Analysis of Pressure Characteristics of Ultra-High Specific Energy Lithium Metal Battery for Flying Electric Vehicles
Abstract
1. Introduction
2. Experiments
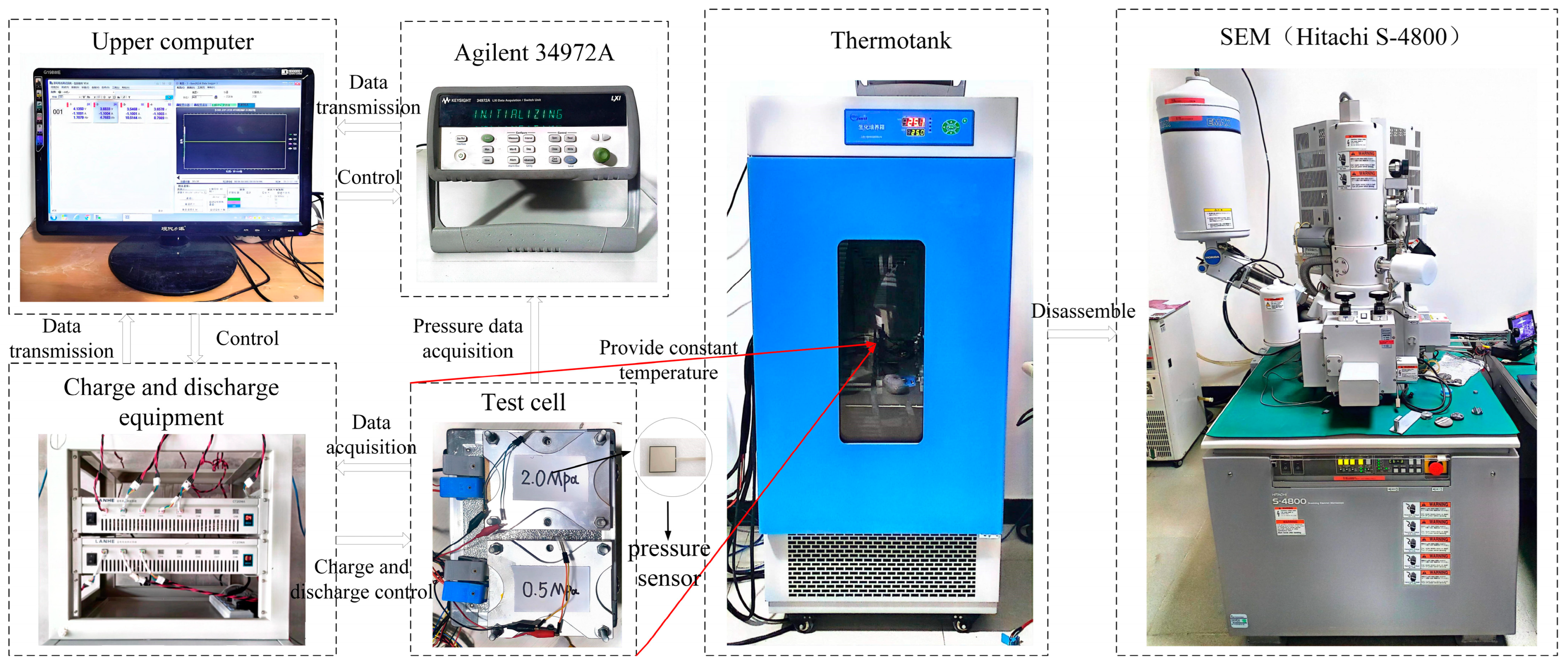
2.1. Experiment Subjects and Experiment Platforms
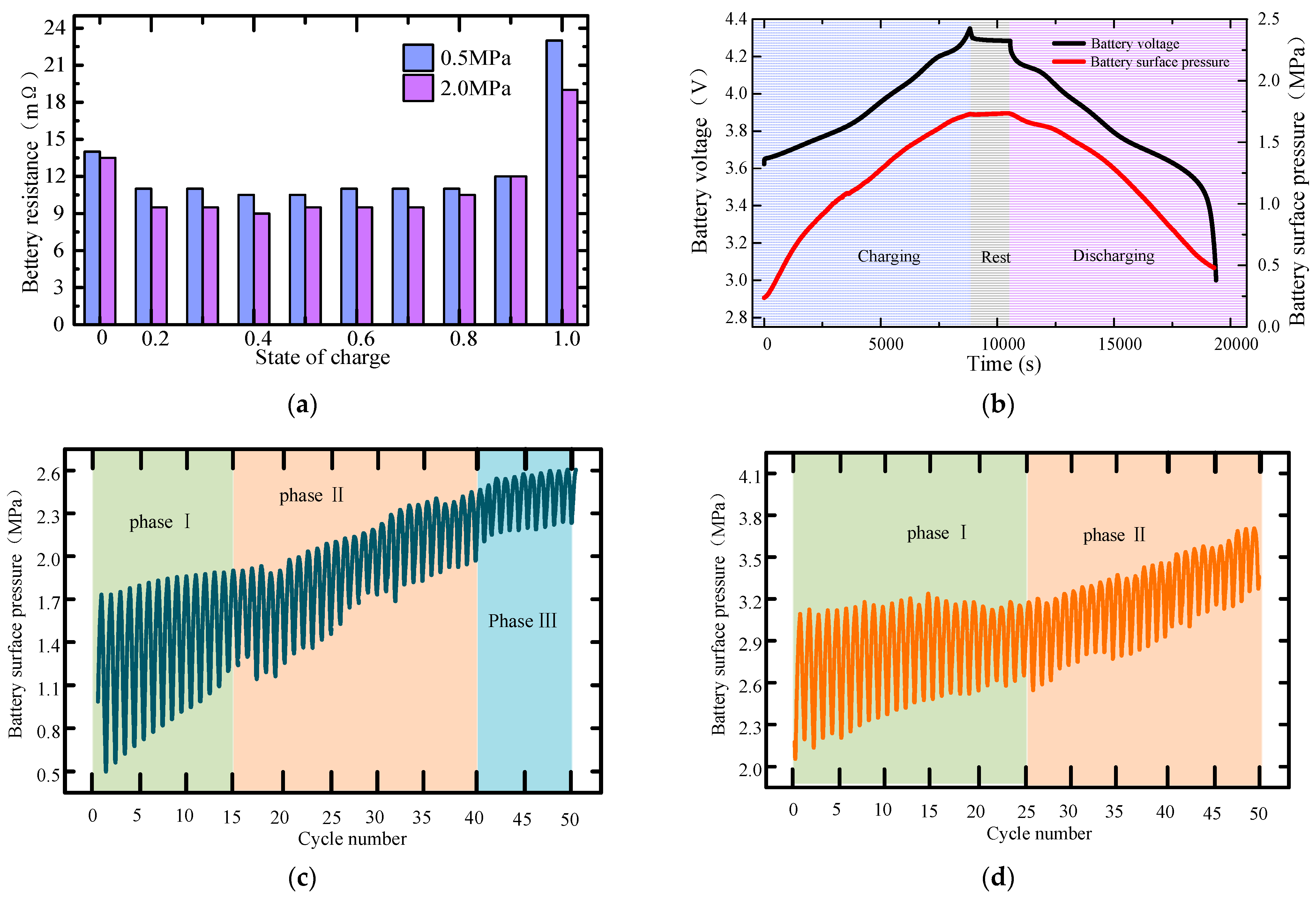
2.2. An Analysis of the Experimental Results
3. Methods
4. Results and Discussion
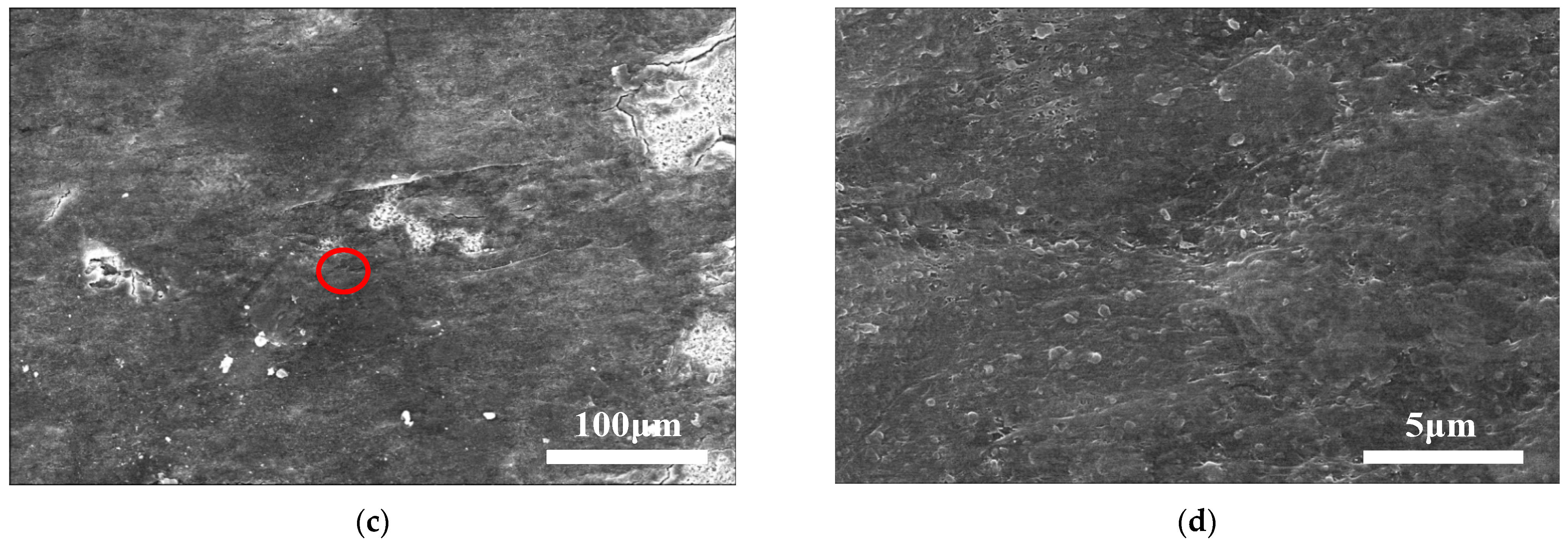
4.1. Initial Lithium Dendrite Morphology
4.2. Crystal Morphology of Lithium Branches at Different Cycle Stages
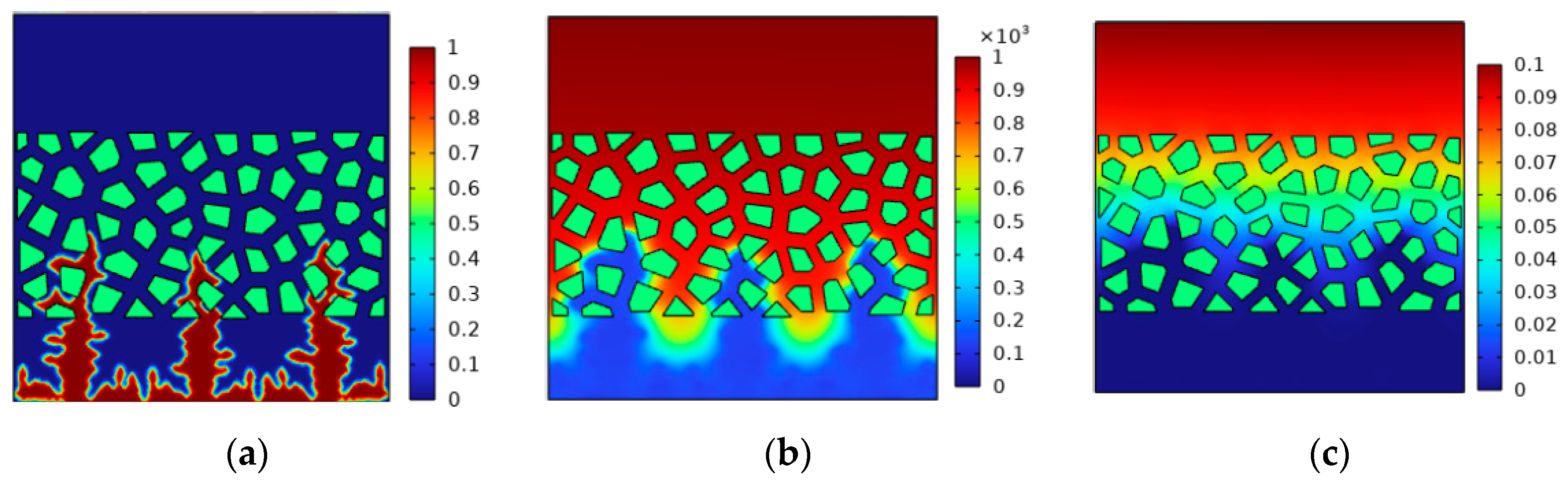
4.3. Effect of Pressure on Lithium Deposition Morphology
5. Conclusions
- (1)
- The maximum pressure on the surface of the lithium metal battery presents three different stages along with the gradual aging of the battery. A large pretension force can extend the cycle of initial stage I, delay the occurrence of stage Ⅲ battery failure, and then improve the battery’s cycle life. The scanning electron microscope further proves that the external pressure can effectively improve the surface structure of the electrode.
- (2)
- With the passage of time and the proceeding of continuous charging and discharging, the diffusion coefficient of the electrolyte gradually decreases. At this time, the lithium dendrite gradually diffuses from the intermediate phase area to produce slender branches and extend on the electrode surface, further increasing the risk of short circuit in the battery.
- (3)
- The increase in external pressure shortens the spindle tip length of the lithium dendrite and the migration curvature in the diaphragm, which promotes the morphology of lithium deposition to develop in a smooth and thick direction. However, as the battery life drops, the diffusion coefficient of the electrolyte goes down, and the battery’s internal stress increases gradually, thus greater external pressure is needed to exert a significant inhibitory effect on the growth of the lithium dendrite.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hammadi, M.A.; AlMesafri, N.; Zafar, S.; Santos, G. Design and Analytical Analyses of eVTOL UAV Performance Calculator for Power and Energy. In Proceedings of the 2023 10th International Conference on Recent Advances in Air and Space Technologies (RAST), Istanbul, Turkey, 7–9 June 2023; pp. 1–6. [Google Scholar]
- Wang, F.; Bai, J.; Yang, L.; Rao, B.; Huang, L.B. Analysis of urban air traffic development by exploring new modes of flying car commuting. J. Beijing Inst. Technol. 2023, 43, 665–675. [Google Scholar]
- Liu, W.X.; Hou, C.; Yang, Y.L.; Chen, Z.H.; Han, J.; Hu, X.S. Analysis of key performance indicators of electric flying vehicles for urban air traffic. J. Mech. Eng. 2024, 60, 1–19. [Google Scholar]
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef] [PubMed]
- Machín, A.; Morant, C.; Márquez, F. Advancements and Challenges in Solid-State Battery Technology: An In-Depth Review of Solid Electrolytes and Anode Innovations. Batteries 2024, 10, 29. [Google Scholar] [CrossRef]
- Cheng, X.B.; Zhang, R.; Zhao, C.Z.; Wei, F.; Zhang, J.G.; Zhang, Q. A review of solid electrolyte interphases on lithium metal anode. Adv. Sci. 2016, 3, 1500213. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, S.; Fu, J.; Liu, Y.; Chen, Z. Manufacturing High-Energy-Density Sulfidic Solid-State Batteries. Batteries 2023, 9, 347. [Google Scholar] [CrossRef]
- Ni, J.; Lei, Y.; Han, Y.; Zhang, Y.; Zhang, C.; Geng, Z.; Xiao, Q. Prefabrication of a Lithium Fluoride Interfacial Layer to Enable Dendrite-Free Lithium Deposition. Batteries 2023, 9, 283. [Google Scholar] [CrossRef]
- Ramasubramanian, A.; Yurkiv, V.; Foroozan, T.; Ragone, M.; Shahbazian-Yassar, R.; Mashayek, F. Lithium diffusion mechanism through solid–electrolyte interphase in rechargeable lithium batteries. J. Phys. Chem. C 2019, 123, 10237–10245. [Google Scholar] [CrossRef]
- Mu, W.; Liu, X.; Wen, Z.; Liu, L. Numerical simulation of the factors affecting the growth of lithium dendrites. J. Energy Storage 2019, 26, 100921. [Google Scholar] [CrossRef]
- Verma, P.; Puravankara, S.; Nandanwar, M.N.; Chakraborty, J. Insights into the Morphological Evolution of Mossy Dendrites in Lithium Metal Symmetric and Full Cell: A Modelling Study. J. Electrochem. Soc. 2023, 170, 030529. [Google Scholar] [CrossRef]
- Cheng, F.; Hu, Y.; Zhao, L. Analysis of weak solutions for the phase-field model for lithium-ion batteries. Appl. Math. Model. 2020, 78, 185–199. [Google Scholar] [CrossRef]
- Ren, Y.; Zhou, Y.; Cao, Y. Inhibit of lithium dendrite growth in solid composite electrolyte by phase-field modeling. J. Phys. Chem. C 2020, 124, 12195–12204. [Google Scholar] [CrossRef]
- Arguello, M.E.; Gumulya, M.; Derksen, J.; Utikar, R.; Calo, V.M. Phase-field modeling of planar interface electrodeposition in lithium-metal batteries. J. Energy Storage 2022, 50, 104627. [Google Scholar] [CrossRef]
- Jing, H.; Xing, H.; Dong, X.; Han, Y. Nonlinear phase-field modeling of lithium dendritic growth during electrodeposition. J. Electrochem. Soc. 2022, 169, 032511. [Google Scholar] [CrossRef]
- Monroe, C.; Newman, J. The impact of elastic deformation on deposition kinetics at lithium/polymer interfaces. J. Electrochem. Soc. 2005, 152, A396. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, H.W.; Liang, L.Y.; Liu, Z.; Qi, Y.; Lu, P.; Chen, J.; Chen, L.-Q. Modulation of dendritic patterns during electrodeposition: A nonlinear phase-field model. J. Power Sources 2015, 300, 376–385. [Google Scholar] [CrossRef]
- Hong, Z.; Viswanathan, V. Prospect of thermal shock induced healing of lithium dendrite. ACS Energy Lett. 2019, 4, 1012–1019. [Google Scholar] [CrossRef]
- Zhang, R.; Shen, X.; Cheng, X.-B.; Zhang, Q. The dendrite growth in 3D structured lithium metal anodes: Electron or ion transfer limitation? Energy Storage Mater. 2019, 23, 556–565. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, R.; Shi, P.; Chen, X.; Zhang, Q. How does external pressure shape Li dendrites in Li metal batteries? Adv. Energy Mater. 2021, 11, 2003416. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Q.J.; Harrison, K.L.; Roberts, S.A.; Harris, S.J. Pressure-driven interface evolution in solid-state lithium metal batteries. Cell Rep. Phys. Sci. 2020, 1, 100012. [Google Scholar] [CrossRef]
- Yurkiv, V.; Foroozan, T.; Ramasubramanian, A.; Shahbazian-Yassar, R.; Mashayek, F. Phase-field modeling of solid electrolyte interface (SEI) influence on Li dendritic behavior. Electrochim. Acta 2018, 265, 609–619. [Google Scholar] [CrossRef]
- Ganser, M.; Hildebrand, F.E.; Klinsmann, M.; Hanauer, M.; Kamlah, M.; McMeeking, R.M. An extended formulation of butler-volmer electrochemical reaction kinetics including the influence of mechanics. J. Electrochem. Soc. 2019, 166, H167. [Google Scholar] [CrossRef]
- Geng, X.B.; Li, D.G.; Xu, B. Mechanical stress-thermodynamic phase-field simulation of lithium dendrite growth in solid electrolyte battery. Acta Phys. Sin. 2023, 72, 220201. [Google Scholar] [CrossRef]
- Li, Y.; Sha, L.; Lv, P.; Qiu, N.; Zhao, W.; Chen, B.; Hu, P.; Zhang, G. Influences of separator thickness and surface coating on lithium dendrite growth: A phase-field study. Materials 2022, 15, 7912. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Li, Y.; Wang, D.; Zhao, W.; Zhang, G.; Yu, J.; Sha, L.; Shi, S. Understanding the separator pore size inhibition effect on lithium dendrite via phase-field simulations. Chin. Chem. Lett. 2022, 33, 3287–3290. [Google Scholar]
- Jana, A.; García, R.E. Lithium dendrite growth mechanisms in liquid electrolytes. Nano Energy 2017, 41, 552–565. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, Y.-N.; Han, D.; Zhou, J.; Zhou, D.; Tang, W.; Goodenough, J.B. Thermodynamic understanding of Li-dendrite formation. Joule 2020, 4, 1864–1879. [Google Scholar] [CrossRef]
- Sharon, D.; Bennington, P.; Patel, S.N.; Nealey, P.F. Stabilizing dendritic electrodeposition by limiting spatial dimensions in nanostructured electrolytes. ACS Energy Lett. 2020, 5, 2889–2896. [Google Scholar] [CrossRef]
- Qiao, D.; Liu, X.; Wen, Z.; Dou, R.; Zhou, W. Numerical analysis of the inhibition of heating and pulse charging on the growth of lithium dendrites. Energy Storage Sci. Technol. 2022, 11, 1008–1018. [Google Scholar]
- Wilkinson, D.; Wainwright, D. In-situ study of electrode stack growth in rechargeable cells at constant pressure. J. Electroanal. Chem. 1993, 355, 193–203. [Google Scholar] [CrossRef]
- Louli, A.J.; Genovese, M.; Weber, R.; Hames, S.; Logan, E.; Dahn, J. Exploring the impact of mechanical pressure on the performance of anode-free lithium metal cells. J. Electrochem. Soc. 2019, 166, A1291–A1299. [Google Scholar] [CrossRef]
- McDowell, M.T.; Cortes, F.J.Q.; Thenuwara, A.C.; Lewis, J.A. Toward High-Capacity Battery Anode Materials: Chemistry and Mechanics Intertwined. Chem. Mater. 2020, 32, 8755–8771. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, L.; Chen, J.; Sun, H.; Yang, T.; Liu, Q.; Huang, Q.; Zhu, T.; Huang, J. Electro-chemo-mechanics of lithium in solid state lithium metal batteries. Energy Environ. Sci. 2021, 14, 602–642. [Google Scholar] [CrossRef]
- Wang, M.J.; Kazyak, E.; Dasgupta, N.P.; Sakamoto, J. Transitioning solid-state batteries from lab to market: Linking electro-chemo-mechanics with practical considerations. Joule 2021, 5, 1371–1390. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, R.; Zhao, C.; Wu, P.; Zhang, Y.T.; Zhang, J.D.; Fan, L.Z.; Liu, Q.B.; Chen, A.B.; Zhang, Q. Progress in the medium force-electrochemical mechanism of lithium metal batteries. Energy Storage Sci. Technol. 2022, 11, 2781–2797. [Google Scholar]




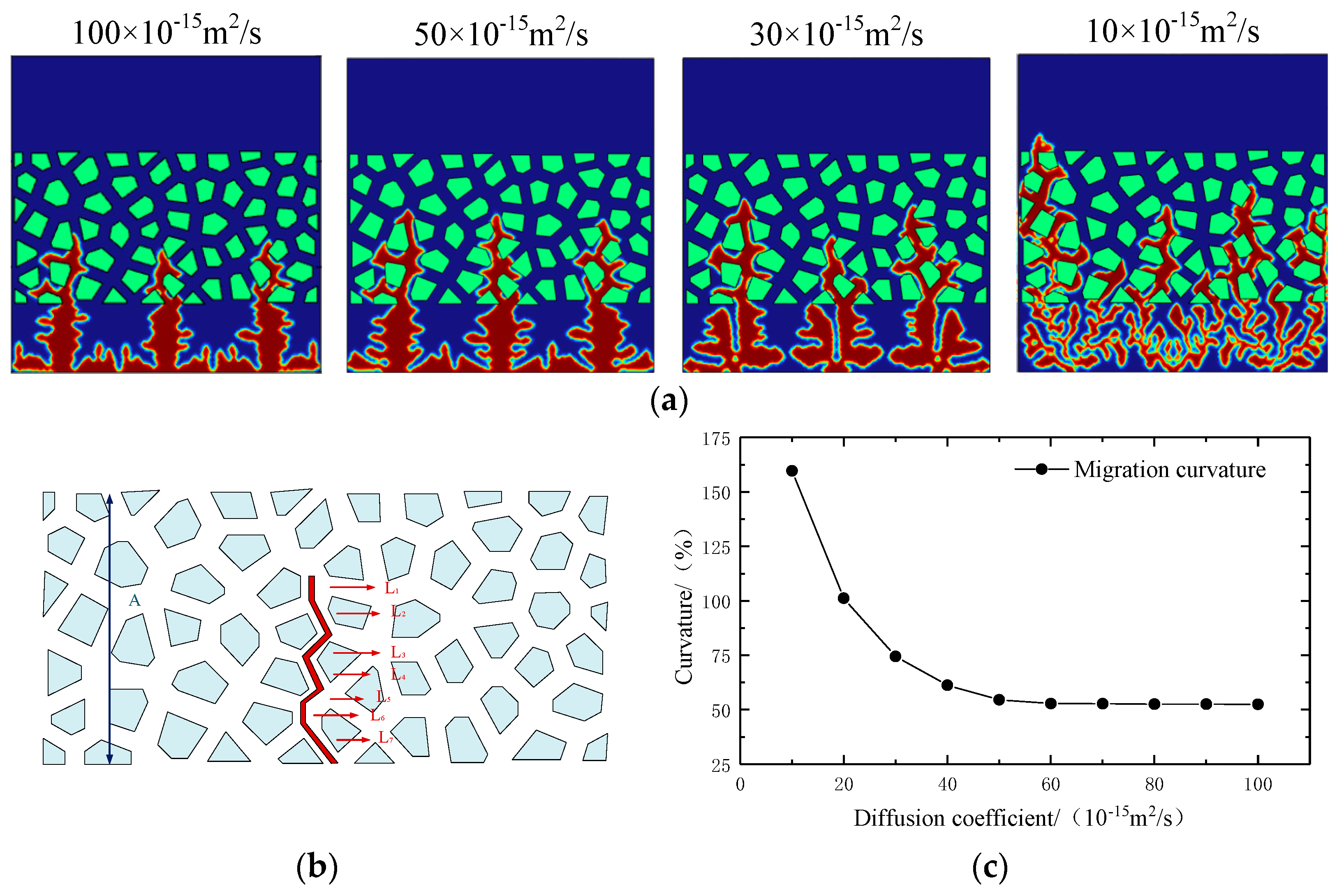
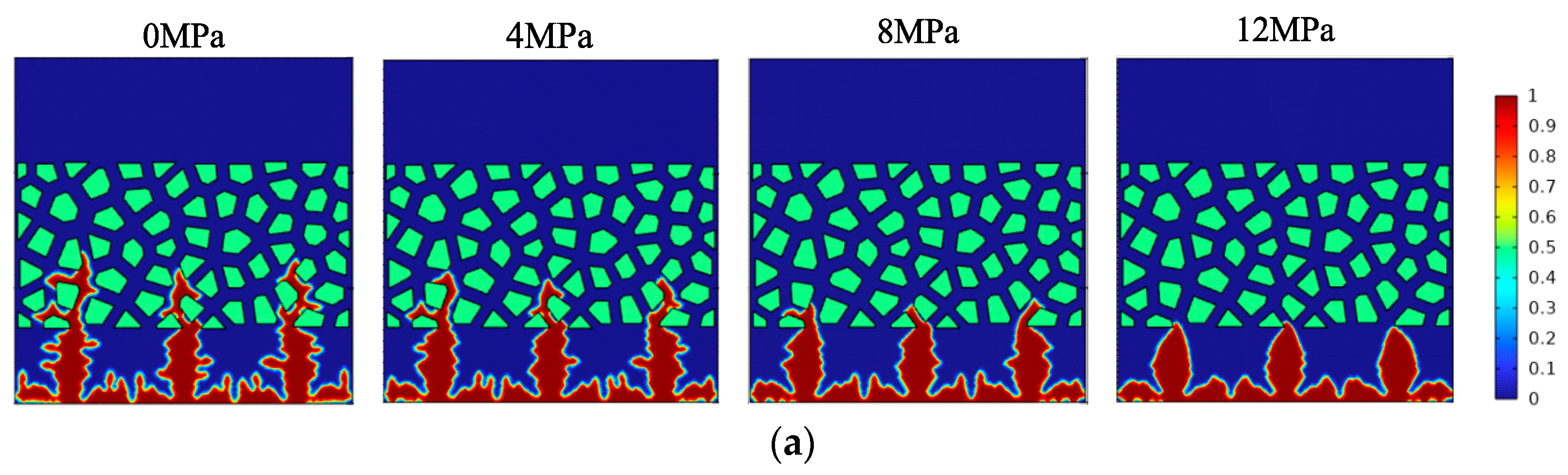
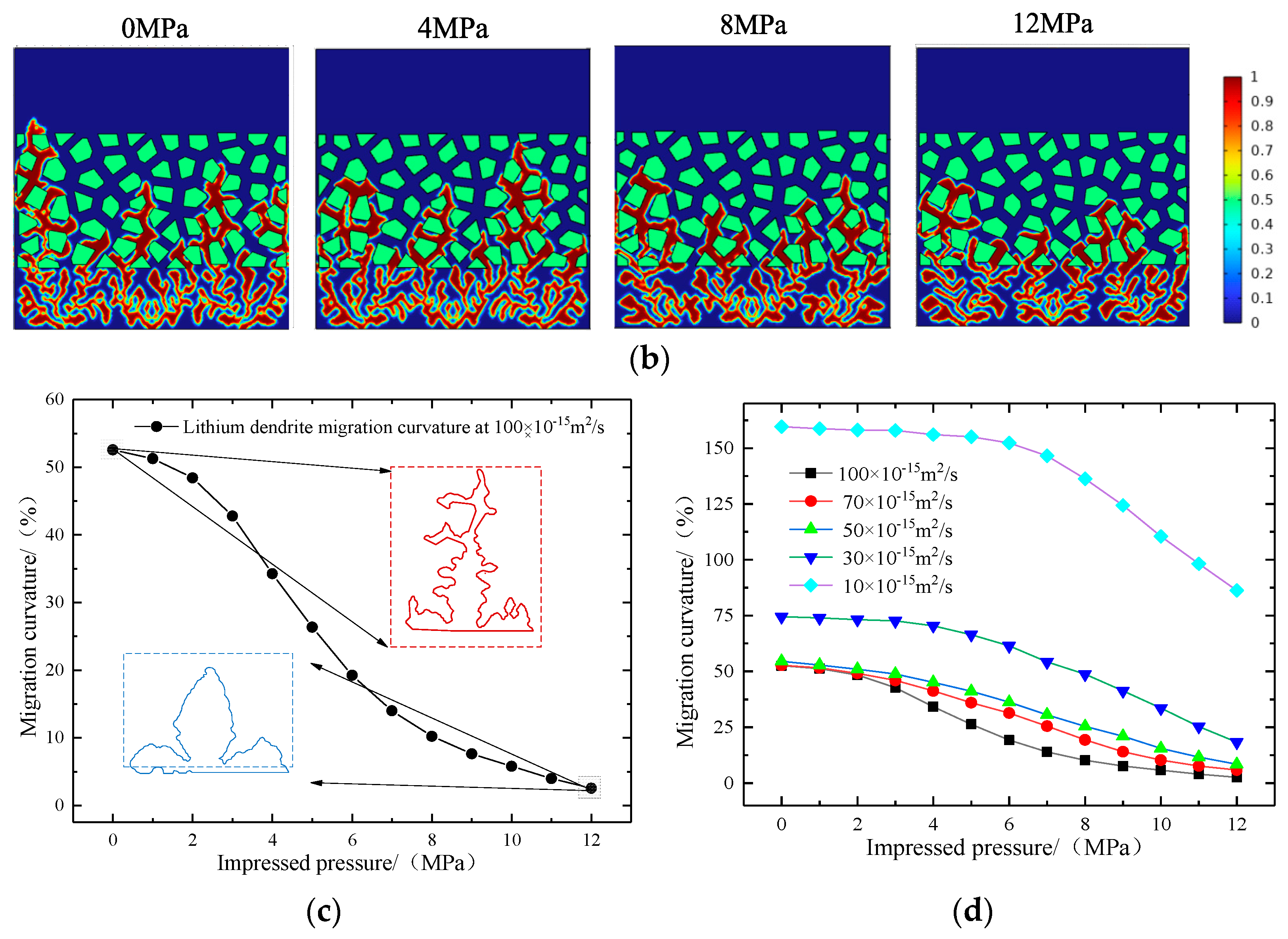
| Parameter | Symbol | Numeric Value | References |
|---|---|---|---|
| Electrode interface mobility | 10−6 [m3·(J*s)−1] | [25,26] | |
| Mobility at the diaphragm interface | 5 × 10−7 [m3·(J*s)−1] | [26] | |
| Constant of action | 0.5 [s−1] | [25,26] | |
| Energy gradient coefficient | 1.5 × 10−6 [J·m−1] | [25,26] | |
| Barrier height | 3.5 × 105 [J·m−3] | [25,26] | |
| Intensity of anisotropy | 0.03 | [25,26] | |
| Anisotropic modulus | 4 | [25,26] | |
| Symmetric factor | 0.5 | [25,26] | |
| Initial electrolyte concentration | 1 × 103 [mol·m−3] | [25,26] | |
| Initial lithium atom concentration in lithium metal | 7.69 × 104 [mol·m−3] | [25,26] | |
| Electrode diffusion coefficient | 1 × 10−13 [m2·s−1] | [25,26] | |
| Electrolyte diffusion coefficient | 1 × 10−13 [m2·s−1] | [25,26] | |
| Electrode conductivity | 107 [S·m−1] | [25,26] | |
| Electrolytic conductivity | 0.1 [S·m−1] | [25,26] | |
| Electrode Young’s modulus | 7.8 [GPa] | [20] | |
| Electrolyte Young’s modulus | 1.0 [GPa] | [20] | |
| Electrode Poisson ratio | 0.42 | [20] | |
| Electrolyte Poisson ratio | 0.3 | [20] | |
| Vegard Strain coefficient | −8.66 × 10−4 −7.73 × 10−4 −5.29 × 10−4 | [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, W.; Chai, J.; Xu, R. Analysis of Pressure Characteristics of Ultra-High Specific Energy Lithium Metal Battery for Flying Electric Vehicles. Electronics 2024, 13, 1505. https://doi.org/10.3390/electronics13081505
Shi W, Chai J, Xu R. Analysis of Pressure Characteristics of Ultra-High Specific Energy Lithium Metal Battery for Flying Electric Vehicles. Electronics. 2024; 13(8):1505. https://doi.org/10.3390/electronics13081505
Chicago/Turabian StyleShi, Wei, Jin Chai, and Ruofan Xu. 2024. "Analysis of Pressure Characteristics of Ultra-High Specific Energy Lithium Metal Battery for Flying Electric Vehicles" Electronics 13, no. 8: 1505. https://doi.org/10.3390/electronics13081505
APA StyleShi, W., Chai, J., & Xu, R. (2024). Analysis of Pressure Characteristics of Ultra-High Specific Energy Lithium Metal Battery for Flying Electric Vehicles. Electronics, 13(8), 1505. https://doi.org/10.3390/electronics13081505





