Hydrogen Energy in Electrical Power Systems: A Review and Future Outlook
Abstract
:1. Introduction
2. Current Status of Hydrogen Production and Storage
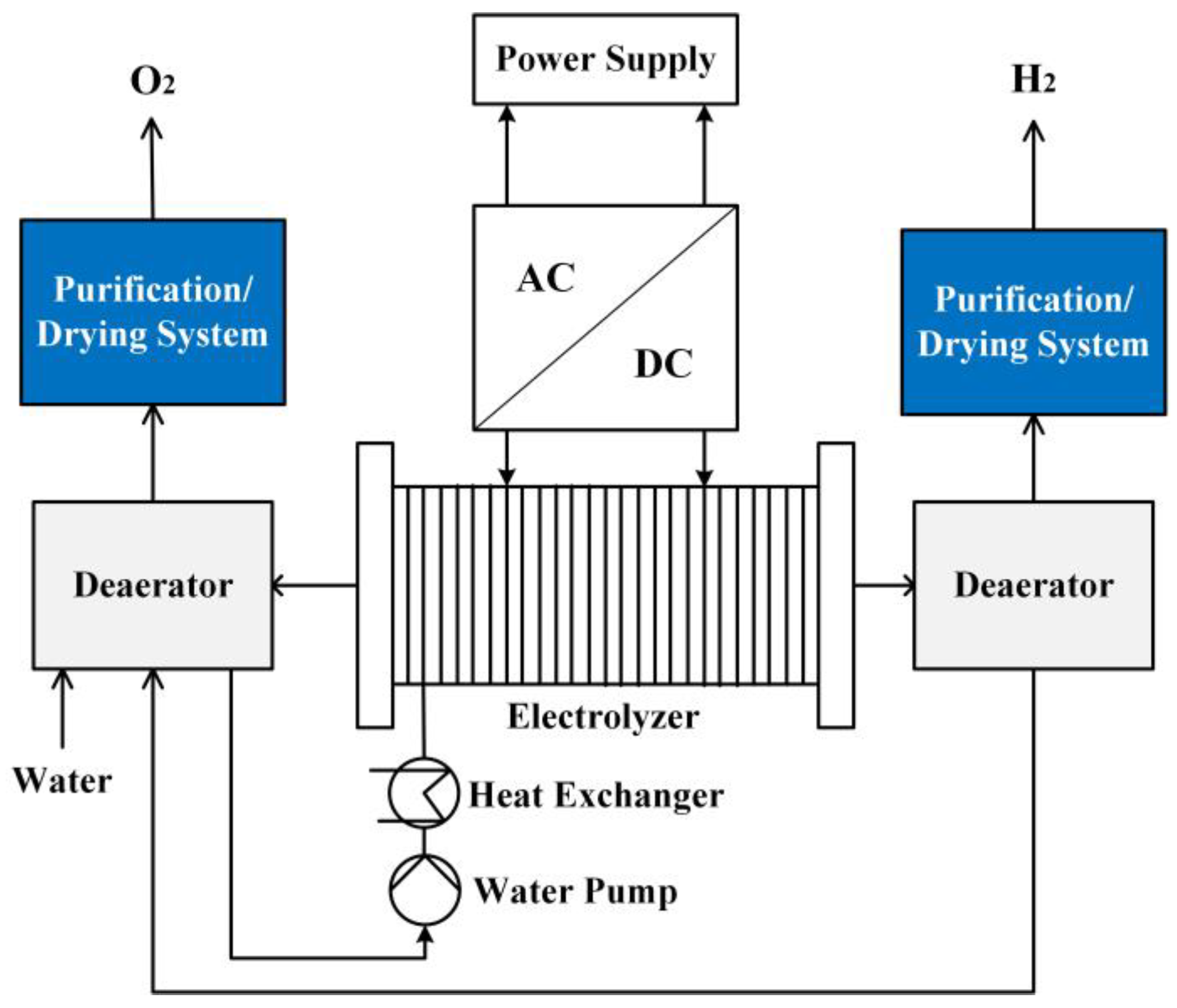
2.1. Review of Production of Hydrogen from Electrolytic Water
- (a)
- Technology for electrolyzing water
- (b)
- Electrolyzer technology
2.2. Developments in Hydrogen Storage Technologies
- (a)
- Compressed Gaseous Hydrogen
- (b)
- Liquid Hydrogen
- (c)
- Chemical Hydrogen Carriers
- (d)
- Metal Hydrides
- (e)
- Solid Hydrides
2.3. Application of Integration of Hydrogen Storage with Renewable Energy Sources
3. Key Technologies for Hydrogen Electrification
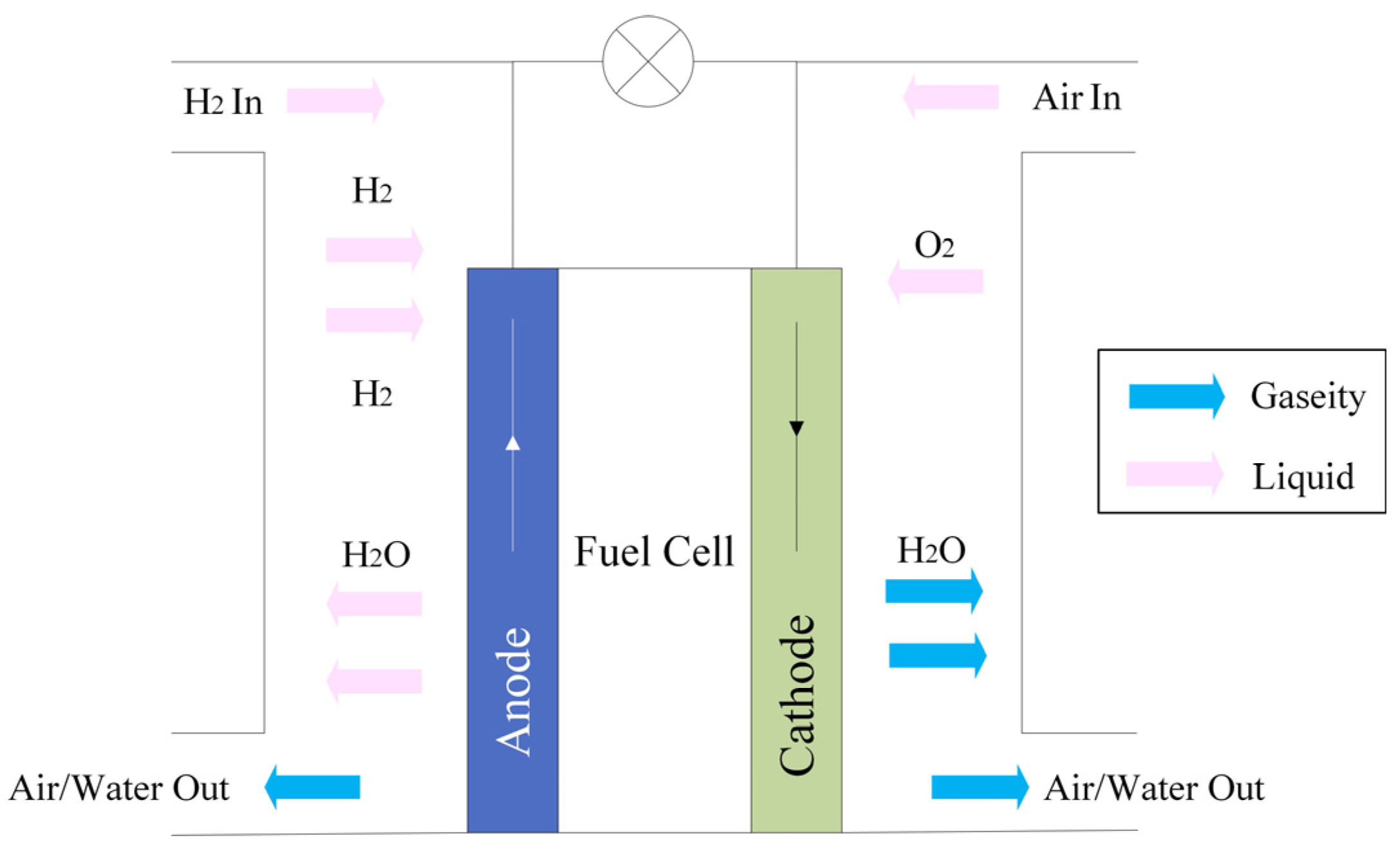
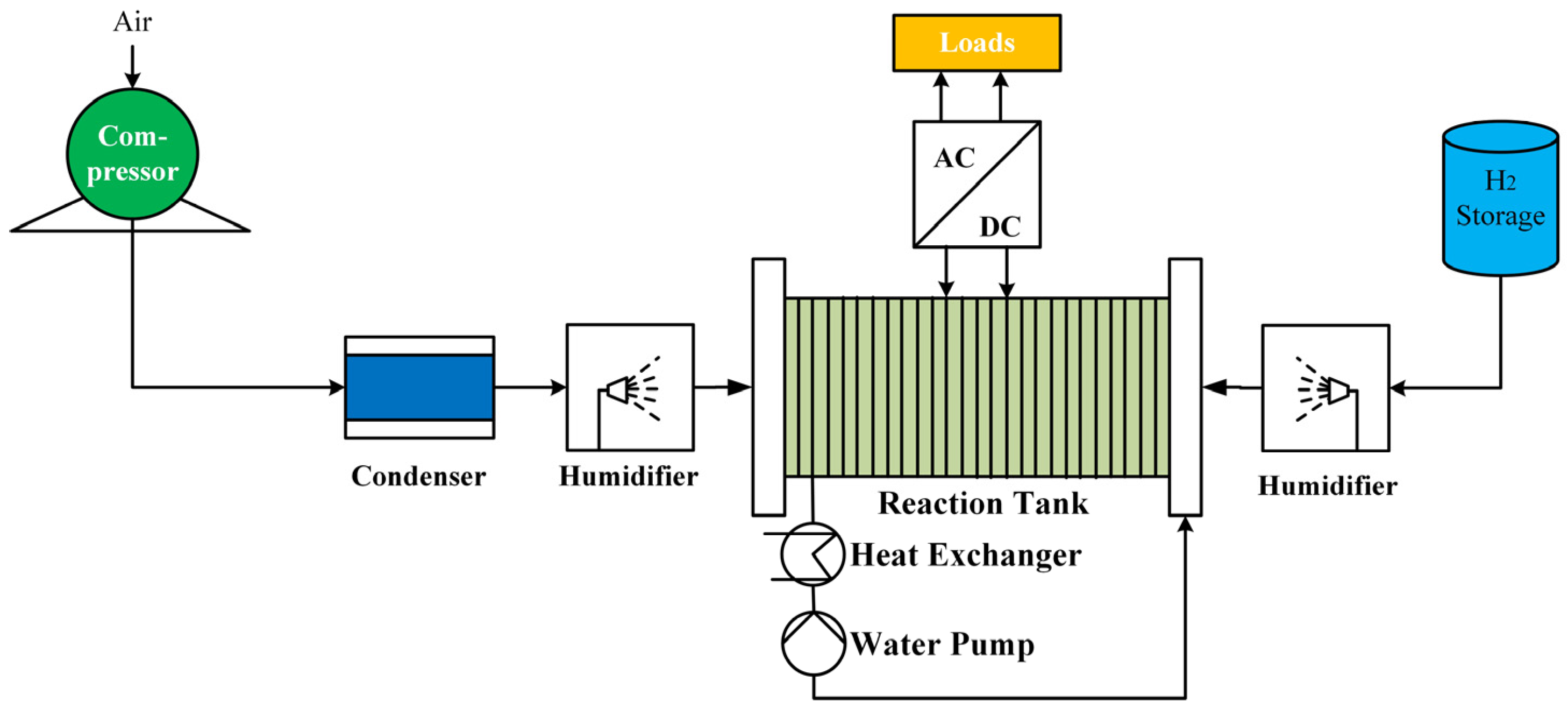
3.1. Hydrogen Electrification Technology
- (a)
- Principle of operation
- (b)
- Structure of Fuel Cell Systems
3.2. Hydrogen Ancillary Service Technologies
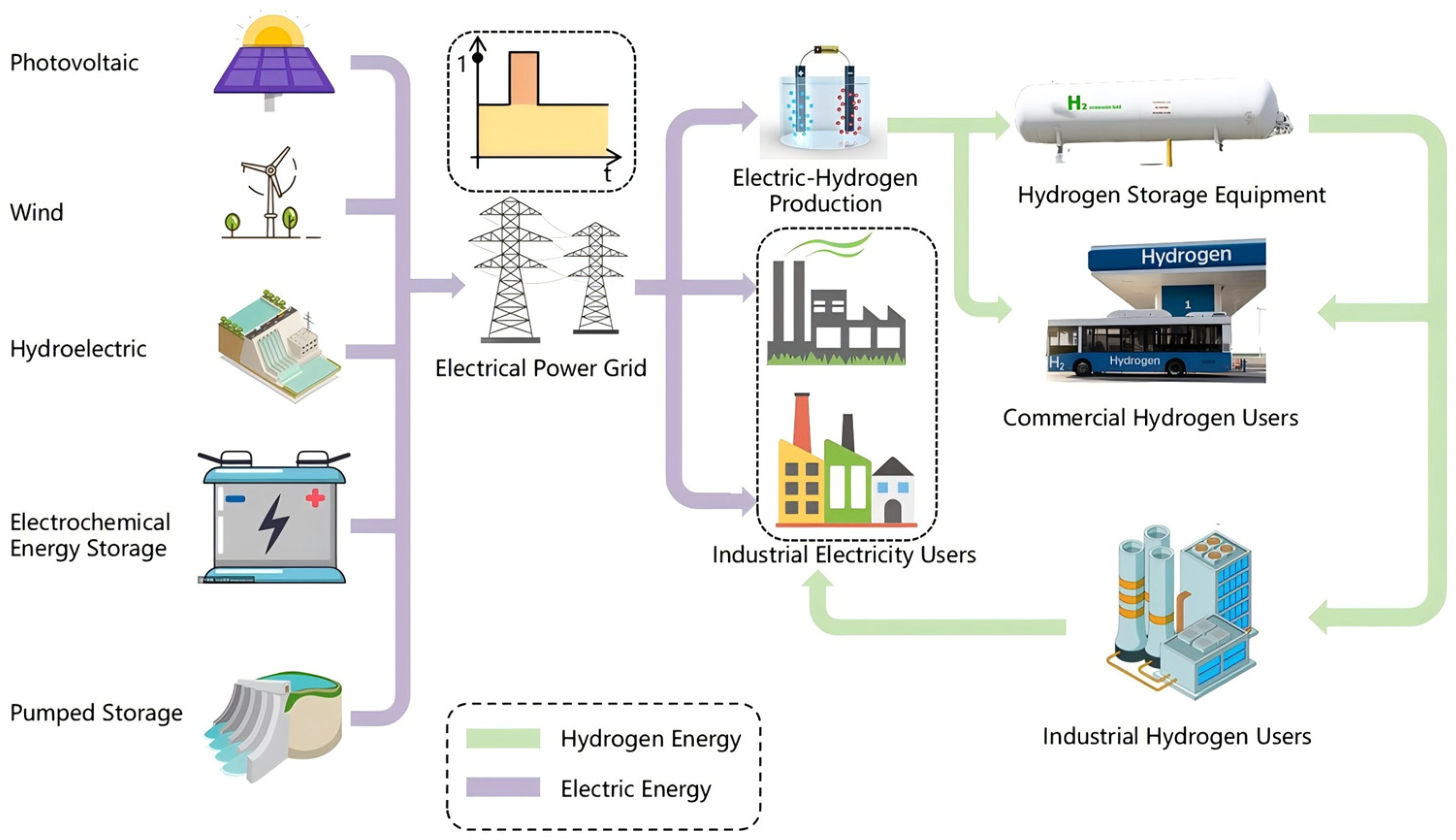
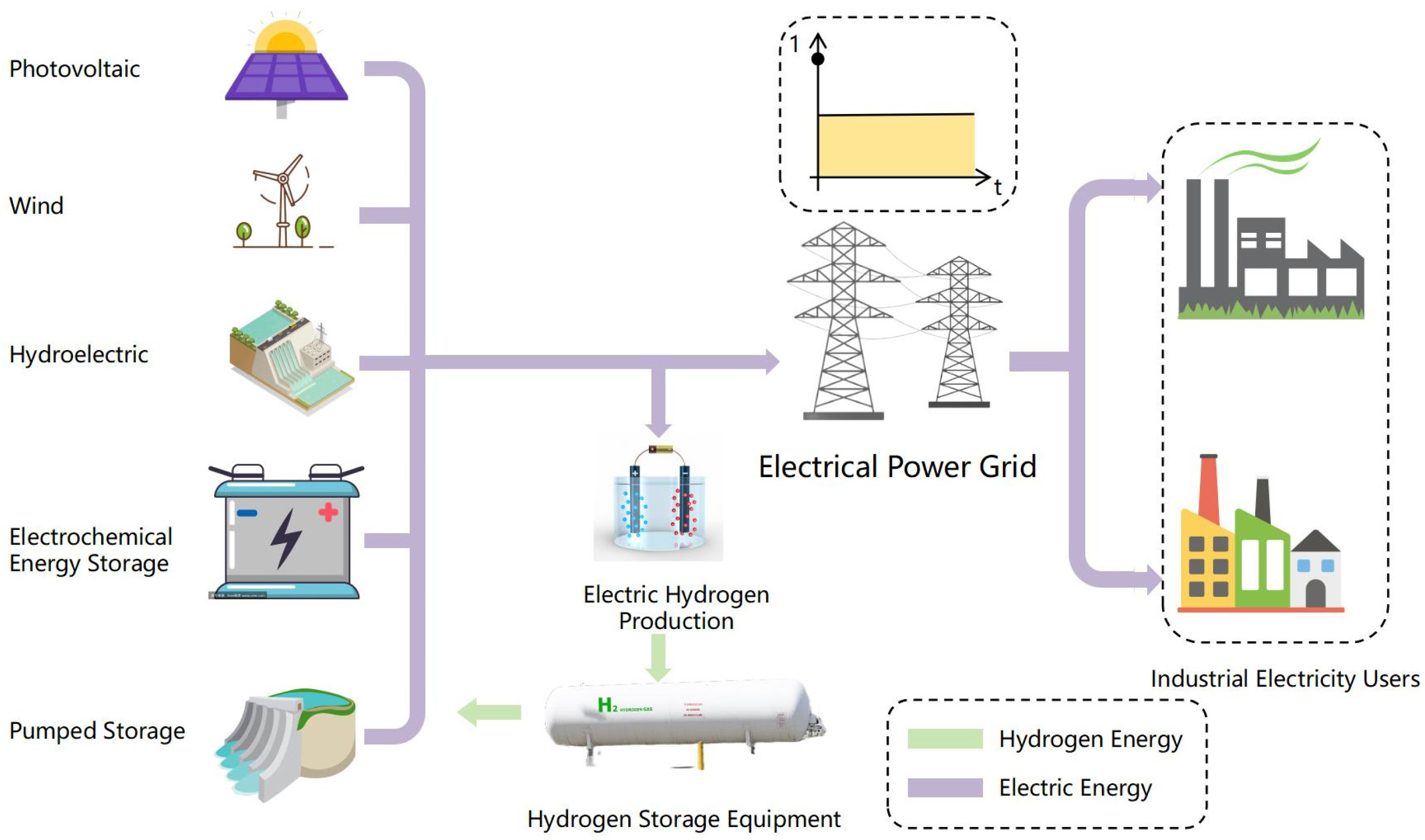
3.3. Hydrogen–Electric Power Systems
4. Key Technologies for Hydrogen–Electric Coupling
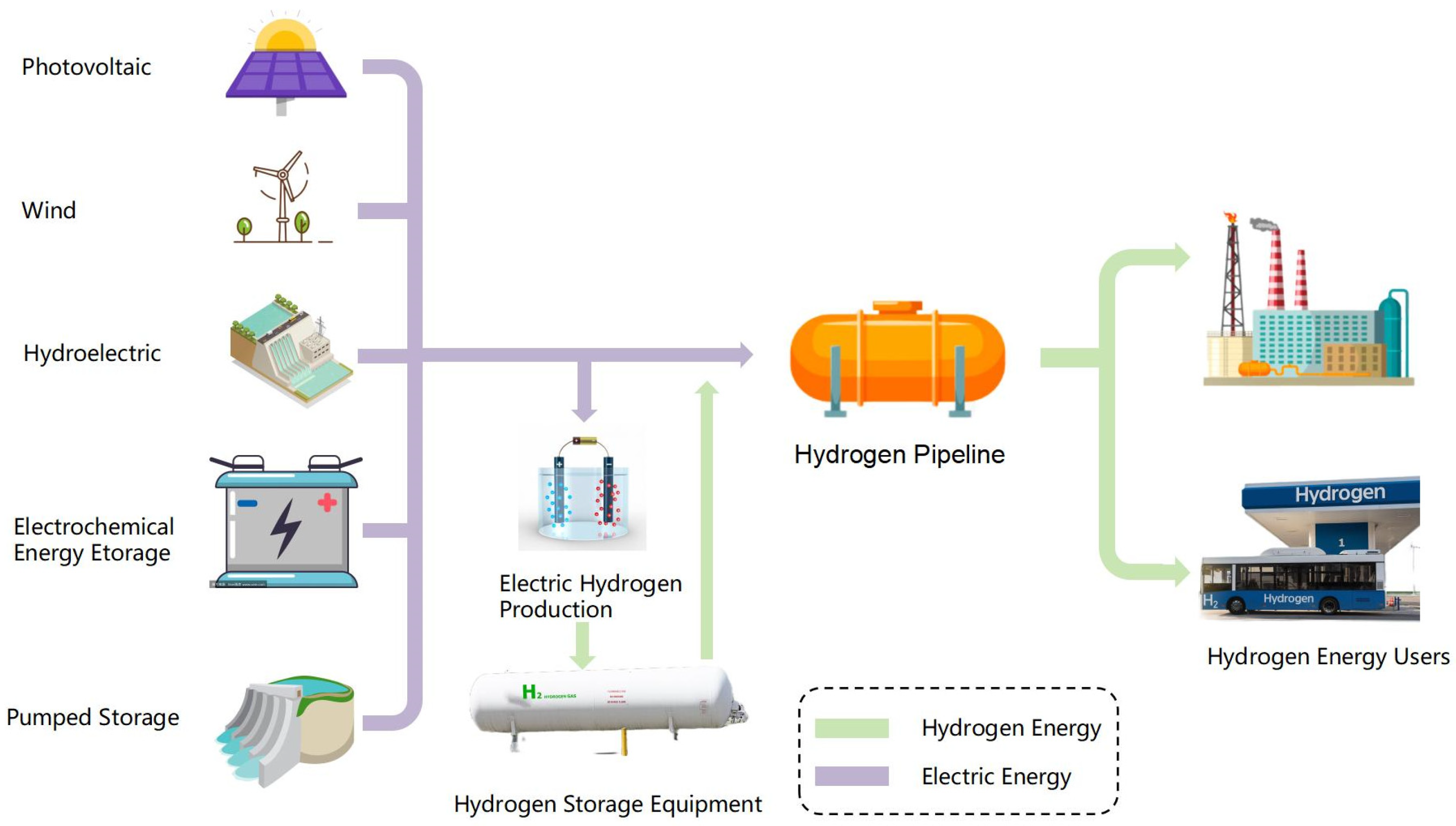
4.1. Load-Side Electric–Hydrogen Coupling
4.2. Power-Side Electric–Hydrogen Coupling
- (a)
- Electricity-transmission method
- (b)
- Hydrogen-delivery mode
- (c)
- Local equilibrium
5. Outlook for the Development of Hydrogen Energy Applications
5.1. Reducing Electrolysis Costs and Improving Preparation Yields
5.2. Development of New Materials to Enhance Hydrogen Storage Capacity
5.3. Optimizing Control Methods to Improve Power Generation Efficiency
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Li, Z.; Mandapaka, P.V.; Lo, E.Y. Risk-averse restoration of coupled power and water systems with small pumped-hydro storage and stochastic rooftop renewables. Appl. Energy 2023, 339, 120953. [Google Scholar] [CrossRef]
- Saidi, K.; Omri, A. The impact of renewable energy on carbon emissions and economic growth in 15 major renewable energy-consuming countries. Environ. Res. 2020, 186, 109567. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Song, M.; Shen, Z. The evolution of renewable energy and its impact on carbon reduction in China. Energy 2021, 237, 121639. [Google Scholar] [CrossRef]
- Sun, Y.; Li, H.; Andlib, Z.; Genie, M.G. How do renewable energy and urbanization cause carbon emissions? Evidence from advanced panel estimation techniques. Renew. Energy 2022, 185, 996–1005. [Google Scholar] [CrossRef]
- Behabtu, H.A.; Messagie, M.; Coosemans, T.; Berecibar, M.; Anlay Fante, K.; Kebede, A.A.; Mierlo, J. V A review of energy storage technologies’ application potentials in renewable energy sources grid integration. Sustainability 2020, 12, 10511. [Google Scholar] [CrossRef]
- Kebede, A.A.; Kalogiannis, T.; Van Mierlo, J.; Berecibar, M. A comprehensive review of stationary energy storage devices for large scale renewable energy sources grid integration. Renew. Sustain. Energy Rev. 2022, 159, 112213. [Google Scholar] [CrossRef]
- Le, T.T.; Sharma, P.; Bora, B.J.; Tran, V.D.; Truong, T.H.; Le, H.C.; Nguyen, P.Q.P. Fueling the future: A comprehensive review of hydrogen energy systems and their challenges. Int. J. Hydrogen Energy 2024, 54, 791–816. [Google Scholar] [CrossRef]
- Nicoletti, G.; Arcuri, N.; Nicoletti, G.; Bruno, R. A technical and environmental comparison between hydrogen and some fossil fuels. Energy Convers. Manag. 2015, 89, 205–213. [Google Scholar] [CrossRef]
- Capurso, T.; Stefanizzi, M.; Torresi, M.; Camporeale, S.M. Perspective of the role of hydrogen in the 21st century energy transition. Energy Convers. Manag. 2022, 251, 114898. [Google Scholar] [CrossRef]
- Chai, S.; Zhang, G.; Li, G.; Zhang, Y. Industrial hydrogen production technology and development status in China: A review. Clean. Technol. Environ. Policy 2021, 23, 1931–1946. [Google Scholar] [CrossRef]
- Younas, M.; Shafique, S.; Hafeez, A.; Javed, F.; Rehman, F. An overview of hydrogen production: Current status, potential, and challenges. Fuel 2022, 316, 123317. [Google Scholar] [CrossRef]
- Li, J.; Cheng, W. Comparative life cycle energy consumption, carbon emissions and economic costs of hydrogen production from coke oven gas and coal gasification. Int. J. Hydrogen Energy 2020, 45, 27979–27993. [Google Scholar] [CrossRef]
- Yang, K.; Gu, Z.; Long, Y.; Lin, S.; Lu, C.; Zhu, X.; Wang, H.; Li, K. Hydrogen production via chemical looping reforming of coke oven gas. Green Energy Environ. 2021, 6, 678–692. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Rashid, M.D.; Al Mesfer, M.K.; Naseem, H.; Danish, M. Hydrogen production by water electrolysis: A review of alkaline water electrolysis, PEM water electrolysis and high temperature water electrolysis. Int. J. Eng. Adv. Technol. 2015, 4, 2249–8958. [Google Scholar]
- Gong, Y.; Yao, J.; Wang, P.; Li, Z.; Zhou, H.; Xu, C. Perspective of hydrogen energy and recent progress in electrocatalytic water splitting. Chin. J. Chem. Eng. 2022, 43, 282–296. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, W.; Zhang, R.; Sun, H. Development of renewable energy multi-energy complementary hydrogen energy system (A Case Study in China): A review. Energy Explor. Exploit. 2020, 38, 2099–2127. [Google Scholar] [CrossRef]
- Hassan, Q.; Sameen, A.Z.; Salman, H.M.; Jaszczur, M. Large-scale green hydrogen production via alkaline water electrolysis using solar and wind energy. Int. J. Hydrogen Energy 2023, 48, 34299–34315. [Google Scholar] [CrossRef]
- Mandal, M. Recent Advancement on Anion Exchange Membranes for Fuel Cell and Water Electrolysis. ChemElectroChem 2021, 8, 36–45. [Google Scholar] [CrossRef]
- Anwar, S.; Khan, F.; Zhang, Y.; Djire, A. Recent developments in electrocatalysts for hydrogen production through water electrolysis. Int. J. Hydrogen Energy 2021, 46, 32284–32317. [Google Scholar] [CrossRef]
- González, G.M.C.; Toharias, B.; Iranzo, A.; Suárez, C.; Rosa, F. Voltage Distribution Analysis and Non-uniformity Assessment in a 100 cm² PEM Fuel Cell Stack. Energy 2023, 282, 128781. [Google Scholar] [CrossRef]
- Sazali, N.; Wan Salleh, W.N.; Jamaludin, A.S.; Mhd Razali, M.N. New Perspectives on Fuel Cell Technology: A Brief Review. Membranes 2020, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Yodwong, B.; Guilbert, D.; Phattanasak, M.; Kaewmanee, W.; Hinaje, M.; Vitale, G. Faraday’s Efficiency Modeling of a Proton Exchange Membrane Electrolyzer Based on Experimental Data. Energies 2020, 13, 4792. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, C.; Wan, Z.; Chen, X.; Chan, S.H.; Tu, Z. Progress and Perspectives of Integrated Thermal Management Systems in PEM Fuel Cell Vehicles: A Review. Renew. Sustain. Energy Rev. 2022, 155, 111908. [Google Scholar] [CrossRef]
- Pareek, A.; Dom, R.; Gupta, J.; Chandran, J.; Adepu, V.; Borse, P.H. Insights into renewable hydrogen energy: Recent advances and prospects. Mater. Sci. Energy Technol. 2020, 3, 319–327. [Google Scholar] [CrossRef]
- Nasser, M.; Megahed, T.F.; Ookawara, S.; Hassan, H. A review of water electrolysis–based systems for hydrogen production using hybrid/solar/wind energy systems. Environ. Sci. Pollut. Res. 2022, 29, 86994–87018. [Google Scholar] [CrossRef]
- Gutiérrez-Martín, F.; Amodio, L.; Pagano, M. Hydrogen production by water electrolysis and off-grid solar PV. Int. J. Hydrogen Energy 2021, 46, 29038–29048. [Google Scholar] [CrossRef]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Graphene-based electrocatalysts: Hydrogen evolution reactions and overall water splitting. Int. J. Hydrogen Energy 2021, 46, 21401–21418. [Google Scholar] [CrossRef]
- Das, C.K.; Bass, O.; Kothapalli, G.; Mahmoud, T.S.; Habibi, D. Overview of energy storage systems in distribution networks: Placement, sizing, operation, and power quality. Renew. Sustain. Energy Rev. 2018, 91, 1205–1230. [Google Scholar] [CrossRef]
- Palizban, O.; Kauhaniemi, K. Energy storage systems in modern grids—Matrix of technologies and applications. J. Energy Storage 2016, 6, 248–259. [Google Scholar] [CrossRef]
- Yao, L.; Yang, B.; Cui, H.; Zhuang, J.; Ye, J.; Xue, J. Challenges and progresses of energy storage technology and its application in power systems. J. Mod. Power Syst. Clean Energy 2016, 4, 519–528. [Google Scholar] [CrossRef]
- Sabihuddin, S.; Kiprakis, A.E.; Mueller, M. A numerical and graphical review of energy storage technologies. Energies 2015, 8, 172–216. [Google Scholar] [CrossRef]
- Nadeem, F.; Hussain, S.M.S.; Tiwari, P.K.; Goswami, A.K.; Ustun, T.S. Comparative review of energy storage systems, their roles, and impacts on future power systems. IEEE Access 2019, 7, 4555–4585. [Google Scholar] [CrossRef]
- Maisanam, A.K.S.; Biswas, A.; Sharma, K.K. An innovative framework for electrical energy storage system selection for remote area electrification with renewable energy system: Case of a remote village in India. J. Renew. Sustain. Energy 2020, 12, 024101. [Google Scholar] [CrossRef]
- Koohi-Fayegh, S.; Rosen, M.A. A review of energy storage types, applications and recent developments. J. Energy Storage 2020, 27, 101047. [Google Scholar] [CrossRef]
- Alves, M.P.; Gul, W.; Cimini Junior, C.A.; Ha, S.K. A review on industrial perspectives and challenges on material, manufacturing, design and development of compressed hydrogen storage tanks for the transportation sector. Energies 2022, 15, 5152. [Google Scholar] [CrossRef]
- Hassan, I.A.; Ramadan, H.S.; Saleh, M.A.; Hissel, D. Hydrogen storage technologies for stationary and mobile applications: Review, analysis and perspectives. Renew. Sust. Energ Rev. 2021, 149, 111311. [Google Scholar] [CrossRef]
- Rahman, M.M.; Oni, A.O.; Gemechu, E.; Kumar, A. Assessment of energy storage technologies: A review. Energy Convers. Manag. 2020, 223, 113295. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, J.; Li, G.; Zhou, W.; Ni, Z. Review on linerless type V cryo-compressed hydrogen storage vessels: Resin toughening and hydrogen-barrier properties control. Renew. Sustain. Energy Rev. 2024, 189, 114009. [Google Scholar] [CrossRef]
- Noh, H.; Kang, K.; Seo, Y. Environmental and Energy Efficiency Assessments of Offshore Hydrogen Supply Chains Utilizing Compressed Gaseous Hydrogen, Liquefied Hydrogen, Liquid Organic Hydrogen Carriers and Ammonia. Int. J. Hydrogen Energy 2023, 48, 7515–7532. [Google Scholar] [CrossRef]
- Chang, J.; Huang, Q.; Gao, Y.; Zheng, Z. Pathways of Developing High-Energy-Density Flexible Lithium Batteries. Adv. Mater. 2021, 33, 2004419. [Google Scholar] [CrossRef]
- Cigolotti, V.; Genovese, M.; Fragiacomo, P. Comprehensive Review on Fuel Cell Technology for Stationary Applications as Sustainable and Efficient Poly-Generation Energy Systems. Energies 2021, 14, 4963. [Google Scholar] [CrossRef]
- Cheng, X.B.; Liu, H.; Yuan, H.; Peng, H.J.; Tang, C.; Huang, J.Q.; Zhang, Q. A Perspective on Sustainable Energy Materials for Lithium Batteries. SusMat 2021, 1, 38–50. [Google Scholar] [CrossRef]
- Lai, X.; Huang, Y.; Deng, C.; Gu, H.; Han, X.; Zheng, Y.; Ouyang, M. Sorting, Regrouping, and Echelon Utilization of the Large-Scale Retired Lithium Batteries: A Critical Review. Renew. Sustain. Energy Rev. 2021, 146, 111162. [Google Scholar] [CrossRef]
- Jiao, K.; Xuan, J.; Du, Q.; Bao, Z.; Xie, B.; Wang, B.; Zhao, Y.; Fan, L.; Wang, H.; Hou, Z.; et al. Designing the Next Generation of Proton-Exchange Membrane Fuel Cells. Nature 2021, 595, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wu, Y.; Li, B.; Mo, T.; Li, Y.; Feng, S.P.; Qu, J.; Chu, P.K. Development and Application of Fuel Cells in the Automobile Industry. J. Energy Storage 2021, 42, 103124. [Google Scholar] [CrossRef]
- Zhao, X.; Yan, Y.; Zhang, J.; Zhang, F.; Wang, Z.; Ni, Z. Analysis of multilayered carbon fiber winding of cryo-compressed hydrogen storage vessel. Int. J. Hydrogen Energy 2022, 47, 10934–10946. [Google Scholar] [CrossRef]
- Tarkowski, R.; Uliasz-Misiak, B. Towards underground hydrogen storage: A review of barriers. Renew. Sustain. Energy Rev. 2022, 162, 112451. [Google Scholar] [CrossRef]
- Zivar, D.; Kumar, S.; Foroozesh, J. Underground hydrogen storage: A comprehensive review. Renew. Sustain. Energy Rev. 2021, 46, 23436–23462. [Google Scholar] [CrossRef]
- Thiyagarajan, S.R.; Emadi, H.; Hussain, A.; Patange, P.; Watson, M. A comprehensive review of the mechanisms and efficiency of underground hydrogen storage. J. Energy Storage 2022, 51, 104490. [Google Scholar] [CrossRef]
- Kovač, A.; Paranos, M.; Marciuš, D. Hydrogen in energy transition: A review. Int. J. Hydrogen Energy 2021, 46, 10016–10035. [Google Scholar] [CrossRef]
- Yin, L.; Ju, Y. Review on the design and optimization of hydrogen liquefaction processes. Front. Energy 2020, 14, 530–544. [Google Scholar] [CrossRef]
- Milanese, C.; Jensen, T.R.; Hauback, B.C.; Pistidda, C.; Dornheim, M.; Yang, H.; Lombardo, L.; Zuettel, A.; Filinchuk, Y.; Ngene, P.; et al. Complex hydrides for energy storage. Int. J. Hydrogen Energy 2019, 44, 7860–7874. [Google Scholar] [CrossRef]
- Shang, Y.; Pistidda, C.; Gizer, G.; Klassen, T.; Dornheim, M. Mg-based materials for hydrogen storage. J. Magnes Alloy 2021, in press. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Tan, H. An energy-saving hydrogen liquefaction process with efficient utilization of liquefied natural gas cold energy. Int. J. Hydrogen Energy 2024, 49, 1482–1496. [Google Scholar] [CrossRef]
- Morales-Ospino, R.; Celzard, A.; Fierro, V. Strategies to recover and minimize boil-off losses during liquid hydrogen storage. Renew. Sustain. Energy Rev. 2023, 182, 113360. [Google Scholar] [CrossRef]
- Weckerle, C.; Dörr, M.; Linder, M.; Bürger, I. A compact thermally driven cooling system based on metal hydrides. Energies 2020, 13, 2482. [Google Scholar] [CrossRef]
- Wang, J.; Qi, M.; Liu, S.; Zhao, D. Comprehensive safety assessment of a hydrogen liquefaction system based on an integrated system-theoretic process analysis (STPA) and best-worst method (BWM). Int. J. Hydrogen Energy 2024, 66, 479–489. [Google Scholar] [CrossRef]
- Argyrou, M.C.; Christodoulides, P.; Wongwises, S.A. Energy storage for electricity generation and related processes: Technologies appraisal and grid scale applications. Renew. Sustain. Energy Rev. 2018, 94, 804–821. [Google Scholar] [CrossRef]
- Ghorbani, B.; Zendehboudi, S.; Saady, N.M.C.; Duan, X.; Albayati, T.M. Strategies to improve the performance of hydrogen storage systems by liquefaction methods: A comprehensive review. ACS Omega 2023, 8, 18358–18399. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Wu, K.; Luo, B.; Cao, Q.; Zhang, H. Hydrogen storage by liquid organic hydrogen carriers: Catalyst, renewable carrier, and technology–a review. Carbon Resour. Convers. 2023, 6, 334–351. [Google Scholar] [CrossRef]
- Tan, K.C.; Chua, Y.S.; He, T.; Chen, P. Strategies of thermodynamic alternation on organic hydrogen carriers for hydrogen storage application: A review. Green Energy Resour. 2023, 1, 100020. [Google Scholar] [CrossRef]
- Pingkuo, P.L.; Xue, H. Comparative analysis on similarities and differences of hydrogen energy development in the world’s top 4 largest economies: A novel framework. Int. J. Hydrogen Energy 2022, 47, 9485–9503. [Google Scholar] [CrossRef]
- Zhai, L.; Liu, S.; Xiang, Z. Ammonia as a carbon-free hydrogen carrier for fuel cells: A perspective. Ind. Chem. Mater. 2023, 1, 332–342. [Google Scholar] [CrossRef]
- Negro, V.; Noussan, M.; Chiaramonti, D. The potential role of ammonia for hydrogen storage and transport: A critical review of challenges and opportunities. Energies 2023, 16, 6192. [Google Scholar] [CrossRef]
- Kojima, Y. Safety of ammonia as a hydrogen energy carrier. Int. J. Hydrogen Energy 2024, 50, 732–739. [Google Scholar] [CrossRef]
- Ramezani, R.; Di Felice, L.; Gallucci, F. A review of chemical looping reforming technologies for hydrogen produc-tion: Recent advances and future challenges. J. Phys. Energy 2023, 5, 024010. [Google Scholar] [CrossRef]
- Guan, D.; Wang, B.; Zhang, J.; Shi, R.; Jiao, K.; Li, L.; Wang, Y.; Xie, B.; Zhang, Q.; Yu, J.; et al. Hydrogen society: From present to future. Energy Environ. Sci. 2023, 16, 4926–4943. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, C.; Bai, F.; Wang, W.; An, S.; Zhao, K.; Li, Z.; Li, J.; Sun, H. A comprehensive review of the promising clean energy carrier: Hydrogen production, transportation, storage, and utilization (HPTSU) technologies. Fuel 2024, 355, 129455. [Google Scholar] [CrossRef]
- Klopčič, N.; Grimmer, I.; Winkler, F.; Sartory, M.; Trattner, A. A review on metal hydride materials for hydrogen storage. J Energy Storage 2023, 72, 108456. [Google Scholar] [CrossRef]
- Srinivasan, M.N.; Babu, M.D.; Naveena, S.S.; Malini, A.S.; Ilyos, K.; Dixit, K.K.; Husain, S.O.; Dev, B.P.V. Metal hydride hydrogen storage and compression systems for energy storage: Modeling, synthesis, and properties. In Proceedings of the 2023 International Conference for Technological Engineering and its Applications in Sustainable Development (ICTEASD), Al-Najaf, Iraq, 14–15 November 2023; IEEE: Piscataway, NJ, USA, 2023; pp. 256–261. [Google Scholar]
- Zhang, X.; Lou, Z.; Gao, M.; Pan, H.; Liu, Y. Metal hydrides for advanced hydrogen/lithium storage and ionic conduction applications. Acc. Mater. Res. 2024, 5, 371–384. [Google Scholar] [CrossRef]
- Dubey, S.K.; Ravi Kumar, K.; Tiwari, V.; Srivastva, U. Impacts, barriers, and future prospective of metal hydride-based thermochemical energy storage system for high-temperature applications: A comprehensive review. Energy Technol. 2024, 12, 2300768. [Google Scholar] [CrossRef]
- Franke, F.; Kazula, S.; Enghardt, L. Review and evaluation of metal-hydride-based hydrogen sensors as safety devices for future sustainable aviation. Proc. J. Phys. Conf. Ser. 2023, 2454, 012001. [Google Scholar] [CrossRef]
- Nivedhitha, K.S.; Beena, T.; Banapurmath, N.R.; Umarfarooq, M.A.; Ramasamy, V.; Soudagar, M.E.M.; Ağbulut, Ü. Advances in hydrogen storage with metal hydrides: Mechanisms, materials, and challenges. Int. J. Hydrogen Energy 2024, 61, 1259–1273. [Google Scholar] [CrossRef]
- Evans, A.; Strezov, V.; Evans, T.J. Assessment of utility energy storage options for increased renewable energy penetration Sustain. Energy Rev. 2012, 16, 4141–4147. [Google Scholar]
- Gao, J.; Zhou, S.; Lu, Y.; Shen, W. Simulation of a Novel Integrated Multi-Stack Fuel Cell System Based on a Double-Layer Multi-Objective Optimal Allocation Approach. Appl. Sci. 2024, 14, 2961. [Google Scholar] [CrossRef]
- Xie, Z.; Zhou, S.; Gao, J.; Zhang, G.; Shen, W. Structural Design, Matching, and Analysis of Air Supply Devices for Multi-Stack Fuel Cell Systems. Energy Technol. 2023, 11, 2201331. [Google Scholar] [CrossRef]
- Ghaderi, R.; Kandidayeni, M.; Boulon, L.; Trovão, J.P. Q-learning based energy management strategy for a hybrid multi-stack fuel cell system considering degradation. Energy Convers. Manag. 2023, 293, 117524. [Google Scholar] [CrossRef]
- Ding, D.; Wu, X.Y. Hydrogen Fuel Cell Electric Trains: Technologies, Current Status, and Future. Appl. Energy Combust. Sci. 2024, 17, 100255. [Google Scholar] [CrossRef]
- Li, H.; Guo, H.; Yousefi, N. A Hybrid Fuel Cell/Battery Vehicle by Considering Economy Considerations Optimized by Converged Barnacles Mating Optimizer (CBMO) Algorithm. Energy Rep. 2020, 6, 2441–2449. [Google Scholar] [CrossRef]
- Hoskins, A.L.; Millican, S.L.; Czernik, C.E.; Alshankiti, I.; Netter, J.C.; Wendelin, T.J.; Musgrave, C.B.; Weimer, A.W. Continuous On-Sun Solar Thermochemical Hydrogen Production via an Isothermal Redox Cycle. Appl. Energy 2019, 249, 368–376. [Google Scholar] [CrossRef]
- Jacobsen, A.; Godvik, M. Influence of Wakes and Atmospheric Stability on the Floater Responses of the Hywind Scotland Wind Turbines. Wind Energy 2021, 24, 149–161. [Google Scholar] [CrossRef]
- Sharpe, O.; Raman, K. The Australian Hydrogen Centre–Feasibility Studies for Achieving 10 and 100% Renewable Hydrogen in South Australia and Victoria. Aust. Energy Prod. J. 2024, 64, S191–S196. [Google Scholar] [CrossRef]
- Biabani, H.; Aminlou, A.; Hayati, M.M.; Majidi-Gharehnaz, H.; Abapour, M. Green Hydrogen Research and Development Projects in the European Union. In Green Hydrogen in Power Systems; Springer International Publishing: Berlin/Heidelberg, Germany, 2024; pp. 301–320. [Google Scholar]
- Durakovic, G.; del Granado, P.C.; Tomasgard, A. Powering Europe with North Sea Offshore Wind: The Impact of Hydrogen Investments on Grid Infrastructure and Power Prices. Energy 2023, 263, 125654. [Google Scholar] [CrossRef]
- Ishaq, H.; Dincer, I.; Crawford, C. A review on hydrogen production and utilization: Challenges and opportunities. Int. J. Hydrogen Energy 2022, 47, 26238–26264. [Google Scholar] [CrossRef]
- Ragab, A.; Marei, M.I.; Mokhtar, M. Comprehensive Study of Fuel Cell Hybrid Electric Vehicles: Classification, Topologies, and Control System Comparisons. Appl. Sci. 2023, 13, 13057. [Google Scholar] [CrossRef]
- Ma, Y.; Li, C.; Wang, S. Multi-Objective Energy Management Strategy for Fuel Cell Hybrid Electric Vehicle Based on Stochastic Model Predictive Control. ISA Trans. 2022, 131, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shang, Z.; Peng, F.; Li, L.; Zhao, Y.; Liu, Z. Increment-oriented online power distribution strategy for multi-stack proton exchange membrane fuel cell systems aimed at collaborative performance enhancement. J. Power Sources 2021, 512, 230512–230528. [Google Scholar] [CrossRef]
- Ameli, H.; Strbac, G.; Pudjianto, D.; Ameli, M.T. A Review of the Role of Hydrogen in the Heat Decarbonization of Future Energy Systems: Insights and Perspectives. Energies 2024, 17, 1688. [Google Scholar] [CrossRef]
- Shi, W.; Huangfu, Y.; Xu, L.; Pang, S. Online energy management strategy considering fuel cell fault for multi-stack fuel cell hybrid vehicle based on multi-agent reinforcement learning. Appl. Energy 2022, 328, 120234. [Google Scholar] [CrossRef]
- İskenderoğlu, F.C.; Baltacioğlu, M.F.; Demir, M.H.; Baldinelli, A.; Barelli, L.; Bidini, G. Comparison of support vector regression and random forest algorithms for estimating the SOFC output voltage by considering hydrogen flow rates. Int. J. Hydrogen Energy 2020, 45, 35023–35038. [Google Scholar] [CrossRef]
- Ban, M.; Yu, J.; Shahidehpour, M.; Yao, Y. Integration of power-to-hydrogen in day-ahead security-constrained unit commitment with high wind penetration. J. Mod. Power Syst. Clean Energy 2017, 5, 337–349. [Google Scholar] [CrossRef]
- Boretti, A. A high-efficiency internal combustion engine using oxygen and hydrogen. Int. J. Hydrogen Energy 2024, 50, 847–856. [Google Scholar] [CrossRef]
- Zhang, S.W.; Sun, B.G.; Lin, S.L.; Li, Q.; Wu, X.; Hu, T.; Bao, B.-Z.; Wang, X.; Luo, Q.-H. Energy and exergy analysis for a turbocharged direct-injection hydrogen engine to achieve efficient and high-economy performances. Int. J. Hydrogen Energy 2024, 54, 601–612. [Google Scholar] [CrossRef]
- Yu, Z.; Han, J.; Cao, X. Investigation on performance of an integrated solid oxide fuel cell and absorption chiller tri-generation system. Int. J. Hydrogen Energy 2011, 36, 12561–12573. [Google Scholar] [CrossRef]
- Da Silva, D.C.; Kefsi, L.; Sciarretta, A. Closed-Form Expression to Estimate the Hydrogen Consumption of a Fuel Cell Hybrid Electric Vehicle. IEEE Trans. Veh. Technol. 2024, 73, 4717–4728. [Google Scholar] [CrossRef]
- Hydrogen Council Path to Hydrogen Competitiveness: A Cost Perspective. 2020. Available online: https://hydrogencouncil.com/en/path-to-hydrogen-competitiveness-a-cost-perspective// (accessed on 20 January 2020).
- Gu, Z.; Pan, G.; Gu, W.; Qiu, H.; Lu, S. Robust Optimization of Scale and Revenue for Integrated Power-to-Hydrogen Systems within Energy, Ancillary Services, and Hydrogen Markets. IEEE Trans. Power Syst. 2023, 39, 5008–5023. [Google Scholar] [CrossRef]
- Yan, C.; Zou, Y.; Wu, Z.; Maleki, A. Effect of Various Design Configurations and Operating Conditions for Optimization of a Wind/Solar/Hydrogen/Fuel Cell Hybrid Microgrid System by a Bio-Inspired Algorithm. Int. J. Hydrogen Energy 2024, 60, 378–391. [Google Scholar] [CrossRef]
- Deng, W.; Zhang, Y.; Tang, Y.; Li, Q.; Yi, Y. A Neural Network-Based Adaptive Power-Sharing Strategy for Hybrid Frame Inverters in a Microgrid. Front. Energy Res. 2023, 10, 1082948. [Google Scholar] [CrossRef]
- Fu, Z.; Lu, L.; Zhang, C.; Xu, Q.; Zhang, X.; Gao, Z.; Li, J. Fuel Cell and Hydrogen in Maritime Application: A Review on Aspects of Technology, Cost, and Regulations. Sustain. Energy Technol. Assess. 2023, 57, 103181. [Google Scholar] [CrossRef]
- Yang, X.; Sun, J.; Meng, X.; Sun, S.; Shao, Z. Cold Start Degradation of Proton Exchange Membrane Fuel Cell: Dynamic and Mechanism. Chem. Eng. J. 2023, 455, 140823. [Google Scholar] [CrossRef]
- Teoh, Y.H.; How, H.G.; Le, T.D.; Nguyen, H.T.; Loo, D.L.; Rashid, T.; Sher, F. A Review on Production and Implementation of Hydrogen as a Green Fuel in Internal Combustion Engines. Fuel 2023, 333, 126525. [Google Scholar] [CrossRef]
- Park, Y.K.; Kim, B.S. Catalytic Removal of Nitrogen Oxides (NO, NO2, N2O) from Ammonia-Fueled Combustion Exhaust: A Review of Applicable Technologies. Chem. Eng. J. 2023, 461, 141958. [Google Scholar] [CrossRef]
- Albatayneh, A.; Juaidi, A.; Jaradat, M.; Manzano-Agugliaro, F. Future of Electric and Hydrogen Cars and Trucks: An Overview. Energies 2023, 16, 3230. [Google Scholar] [CrossRef]
- Dall’Armi, C.; Pivetta, D.; Taccani, R. Hybrid PEM Fuel Cell Power Plants Fuelled by Hydrogen for Improving Sustainability in Shipping: State of the Art and Review on Active Projects. Electronics 2023, 16, 2022. [Google Scholar] [CrossRef]
- Klatzer, T.; Bachhiesl, U.; Wogrin, S. State-of-the-art expansion planning of integrated power, natural gas, and hydrogen systems. Int. J. Hydrogen Energy 2022, 47, 20585–20603. [Google Scholar] [CrossRef]
- Gonzalez-Romero, I.C.; Wogrin, S.; Gómez, T. Review on generation and transmission expansion co-planning models under a market environment. IET Gener. Transm. Dis. 2020, 14, 931–944. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, N.; Dai, H.; Liu, L.; Zhou, Z.; Shi, Q.; Lu, J. Comparison of Different Coupling Modes between the Power System and the Hydrogen System Based on a Power–Hydrogen Coordinated Planning Optimization Model. Energies 2023, 16, 5374. [Google Scholar] [CrossRef]
- Khaleel, M.M.; Adzman, M.R.; Zali, S.M. An Integrated Hydrogen Fuel Cell to Distribution Network System: Challenges and Opportunities for D-STATCOM. Energies 2021, 14, 7073. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, Q.; Ai, Y.; Chen, W.; Benbouzid, M.; Liu, S.; Gao, F. Two-Stage Distributionally Robust Optimization-Based Coordinated Scheduling of Integrated Energy System with Electricity-Hydrogen Hybrid Energy Storage. Prot. Control Mod. Power Syst. 2023, 8, 542–555. [Google Scholar] [CrossRef]
- Yan, Y. Comparative Analysis of the Economics of Hydrogen Storage and Transportation. Master’s Thesis, Huazhong University of Science Technology, Wuhan, China, 2021. [Google Scholar]
- Salama, H.S.; Magdy, G.; Bakeer, A.; Vokony, I. Adaptive Coordination Control Strategy of Renewable Energy Sources, Hydrogen Production Unit, and Fuel Cell for Frequency Regulation of a Hybrid Distributed Power System. Prot. Control Mod. Power Syst. 2022, 7, 472–489. [Google Scholar] [CrossRef]
- Marquez, R.A.; Espinosa, M.; Kalokowski, E.; Son, Y.J.; Kawashima, K.; Le, T.V.; Chukwuneke, C.E.; Mullins, C.B. A Guide to Electrocatalyst Stability Using Lab-Scale Alkaline Water Electrolyzers. ACS Energy Lett. 2024, 9, 547–555. [Google Scholar] [CrossRef]
- Rocha, C.; Knöri, T.; Ribeirinha, P.; Gazdzicki, P. A review on flow field design for proton exchange membrane fuel cells: Challenges to increase the active area for MW applications. Renew. Sustain. Energy Rev. 2024, 192, 114198. [Google Scholar] [CrossRef]
- Caldera, U.; Bogdanov, D.; Afanasyeva, S.; Breyer, C. Role of seawater desalination in the management of an integrated water and 100% renewable energy based power sector in Saudi Arabia. Water 2017, 10, 3. [Google Scholar] [CrossRef]
| Fuel | Ratio of Energy and Mass (MJ/kg) | |
|---|---|---|
| Higher Heating Value | Lower Heating Value | |
| Hydrogen | 142 | 120 |
| Methane | 55.5 | 50 |
| Liquefied Petroleum Gas | 50.0 | 46.1 |
| Gasoline | 46.4 | 44 |
| Natural Gas | 55 | 50 |
| Coal (anthracite) | 24 | 35 |
| Ethanol | 29.7 | 26.8 |
| Parameters | AWEs | PEMEs | SOEs |
|---|---|---|---|
| Technology maturity | Widespread commercialization | commercialization | R&D phase |
| Temperature/°C | 60–80 | 50–80 | 600–1000 |
| Pressure/bar | 10–30 | 20–50 | 1–15 |
| Current density/A·cm−2 | <0.45 | 1.0–2.0 | 0.3–1.0 |
| Individual electric power/MW | 6 | 2 | 0.15 |
| Electrical efficiency/% | 62–82 | 67–82 | 81–86 |
| System energy consumption/kWh·m−1 | 4.2–4.8 | 4.4–5.0 | 2.5–3.5 |
| Area of electric stack/m2 | 3–3.6 | <0.13 | <0.06 |
| Hydrogen production rate/m3·h−1 | 1400 | 400 | <10 |
| Reactor life/kh | 55–120 | 60–100 | 8–20 |
| System life/a | 20–30 | 10–20 | - |
| Hydrogen production purity % | >99.8 | 99.999 | - |
| Cold start time/min | 60–120 | 5–10 | >60 |
| Hot start time/s | 60–300 | <10 | 900 |
| Investment cost/USD·kW−1 | 800–1500 | 400–2100 | >2000 |
| Category | Pros | Cons | Cost | Comment |
|---|---|---|---|---|
| Compressed Gaseous Hydrogen | Mature, suitable for short-distance transportation and distribution | The storage density is relatively low, requiring high-pressure containers, which limits the transportation volume | Short-distance transportation (within 200 km): USD 0.30 to USD 0.50 per kg of H2. Long-distance transportation: USD 1.00 to USD 3.00 per kg of H2 | Suitable for localized or short-distance applications, but the costs for long-distance transportation are higher. |
| Liquid Hydrogen | High storage density, suitable for large-scale, long-distance transportation | The liquefaction process has high energy consumption, strict low-temperature storage with transportation requirements, and may have evaporation loss | Medium distances (around 500 km): USD 1.00–USD 2.00/kg H2 | Liquid hydrogen is suitable for long-distance transportation, but the high cost of the liquefaction process and cryogenic storage equipment has a significant impact on the overall cost. High storage density makes it suitable for long-distance transportation but requires considering the energy consumption during the liquefaction process. |
| Chemical Hydrogen Carriers | Easy to store and transport at room temperature and pressure, with large transportation volume and high safety | Requires additional hydrogen extraction processes, affecting the overall efficiency | Depending on the chemical carrier used, the cost is between USD 1.00 and USD 5.00/kg H2. e.g., the transport cost of using ammonia as a carrier is about USD 1.50–USD 3.00/kg H2, and the additional cost of hydrogen extraction from the carrier should be considered. | Suitable for long-distance or cross-border transportation, safer at normal temperature and pressure, but the overall efficiency is low. |
| Metal Hydrides | High hydrogen storage density, stable storage, suitable for small-scale or portable applications | The hydrogen storage/release process is slow, and the cost of metal hydride material is high | USD 5.00–USD 10.00/kg H2, mainly due to high material cost and low efficiency of hydrogen storage/release process. | High storage density, but the process of releasing hydrogen is complex and usually used for special applications rather than large-scale transportation. |
| Solid Hydrogen | Storage density is extremely high, theoretically | The preparation and processing techniques are complex with high cost, and mainly in the lab stage | At present, it is mainly in the research stage, and the economy has not been fully evaluated. If the practical application is considered, the cost may be extremely high, far more than other forms. | Suitable for experiments and specific applications; commercial and large-scale applications are not yet available. |
| Typology | Operating Temperature | Battery Pack Voltage Efficiency | Advantage | Drawbacks |
|---|---|---|---|---|
| Proton Exchange Membrane Fuel Cell (PEMFC) | 80 °C–100 °C (low temperature) or 200 °C (high temperature) | 50–60% | Fast start-up and versatility | Catalysts are expensive |
| Solid Oxide Fuel Cells (SOFCs) | 800 °C–1000 °C | 60–80% | With solid electrolytes, the reaction heat is reusable and less costly. | Presence of metal corrosion problems |
| Alkaline fuel cell (AFC) | Approx 70 °C | About 60% | Good current response | Limited application scenarios |
| Molten Carbonate Fuel Cell (MCFC) | Approx 650 °C | 60–80% | Good conductivity and high current density | Slow start, only for large-scale use. |
| Phosphoric acid fuel cell (PAFC) | Approx 180 °C | More than 80% | High efficiency | Low current density and high catalyst cost |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, S.; Shen, P.; Deng, W.; Yu, Q. Hydrogen Energy in Electrical Power Systems: A Review and Future Outlook. Electronics 2024, 13, 3370. https://doi.org/10.3390/electronics13173370
Dai S, Shen P, Deng W, Yu Q. Hydrogen Energy in Electrical Power Systems: A Review and Future Outlook. Electronics. 2024; 13(17):3370. https://doi.org/10.3390/electronics13173370
Chicago/Turabian StyleDai, Siting, Pin Shen, Wenyang Deng, and Qing Yu. 2024. "Hydrogen Energy in Electrical Power Systems: A Review and Future Outlook" Electronics 13, no. 17: 3370. https://doi.org/10.3390/electronics13173370




