Analysis of Handwriting for Recognition of Parkinson’s Disease: Current State and New Study
Abstract
1. Introduction
2. Related Works
2.1. Features of Handwriting
2.2. Handwriting Samples
2.3. Condition of Patients
2.4. Algorithms
3. Proposed Approach
4. Material and Methods
4.1. Acquisition of Handwriting Sample Images
4.2. Handwriting Features
5. Generation of Diagnostic Features
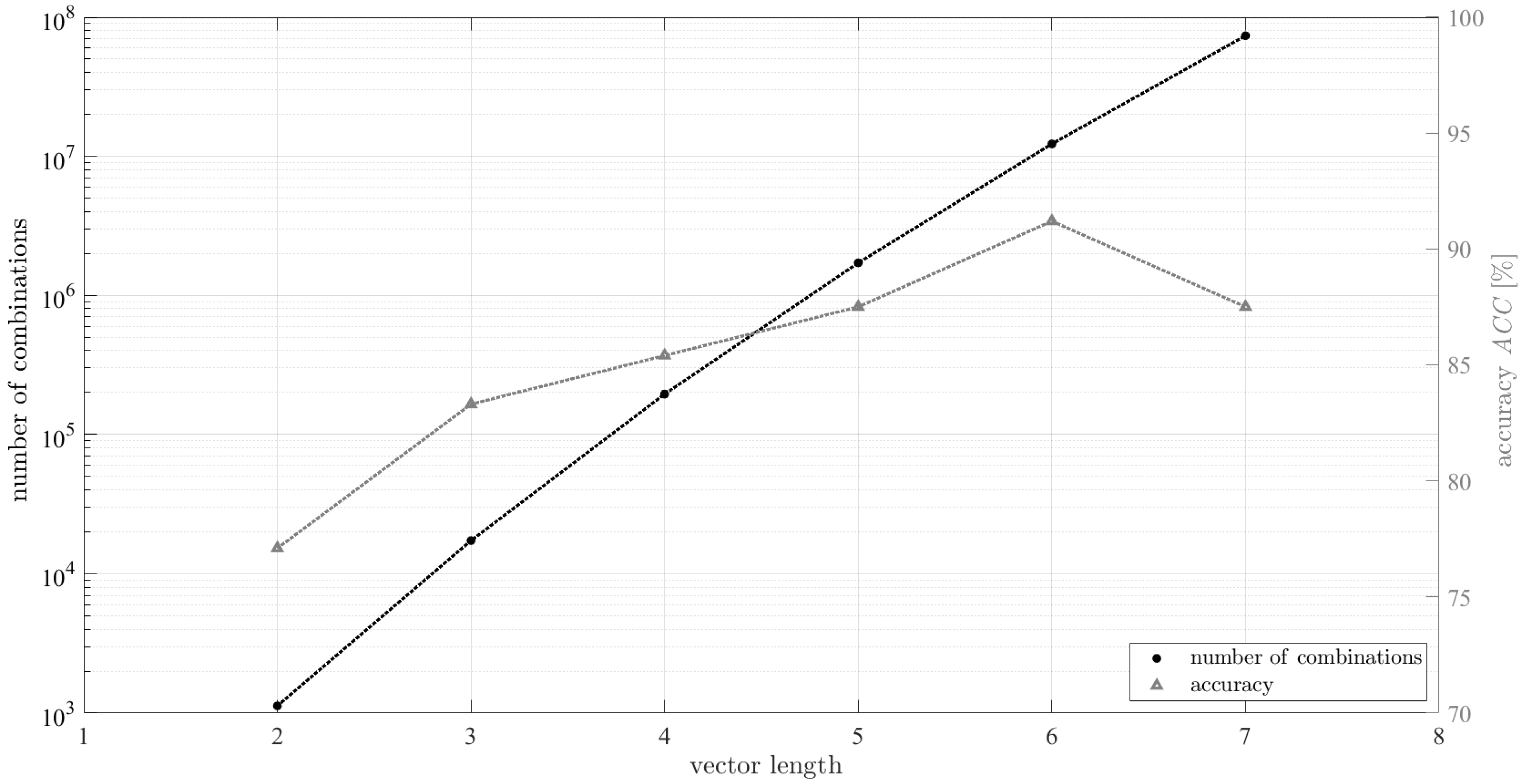
6. A Quantitative Assessment of the Recognition Process
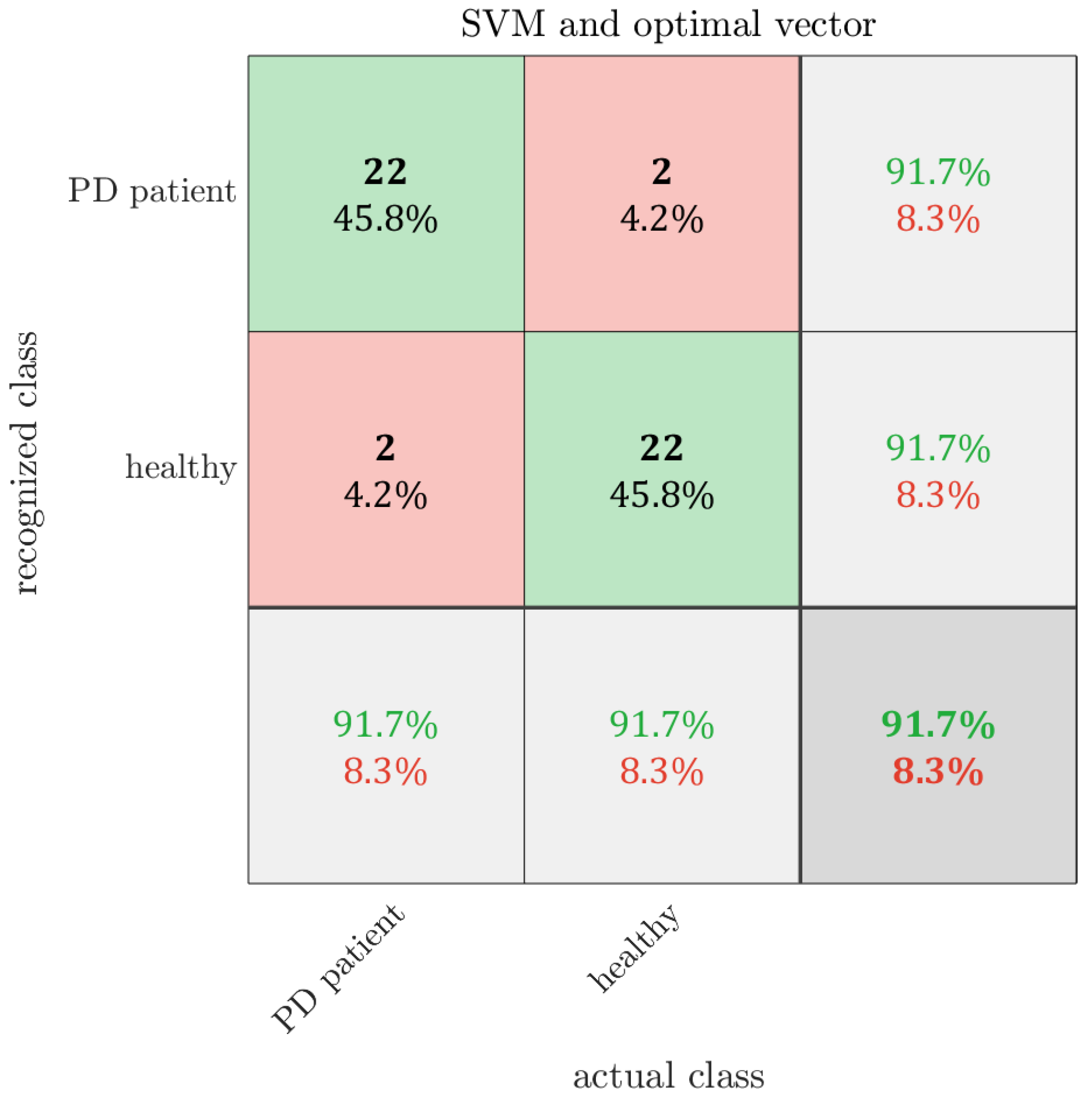
7. Results
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pang, M.; Peng, R.; Wang, Y.; Zhu, Y.; Wang, P.; Moussian, B.; Su, Y.; Liu, X.; Ming, D. Molecular understanding of the translational models and the therapeutic potential natural products of Parkinson’s disease. Biomed. Pharmacother. 2022, 155, 113718. [Google Scholar] [CrossRef] [PubMed]
- Kouli, A.; Torsney, K.M.; Kuan, W. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis; Codon Publications eBooks: Brisbane, Australia, 2018; pp. 3–26. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Constantinescu, R.; Thompson, J.P.; Biglan, K.M.; Holloway, R.G.; Kieburtz, K.; Marshall, F.J.; Ravina, B.M.; Schifitto, G.; Siderowf, A.; et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 2007, 68, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Statistics. Parkinson’s Foundation. Available online: https://www.parkinson.org/understanding-parkinsons/statistics (accessed on 17 August 2024).
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Massano, J.; Bhatia, K.P. Clinical approach to parkinson’s disease: Features, diagnosis, and principles of management. Cold Spring Harb. Perspect. Med. 2012, 2, a008870. [Google Scholar] [CrossRef] [PubMed]
- Van Galen, G.P. Handwriting: Issues for a psychomotor theory. Hum. Mov. Sci. 1991, 10, 165–1911. [Google Scholar] [CrossRef]
- Becker, G.; Müller, A.; Braune, S.; Büttner, T.; Benecke, R.; Greulich, W.; Klein, W.; Mark, G.; Rieke, J.; Thümler, R. Early diagnosis of parkinson’s disease. J. Neurol. 2002, 249, iii40–iii48. [Google Scholar] [CrossRef]
- The Unified Parkinson’s Disease Rating Scale (UPDRS): Status and recommendations. Mov. Disord. 2003, 18, 738–750. [CrossRef]
- McLennan, J.; Nakano, K.; Tyler, H.; Schwab, R. Micrographia in parkinson’s disease. J. Neurol. Sci. 1972, 15, 141–152. [Google Scholar] [CrossRef]
- Margolin, D.I.; Wing, A.M. Agraphia and Micrographia: Clinical manifestations of motor programming and performance disorders. Acta Psychol. 1983, 54, 263–283. [Google Scholar] [CrossRef]
- Shukla, A.W.; Ounpraseuth, S.; Okun, M.S.; Gray, V.; Schwankhaus, J.; Metzer, W.S. Micrographia and related deficits in parkinson’s disease: A cross-sectional study. BMJ Open 2012, 2, e000628. [Google Scholar] [CrossRef]
- Mergl, R.; Tigges, P.; Schröter, A.; Möller, H.-J.; Hegerl, U. Digitized analysis of handwriting and drawing movements in healthy subjects: Methods, results and perspectives. J. Neurosci. Methods 1999, 90, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Lenka, A.; Pal, P.K. Handwriting analysis in parkinson’s disease: Current status and future directions. Mov. Disord. Clin. Pract. 2017, 4, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Teulings, H.-L.; Stelmach, G.E. Force amplitude and force duration in Parkinsonian handwriting. In Tutorials in Motor Neuroscience; Springer: Dordrecht, The Netherlands, 1991; pp. 149–160. [Google Scholar] [CrossRef]
- Teulings, H.-L.; Stelmach, G.E. Signal-to-noise ratio of handwriting size, force, and time: Cues to early markers of parkinson’s disease? Sensorimotor Impair. Elder. 1993, 75, 311–327. [Google Scholar] [CrossRef]
- Oliveira, R.M.; Gurd, J.M.; Nixon, P.; Marshall, J.C.; Passingham, R.E. Micrographia in parkinson’s disease: The effect of providing external cues. J. Neurol. Neurosurg. Psychiatry 1997, 63, 429–433. [Google Scholar] [CrossRef]
- Van Gemmert, A.W.; Teulings, H.L.; Stelmach, G.E. Parkinsonian Patients Reduce Their Stroke Size with Increased Processing Demands. Brain Cogn. 2001, 47, 504–512. [Google Scholar] [CrossRef]
- Van Gemmert, A.W.; Adler, C.H.; Stelmach, G.E. Parkinson’s disease patients undershoot target size in handwriting and similar tasks. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1502–1508. [Google Scholar] [CrossRef]
- Caligiuri, M.P.; Teulings, H.-L.; Filoteo, J.V.; Song, D.; Lohr, J.B. Quantitative measurement of handwriting in the assessment of drug-induced parkinsonism. Hum. Mov. Sci. 2006, 25, 510–522. [Google Scholar] [CrossRef]
- Broeder, S.; Nackaerts, E.; Nieuwboer, A.; Smits-Engelsman, B.; Swinnen, S.; Heremans, E. The effects of dual tasking on handwriting in patients with Parkinson’s disease. Neuroscience 2014, 263, 193–202. [Google Scholar] [CrossRef]
- Teulings, H.-L.; Contreras-Vidal, J.L.; E Stelmach, G.; Adler, C.H. Adaptation of handwriting size under distorted visual feedback in patients with Parkinson’s disease and elderly and young controls. J. Neurol. Neurosurg. Psychiatry 2002, 72, 315–324. [Google Scholar] [CrossRef]
- Contreras-Vidal, J.L.; Teulings, H.-L.; E Stelmach, G.; Adler, C.H. Adaptation to changes in vertical display gain during handwriting in Parkinson’s disease patients, elderly and young controls. Park. Relat. Disord. 2002, 9, 77–84. [Google Scholar] [CrossRef]
- Ponsen, M.M.; Daffertshofer, A.; Wolters, E.C.; Beek, P.J.; Berendse, H.W. Impairment of complex upper limb motor function in de novo Parkinson’s disease. Park. Relat. Disord. 2008, 14, 199–204. [Google Scholar] [CrossRef]
- Smits, E.J.; Tolonen, A.J.; Cluitmans, L.; van Gils, M.; Conway, B.A.; Zietsma, R.C.; Leenders, K.L.; Maurits, N.M. Standardized handwriting to assess bradykinesia, micrographia and tremor in Parkinson’s disease. PLoS ONE 2014, 9, e97614. [Google Scholar] [CrossRef] [PubMed]
- Zham, P.; Raghav, S.; Kempster, P.; Arjunan, S.P.; Wong, K.; Nagao, K.J.; Kumar, D.K. A kinematic study of progressive micrographia in Parkinson’s disease. Front. Neurol. 2019, 10, 403. [Google Scholar] [CrossRef] [PubMed]
- van Gemmert, A.W.; Teulings, H.-L.; Stelmach, G.E. The influence of mental and motor load on handwriting movements in Parkinsonian patients. Acta Psychol. 1998, 100, 161–175. [Google Scholar] [CrossRef]
- Tucha, O.; Mecklinger, L.; Thome, J.; Reiter, A.; Alders, G.L.; Sartor, H.; Naumann, M.; Lange, K.W. Kinematic analysis of dopaminergic effects on skilled handwriting movements in Parkinson’s disease. J. Neural Transm. 2005, 113, 609–623. [Google Scholar] [CrossRef]
- Poluha, P.; Teulings, H.-L.; Brookshire, R. Handwriting and speech changes across the levodopa cycle in Parkinson’s disease. Acta Psychol. 1998, 100, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Broderick, M.P.; Van Gemmert, A.W.A.; Shill, H.A.; Stelmach, G.E. Hypometria and bradykinesia during drawing movements in individuals with Parkinson’s disease. Exp. Brain Res. 2009, 197, 223–233. [Google Scholar] [CrossRef]
- Rosenblum, S.; Samuel, M.; Zlotnik, S.; Erikh, I.; Schlesinger, I. Handwriting as an objective tool for Parkinson’s disease diagnosis. J. Neurol. 2013, 260, 2357–2361. [Google Scholar] [CrossRef]
- Jerkovic, V.M.; Kojic, V.; Miskovic, N.D.; Djukic, T.; Kostic, V.S.; Popovic, M.B. Analysis of on-surface and in-air movement in handwriting of subjects with Parkinson’s disease and atypical parkinsonism. Biomed. Eng. Biomed. Tech. 2018, 64, 187–194. [Google Scholar] [CrossRef]
- Drotár, P.; Mekyska, J.; Rektorová, I.; Masarová, L.; Smékal, Z.; Faundez-Zanuy, M. Analysis of in-air movement in handwriting: A novel marker for Parkinson’s disease. Comput. Methods Programs Biomed. 2014, 117, 405–411. [Google Scholar] [CrossRef]
- Drotar, P.; Mekyska, J.; Rektorova, I.; Masarova, L.; Smekal, Z.; Faundez-Zanuy, M. Decision support Framework for Parkinson’s Disease based on novel handwriting markers. IEEE Trans. Neural Syst. Rehabilit. Eng. 2015, 23, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Drotár, P.; Mekyska, J.; Rektorová, I.; Masarová, L.; Smékal, Z.; Faundez-Zanuy, M. Evaluation of handwriting kinematics and pressure for differential diagnosis of Parkinson’s disease. Artif. Intell. Med. 2016, 67, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Impedovo, D.; Pirlo, G.; Vessio, G. Dynamic handwriting analysis for supporting earlier Parkinson’s disease diagnosis. Information 2018, 9, 247. [Google Scholar] [CrossRef]
- Mucha, J.; Mekyska, J.; Galaz, Z.; Faundez-Zanuy, M.; Lopez-De-Ipina, K.; Zvoncak, V.; Kiska, T.; Smekal, Z.; Brabenec, L.; Rektorova, I. Identification and monitoring of Parkinson’s Disease dysgraphia based on Fractional-Order derivatives of online handwriting. Appl. Sci. 2018, 8, 2566. [Google Scholar] [CrossRef]
- Moetesum, M.; Siddiqi, I.; Vincent, N.; Cloppet, F. Assessing visual attributes of handwriting for prediction of neurological disorders—A case study on Parkinson’s disease. Pattern Recognit. Lett. 2018, 121, 19–27. [Google Scholar] [CrossRef]
- Naseer, A.; Rani, M.; Naz, S.; Razzak, M.I.; Imran, M.; Xu, G. Refining Parkinson’s neurological disorder identification through deep transfer learning. Neural Comput. Appl. 2019, 32, 839–854. [Google Scholar] [CrossRef]
- Arnab, S.R.; Uddin, M.R.; Jawad, A. Enhancing Parkinson’s disease detection through handwriting analysis: A deep learning approach with segmentation and transfer learning. In Proceedings of the 26th International Conference on Computer and Information Technology (ICCIT), Cox’s Bazar, Bangladesh, 13–15 December 2023. [Google Scholar] [CrossRef]
- Naz, S.; Kamran, I.; Gul, S.; Hadi, F.; Khalifa, F. Multi-Model fusion of CNNs for identification of Parkinson’s disease using handwritten samples. IEEE Access 2023, 11, 135600–135608. [Google Scholar] [CrossRef]
- Chmielińska, J.; Białek, K.; Potulska-Chromik, A.; Jakubowski, J.; Majda-Zdancewicz, E.; Nojszewska, M.; Kostera-Pruszczyk, A.; Dobrowolski, A. Multimodal data acquisition set for objective assessment of Parkinson’s disease. Radioelectron. Syst. Conf. 2019, 11442, 115–124. [Google Scholar] [CrossRef]
- Białek, K.; Jakubowski, J.; Białek, R. Parametrization of handwriting for the assessment of neurodegenerative disorders on the example of Parkinson’s disease. Prz. Elektrotechniczny 2024, 1, 277–279. [Google Scholar] [CrossRef]
- Nersisyan, S.; Novosad, V.; Galatenko, A.; Sokolov, A.; Bokov, G.; Konovalov, A.; Alekseev, D.; Tonevitsky, A. ExhauFS: Exhaustive search-based feature selection for classification and survival regression. PeerJ 2022, 10, e13200. [Google Scholar] [CrossRef]
- De La Torre Bucio, J.; Paz, A.R.; Rios, J.A.M. Exhaustive search applied to time series forecasting methods using parallel processing. In Proceedings of the 2022 IEEE International Autumn Meeting on Power, Electronics and Computing (ROPEC), Ixtapa, Mexico, 9–11 November 2022. [Google Scholar] [CrossRef]
- Asci, F.; Scardapane, S.; Zampogna, A.; D’onofrio, V.; Testa, L.; Patera, M.; Falletti, M.; Marsili, L.; Suppa, A. Handwriting Declines with Human aging: A Machine Learning study. Front. Aging Neurosci. 2022, 14, 889930. [Google Scholar] [CrossRef] [PubMed]
- Majda-Zdancewicz, E.; Potulska-Chromik, A.; Jakubowski, J.; Nojszewska, M.; Kostera-Pruszczyk, A. Deep learning vs feature engineering in the assessment of voice signals for diagnosis in Parkinson’s disease. Bull. Pol. Acad. Sci. Tech. Sci. 2021, 69, 137347. [Google Scholar] [CrossRef]
- Jakubowski, J.; Potulska-Chromik, A.; Białek, K.; Nojszewska, M.; Kostera-Pruszczyk, A. A study on the possible diagnosis of Parkinson’s disease on the basis of facial image analysis. Electronics 2021, 10, 2832. [Google Scholar] [CrossRef]
- Jakubowski, J.; Potulska-Chromik, A.; Chmielińska, J.; Nojszewska, M.; Kostera-Pruszczyk, A. Application of imaging techniques to objectify the Finger Tapping test used in the diagnosis of Parkinson’s disease. Bull. Pol. Acad. Sci. Tech. Sci. 2023, 71, 144886. [Google Scholar] [CrossRef]

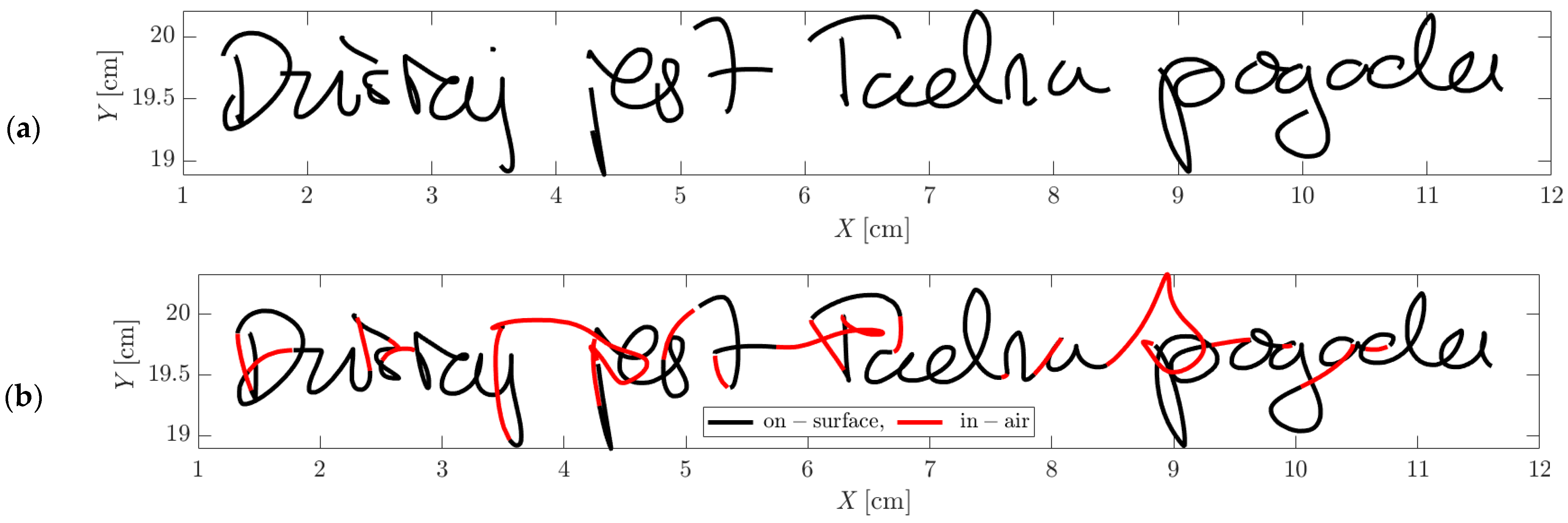
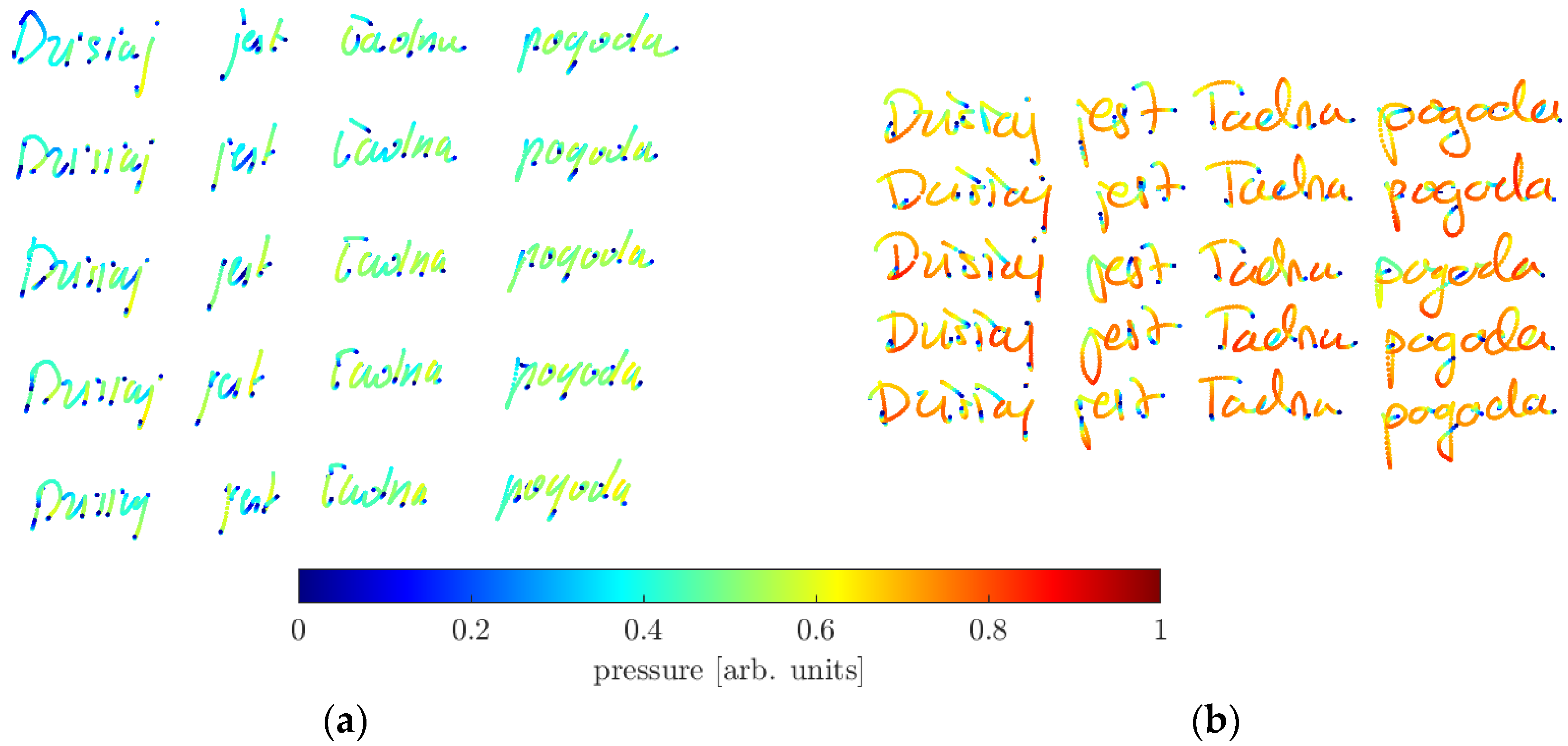
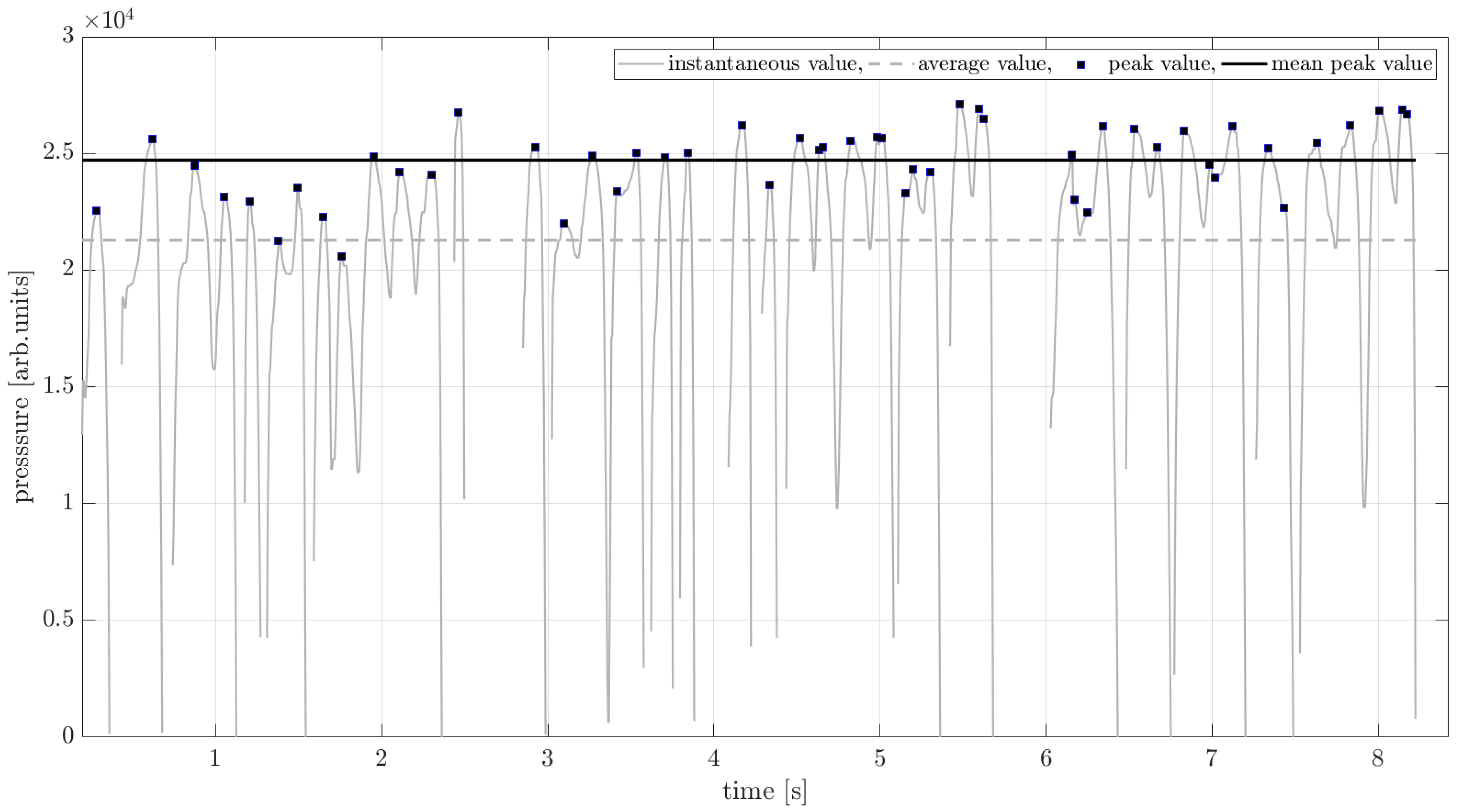

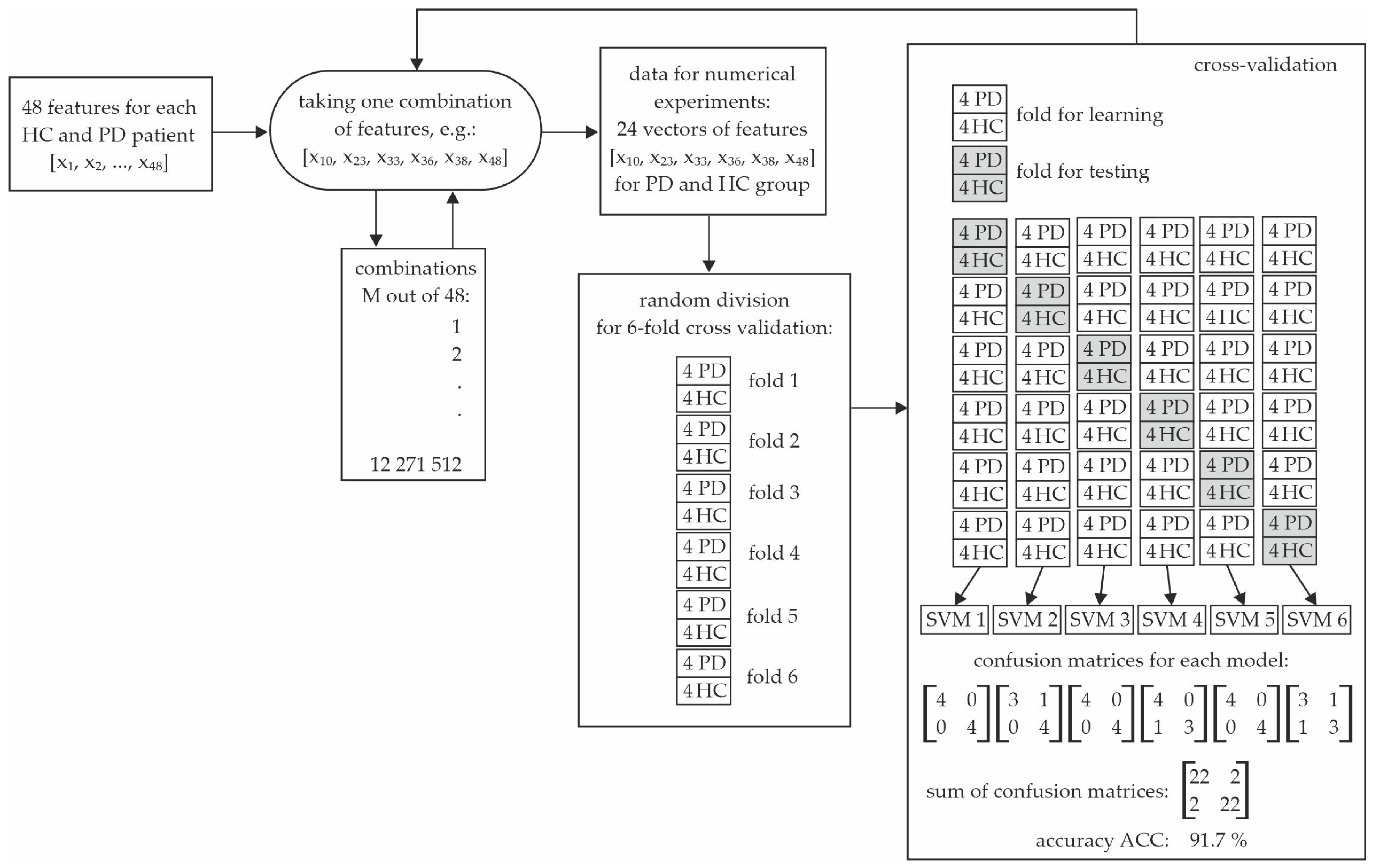
| Volunteers | Male | Female | Total | Age Range |
|---|---|---|---|---|
| PD | 8 | 16 | 24 | 28–84 |
| HC | 19 | 5 | 24 | 25–74 |
| Group of Features | Symptoms of PD | Measured Feature | Description |
|---|---|---|---|
| Time features | Bradykinesia |
|
|
|
| ||
|
| ||
| Velocity features | Bradykinesia |
|
|
| Pressure features | Rest tremor Limb stiffness |
|
|
|
| ||
|
| ||
|
| ||
| Handwriting geometry features | Micrographia Hypometria Kinesthesia |
|
|
|
| ||
|
| ||
|
|
| Class | In-Air Time [s] | On-Surface Time [s] | Total [s] |
|---|---|---|---|
| PD | 4.1 ± 2.0 | 10.3 ± 3.3 | 14.5 ± 4.8 |
| HC | 3.7 ± 1.5 | 7.9 ± 1.6 | 11.5 ± 2.8 |
| Source | Df | Test Statistic | Value | F | p-Value |
|---|---|---|---|---|---|
| State (PD or healthy) | 1 | Pillai | 0.26 | 2.33 | 0.05 |
| Wilks | 0.74 | 2.33 | 0.05 | ||
| Hotteling | 0.36 | 2.33 | 0.05 | ||
| Roy | 0.36 | 2.33 | 0.05 | ||
| Age (younger adults 18–35 years or middle-aged adults 36–55 years or older adults 56 years and older) [46] | 2 | Pillai | 0.16 | 0.58 | 0.85 |
| Wilks | 0.84 | 0.58 | 0.85 | ||
| Hotteling | 0.18 | 0.59 | 0.84 | ||
| Roy | 0.16 | 1.09 | 0.39 |
| Reference | ACC [%] | Sensitivity [%] | Specificity [%] | Number of Features | Additional Information |
|---|---|---|---|---|---|
| [33] Drotár, P. et al., 2014 | 85.61 | 85.95 | 85.26 | 50 | Single sentence, PaHaW database |
| [34] Drotár, P. et al., 2014 | 88.1 | - | - | 162 | 7 different handwriting tasks, PaHaW |
| [35] Drotár, P. et al., 2016 | 81.3 | 80,9 | 87.4 | 20 | 8 different handwriting tasks, PaHaW |
| [36] Impedovo, D. et al., 2018 | 74.76 | 68.97 | 77.78 | unknown | 8 different handwriting tasks, PaHaW |
| [32] Jerkovic, V. M. et al., 2019 | 86.05 | - | - | 20 | Single sentence |
| this paper | 91.67 | 91.67 | 91.67 | 6 | Same 5 sentences |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Białek, K.; Potulska-Chromik, A.; Jakubowski, J.; Nojszewska, M.; Kostera-Pruszczyk, A. Analysis of Handwriting for Recognition of Parkinson’s Disease: Current State and New Study. Electronics 2024, 13, 3962. https://doi.org/10.3390/electronics13193962
Białek K, Potulska-Chromik A, Jakubowski J, Nojszewska M, Kostera-Pruszczyk A. Analysis of Handwriting for Recognition of Parkinson’s Disease: Current State and New Study. Electronics. 2024; 13(19):3962. https://doi.org/10.3390/electronics13193962
Chicago/Turabian StyleBiałek, Kamila, Anna Potulska-Chromik, Jacek Jakubowski, Monika Nojszewska, and Anna Kostera-Pruszczyk. 2024. "Analysis of Handwriting for Recognition of Parkinson’s Disease: Current State and New Study" Electronics 13, no. 19: 3962. https://doi.org/10.3390/electronics13193962
APA StyleBiałek, K., Potulska-Chromik, A., Jakubowski, J., Nojszewska, M., & Kostera-Pruszczyk, A. (2024). Analysis of Handwriting for Recognition of Parkinson’s Disease: Current State and New Study. Electronics, 13(19), 3962. https://doi.org/10.3390/electronics13193962






