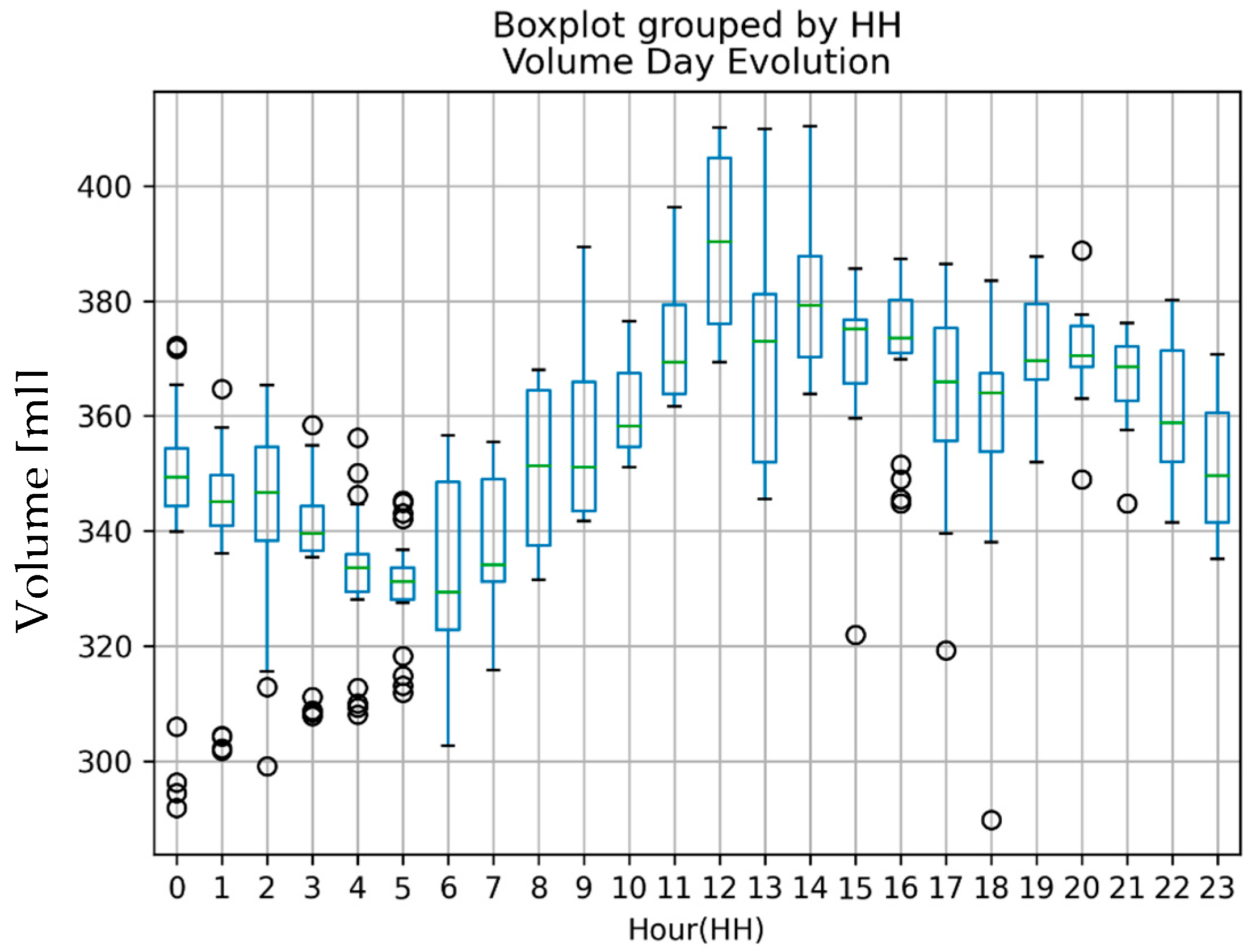
| Variable | Controls | HF Patients |
|---|---|---|
| Subjects | 4 | 2 |
| Mean age (years) | 60 | 79.50 |
| Male | 2 | 2 |
| Mean weight (kg) | 73.5 | 89.90 |
| Mean height (m) | 1.68 | 1.73 |
| Mean heart rate (bpm) | 70.95 | 70.60 |
| Mean systolic blood pressure (mmHg) | 118.98 | 135.40 |
| Mean diastolic blood pressure (mmHg) | 65.93 | 66.45 |
| Mean diuresis (mL) | 1572.75 | 1627.80 |
| Mean temperature (°C) | 36.27 | 36.23 |
| Mean oxygen saturation (%) | 97.30 | 93.00 |
| Mean hemoglobin (g/dL) | 14.05 | 10.15 |
| Mean creatinine (mg/dL) | 0.92 | 1.36 |
| Mean urea (mg/dL) | 31.5 | 54.00 |
| Mean sodium (mEq/L) | 142.5 | 134.00 |
| Mean NT-proBNP (pg/mL) | 131.1 | 2143.00 |
| Comorbid pathologies (ICD-10) | N/A | B18.2; E11.8; E66; E78.5; I08.3; I10; I11.0; I25.9; I48.2; I50.3; J44.9; K74.6; N18.3 |
| Test Point | R_0 [Ω] | R_inf [Ω] | ||||||
|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S1 | S2 | S3 | S4 | |
| MM | 55.6 | 32.1 | 58.4 | 43.9 | 41.2 | 24.4 | 52.1 | 30.8 |
| ME | 51.1 | 30.0 | 43.1 | 56.0 | 24.5 | 24.7 | 37.8 | 39.3 |
| TM | 65.3 | 46.7 | 45.3 | 44.1 | 47.7 | 34.1 | 38.8 | 32.8 |
| TE | 43.6 | na | 75.3 | 351.4 | 25.5 | na | 59.9 | 49.1 |
| WM | 53.6 | 38.0 | 66.9 | 61.9 | 41.4 | 28.5 | 55.3 | 41.4 |
| WE | 53.3 | 37.7 | 56.3 | 51.8 | 4.1 | 27.7 | 49.2 | 36.6 |
| TuM | 45.9 | 56.5 | 49.1 | 61.2 | 33.0 | 39.2 | 41.2 | 40.1 |
| TuE | 37.2 | na | 64.7 | 63.9 | 32.4 | na | 44.2 | 42.1 |
| FM | 47.7 | 21.7 | 82.3 | 57.1 | 34.7 | 34.0 | 62.8 | 39.0 |
| FE | 4260.5 | 37.6 | 57.9 | 802.5 | 45.0 | 27.7 | 49.3 | 56.4 |
| Test Point | Rex [Ω] | Rin [Ω] | ||||||
|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S1 | S2 | S3 | S4 | |
| MM | 55.6 | 32.1 | 58.4 | 43.8 | 159.9 | 102.3 | 477.7 | −122.8 |
| ME | 51.2 | 30,0 | 43.1 | 55.7 | 47.2 | 139.5 | 305.2 | 159.9 |
| TM | 65.4 | 46.7 | 45.3 | 48.8 | 176.3 | 126.1 | 270.2 | 128.7 |
| TE | 43.6 | na | 75.3 | 254.2 | 61.8 | na | 293.4 | 45.2 |
| WM | 53.6 | 38.0 | 66.9 | 61.4 | 180.6 | 113.7 | 319.9 | 161.4 |
| WE | 53.3 | 37.7 | 56.3 | 51.7 | 4.4 | 104.5 | 392.2 | 149.3 |
| TuM | 45.9 | 56.5 | 49.1 | 61.9 | 117.6 | 127.9 | 256.1 | 144.5 |
| TuE | 37.2 | na | 64.7 | 63.3 | 249.6 | na | 140.2 | 154.9 |
| FM | 47.7 | 21.7 | 82.3 | 56.6 | 127.0 | −60.0 | 265.4 | 166.3 |
| FE | 4260.5 | 37.6 | 57.9 | 320.8 | 45.5 | 104.8 | 331.0 | 44.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scaliusi, S.F.; Gimenez, L.; Pérez, P.; Martín, D.; Olmo, A.; Huertas, G.; Medrano, F.J.; Yúfera, A. From Bioimpedance to Volume Estimation: A Model for Edema Calculus in Human Legs. Electronics 2023, 12, 1383. https://doi.org/10.3390/electronics12061383
Scaliusi SF, Gimenez L, Pérez P, Martín D, Olmo A, Huertas G, Medrano FJ, Yúfera A. From Bioimpedance to Volume Estimation: A Model for Edema Calculus in Human Legs. Electronics. 2023; 12(6):1383. https://doi.org/10.3390/electronics12061383
Chicago/Turabian StyleScaliusi, Santiago F., Luis Gimenez, Pablo Pérez, Daniel Martín, Alberto Olmo, Gloria Huertas, F. Javier Medrano, and Alberto Yúfera. 2023. "From Bioimpedance to Volume Estimation: A Model for Edema Calculus in Human Legs" Electronics 12, no. 6: 1383. https://doi.org/10.3390/electronics12061383
APA StyleScaliusi, S. F., Gimenez, L., Pérez, P., Martín, D., Olmo, A., Huertas, G., Medrano, F. J., & Yúfera, A. (2023). From Bioimpedance to Volume Estimation: A Model for Edema Calculus in Human Legs. Electronics, 12(6), 1383. https://doi.org/10.3390/electronics12061383









