Is the Neuromuscular Organization of Throwing Unchanged in Virtual Reality? Implications for Upper Limb Rehabilitation
Abstract
:1. Introduction
2. Materials and Methods
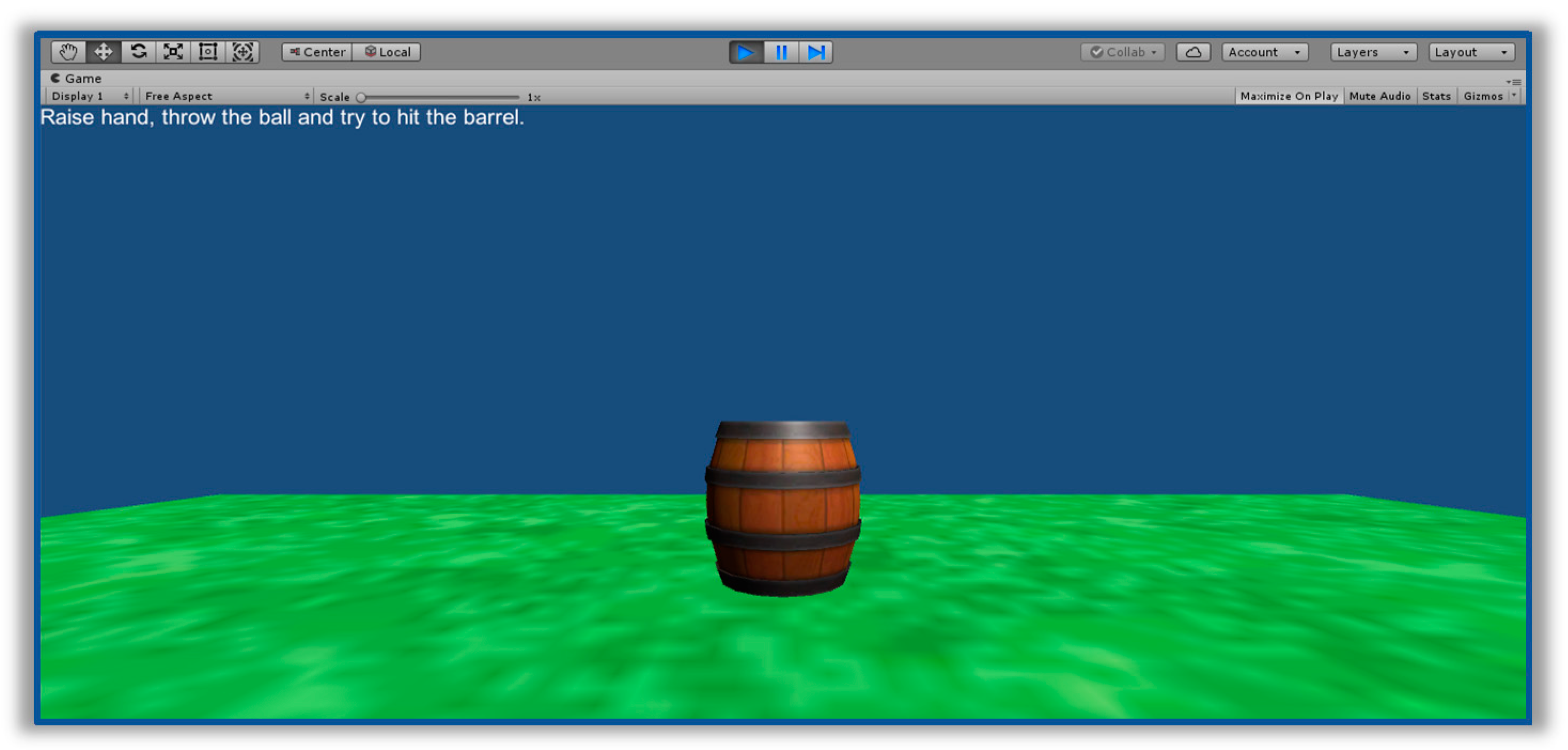
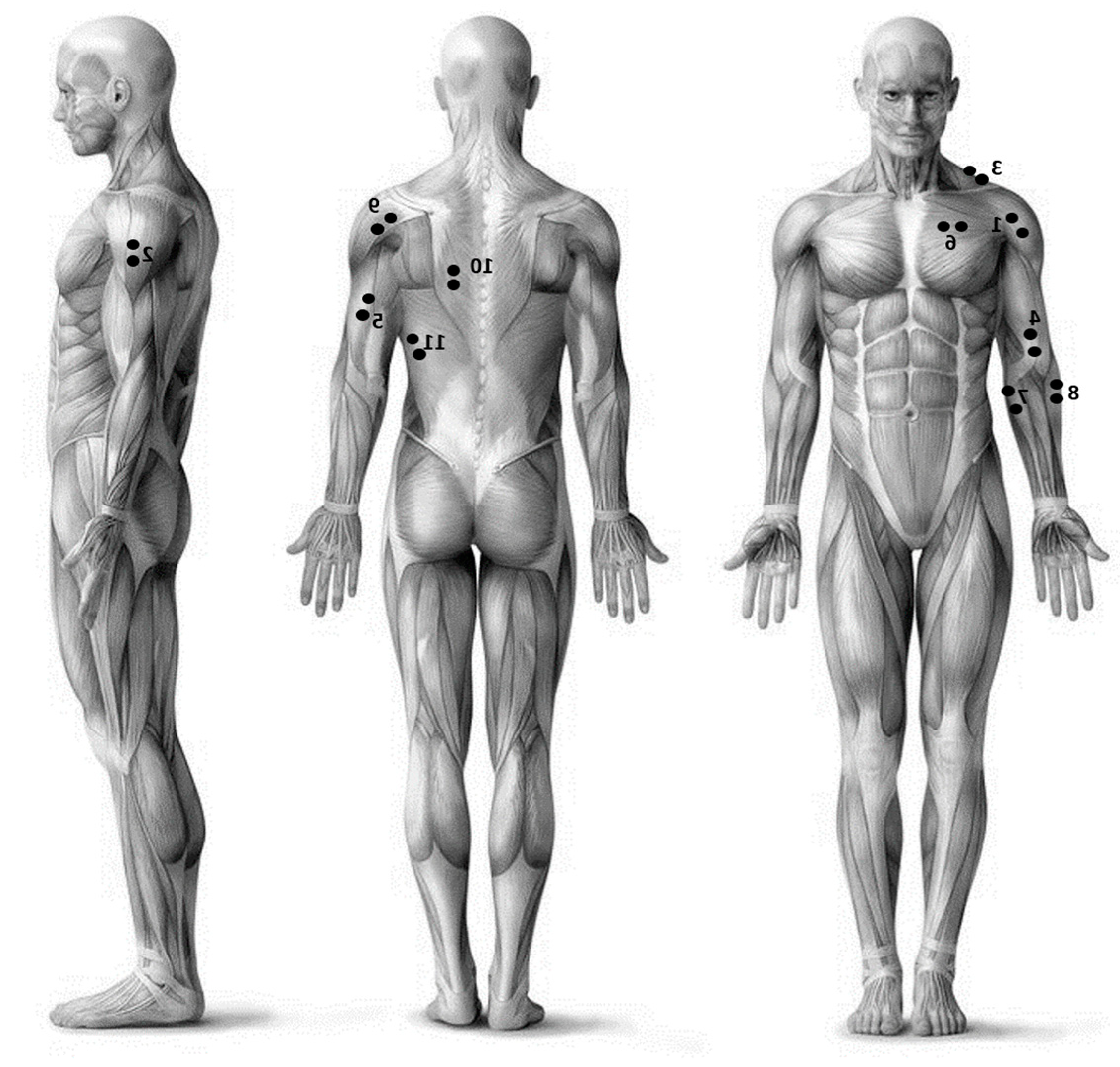
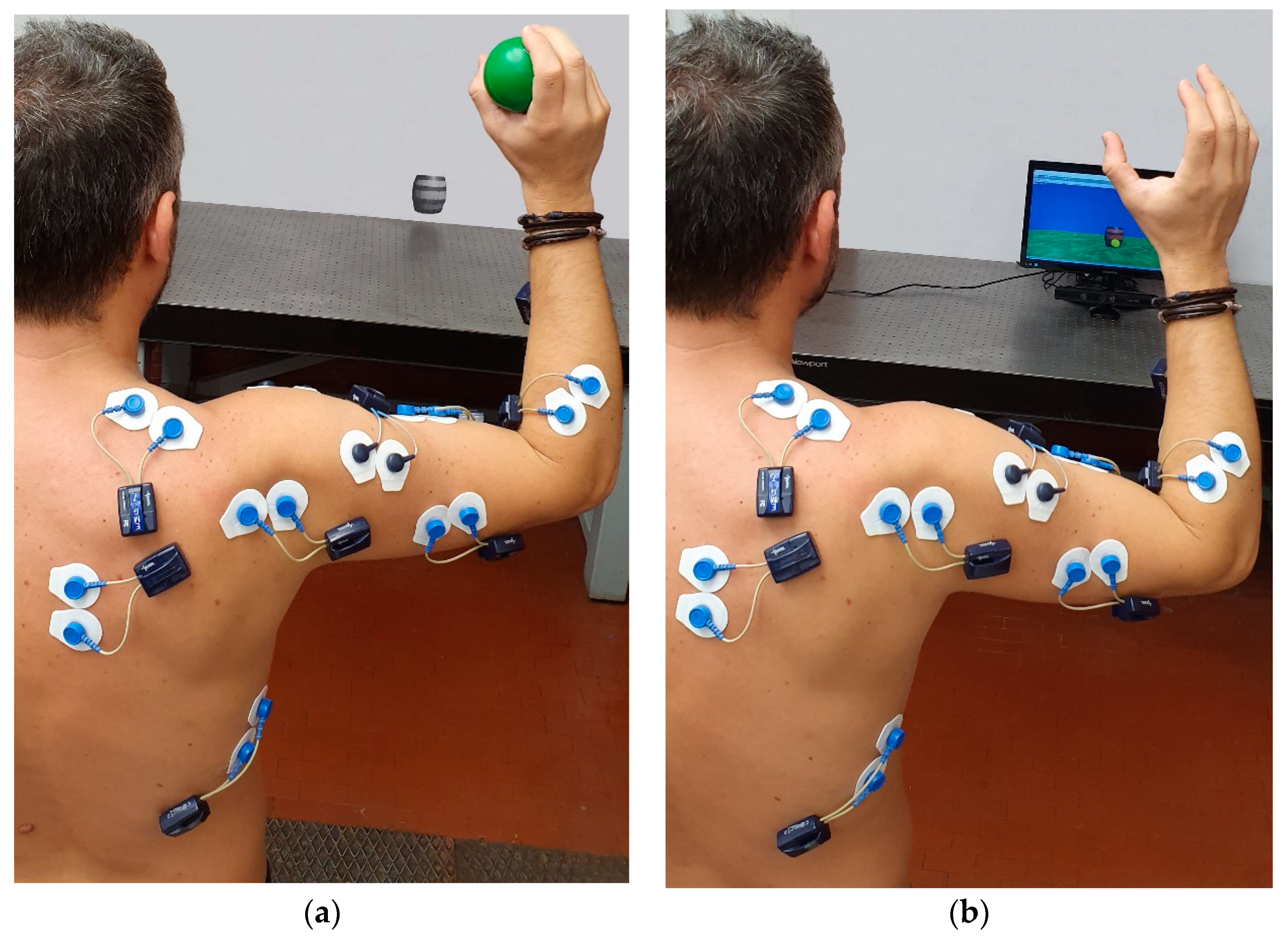
2.1. Experimental Setup
2.2. Experimental Protocol
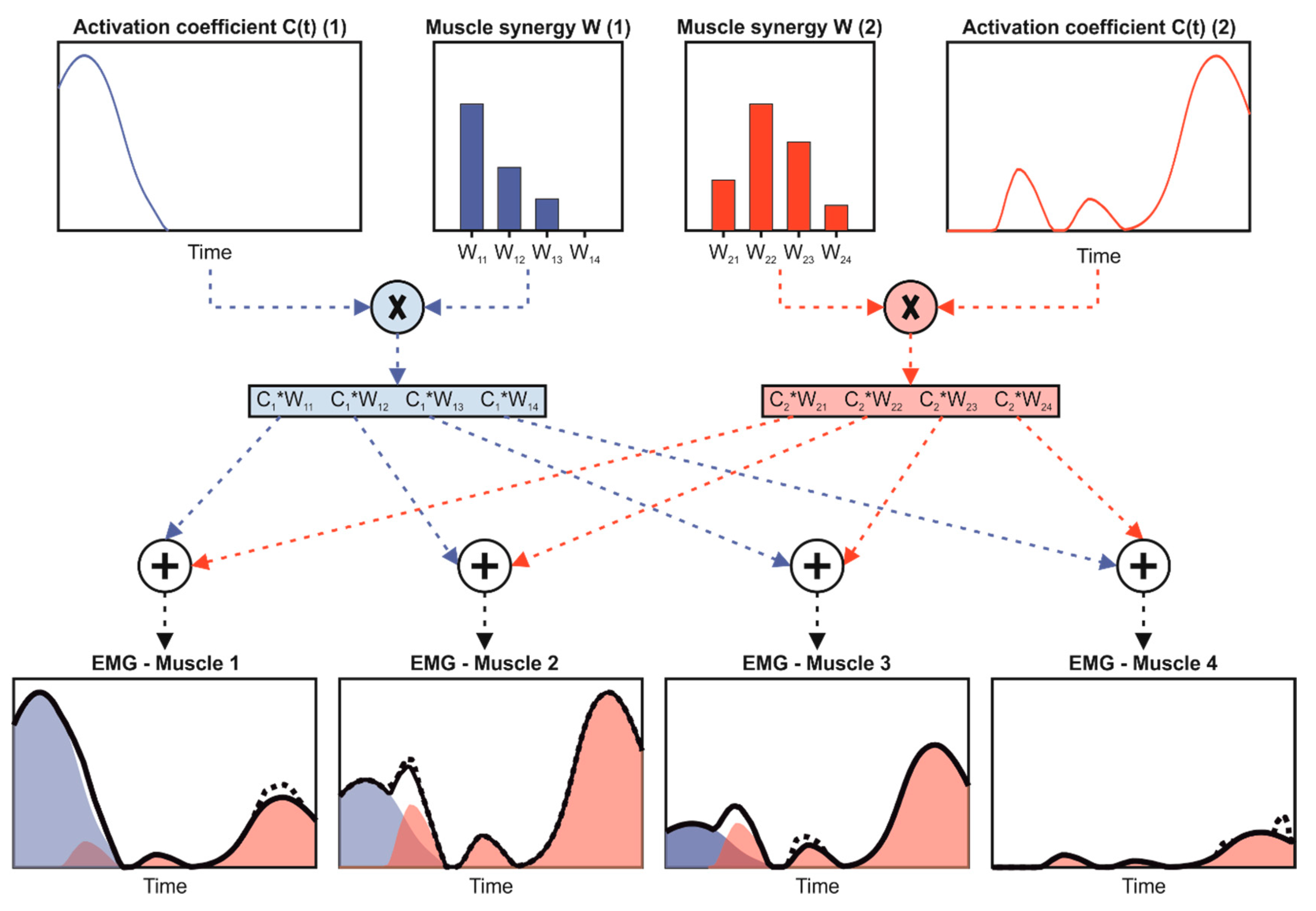
2.3. Data Processing
2.4. Data Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sisto, S.A.; Forrest, G.F.; Glendinning, D. Virtual Reality Applications for Motor Rehabilitation After Stroke. Top. Stroke Rehabil. 2002, 8, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.R.; Desai, P.N.; Ajmera, K.D.; Mehta, K. A Review Paper on Oculus Rift-A Virtual Reality Headset. Int. J. Eng. Trands Technol. 2014, 13, 175–179. [Google Scholar] [CrossRef] [Green Version]
- Connelly, L.; Jia, Y.; Toro, M.L.; Stoykov, M.E.; Kenyon, R.V.; Kamper, D.G. A Pneumatic Glove and Immersive Virtual Reality Environment for Hand Rehabilitative Training After Stroke. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Freina, L.; Ott, M. A Literature Review on Immersive Virtual Reality in Education; State of the Art and Perspectives: New York, NY, USA, 2015. [Google Scholar]
- Schultheis, M.T.; Rizzo, A.A. The application of virtual reality technology in rehabilitation. Rehabil. Psychol. 2001, 46, 296. [Google Scholar] [CrossRef]
- Ma, M.; Zheng, H. Virtual Reality and Serious Games in Healthcare. In Advanced Computational Intelligence Paradigms in Healthcare 6. Virtual Reality in Psychotherapy, Rehabilitation, and Assessment; Springer: Berlin/Heidelberg, Germany, 2011; pp. 169–192. [Google Scholar]
- Feigin, V.; Forouzanfar, M.; Krishnamurthi, R.; Mensah, G.; Connor, M.; Bennet, D.; Moran, A.; Sacco, R.; Anderson, L.; Truelsen, T.; et al. Global and regional burden of stroke during 1990–2010: Findings from the Global Burden of Disease Study 2010. Lancet 2014, 383, 245–254. [Google Scholar] [CrossRef]
- Colombo, R.; Sterpi, I.; Mazzone, A.; Delconte, C.; Pisano, F. Robot-aided neurorehabilitation in sub-acute and chronic stroke: Does spontaneous recovery have a limited impact on outcome? NeuroRehabilitation 2013, 33, 621–629. [Google Scholar] [CrossRef] [Green Version]
- Schaechter, J.D. Motor rehabilitation and brain plasticity after hemiparetic stroke. Prog. Neurobiol. 2004, 73, 61–72. [Google Scholar] [CrossRef]
- Taub, E.; Uswatte, G.; Mark, V.W.; Morris, D.M.; Barman, J.; Bowman, M.H.; Bryson, C.; Delgado, A.; Bishop-McKay, S. Method for Enhancing Real-World Use of a More Affected Arm in Chronic Stroke: Transfer Package of Constraint-Induced Movement Therapy. Stroke 2013, 44, 1383–1388. [Google Scholar] [CrossRef] [Green Version]
- Cappa, P.; Clerico, A.; Nov, O.; Porfiri, M. Can force feedback and science learning enhance the effectiveness of neuro-rehabilitation? An experimental study on using a low-cost 3D joystick and a virtual visit to a zoo. PLoS ONE 2013, 8, e83945. [Google Scholar] [CrossRef]
- Summa, S.; Basteris, A.; Betti, E.; Sanguineti, V. Adaptive training with full-body movements to reduce bradykinesia in persons with Parkinson’s disease: A pilot study. J. Neuroeng. Rehabil. 2015, 12, 16. [Google Scholar] [CrossRef] [Green Version]
- Kwakkel, G.; Kollen, B.J.; Krebs, H.I. Effects of robot-assisted therapy on upper limb recovery after stroke: A systematic review. Neurorehabil. Neural Repair 2007, 22, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Chang, Y.; Chang, H.; Chou, L. An interactive game-based shoulder wheel system for rehabilitation. Patient Prefer. Adher. 2012, 6, 821–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheok, G.; Tan, D.; Low, A.; Hewitt, J. Is Nintendo Wii an Effective Intervention for Individuals With Stroke? A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2015, 16, 923–932. [Google Scholar] [CrossRef]
- Huang, H.; Wolf, S.L.; He, J. Recent developments in biofeedback for neuromotor rehabilitation. J. Neuroeng. Rehabil. 2006, 3, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scalona, E.; Martelli, F.; Del Prete, Z.; Palermo, E.; Rossi, S. A Novel Protocol for the Evaluation of Motor Learning in 3D Reching Tasks Using Novint Falcon. In Proceedings of the 7th IEEE International Conference on Biomedical Robotics and Biomechatronics (Biorob), Overijssel, The Netherlands, 26–29 August 2018; pp. 268–272. [Google Scholar]
- Pastor, I.; Hayes, H.; Bamberg, S. A feasibility study of an upper limb rehabilitation system using kinect and computer games. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2012, 2012, 1286–1289. [Google Scholar]
- Laut, J.; Cappa, F.; Nov, O.; Porfiri, M. Increasing Patient Engagement in Rehabilitation Exercises Using Computer-Based Citizen Science. PLoS ONE 2015, 10, e0117013. [Google Scholar] [CrossRef] [PubMed]
- Scalona, E.; Hayes, D.; Del Prete, Z.; Palermo, E.; Rossi, S. Perturbed Point-to-Point Reaching Tasks in a 3D Environment Using a Portable Haptic Device. Electronics 2019, 8, 32. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.-J.; Chen, S.-F.; Huang, J.-D. A Kinect-based system for physical rehabilitation: A pilot study for young adults with motor disabilities. Res. Dev. Disabil. 2011, 32, 2566–2570. [Google Scholar] [CrossRef]
- Webster, D.; Celik, O. Systematic review of Kinect applications in elderly care and stroke rehabilitation. J. Neuroeng. Rehabil. 2014, 11, 108–111. [Google Scholar] [CrossRef] [Green Version]
- Lange, B.; Chang, C.Y.; Suma, E.; Newman, B.; Rizzo, A.S.; Bolas, M. Development and evaluation of low cost game-based balance rehabilitation tool using the Microsoft Kinect sensor. In Proceedings of the IEEE Engineering in Medicine and Biology Conference, Boston, MA, USA, 30 August–3 September 2011. [Google Scholar]
- Mitra, S.; Acharya, T. Gesture recognition: A survey. IEEE Trans. Syst. Man Cybern. 2007, 37, 311–324. [Google Scholar] [CrossRef]
- Hondori, H.M.; Khademi, M. A review on technical and clinical impact of microsoft kinect on physical therapy and rehabilitation. J. Med. Eng. 2014, 2014, 846514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palermo, E.; Laut, J.; Nov, O.; Cappa, P.; Porfiri, M. A Natural User Interface to Integrate Citizen Science and Physical Exercise. PLoS ONE 2017, 12, e0172587. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, A. Identification of neuromuscular synergies in natural upper-arm movements. Biol. Cybern. 2002, 86, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Park, T.S. Analysis of Muscle On-set Time of Fully Immersive Virtual Reality Motions and Actual Motions in Healthy Adults. Int. J. Pure Appl. Math. 2018, 118, 2367–2380. [Google Scholar]
- Bizzi, E.; Cheung, V.C.K.; d’Avella, A.; Saltiel, P.; Tresch, M. Combining modules for movement. Brain Res. Rev. 2008, 57, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Tresch, M.C. Matrix Factorization Algorithms for the Identification of Muscle Synergies: Evaluation on Simulated and Experimental Data Sets. J. Neurophysiol. 2005, 95, 2199–2212. [Google Scholar] [CrossRef] [Green Version]
- Neptune, R.R.; Clark, D.J.; Kautz, S.A. Modular control of human walking: A simulation study. J. Biomech. 2009, 42, 1282–1287. [Google Scholar] [CrossRef] [Green Version]
- Santuz, A.; Ekizos, A.; Janshen, L.; Baltzopoulos, V.; Arampatzis, A. on the Methodological Implications of Extracting Muscle Synergies From Human Locomotion. Int. J. Neural Syst. 2016, 27, 1–15. [Google Scholar] [CrossRef]
- Ivanenko, Y.P.; Poppele, R.E.; Lacquaniti, F. Five basic muscle activation patterns account for muscle activity during human locomotion. J. Physiol. 2004, 1, 267–282. [Google Scholar] [CrossRef]
- Taborri, J.; Palermo, E.; Masiello, D.; Rossi, S. Factorization of EMG via muscle synergies in walking task: Evaluation of intra-subject and inter-subject variability. In Proceedings of the IEEE International Instrumentation and Measurement Technology Conference, Torino, Italy, 22–25 May 2017. [Google Scholar]
- Ting, L.H.; Macpherson, J.M. A Limited Set of Muscle Synergies for Force Control During a Postural Task. J. Neurophysiol. 2005, 93, 609–613. [Google Scholar] [CrossRef] [Green Version]
- Taborri, J.; Mileti, I.; Del Prete, Z.; Rossi, S.; Palermo, E. Yaw Postural Perturbation Through Robotic Platform: Aging Effects on Muscle Synergies. In Proceedings of the 7th IEEE International Conference on Biomedical Robotics and Biomechatronics (Biorob), Overijssel, The Netherlands, 26–29 August 2018; pp. 916–921. [Google Scholar]
- Torres-oviedo, G.; Ting, L.H. Subject-Specific Muscle Synergies in Human Balance Control Are Consistent Across Different Biomechanical Contexts. J. Neurophysiol. 2010, 103, 3084–3098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taborri, J.; Agostini, V.; Artemiadis, P.K.; Ghislieri, M.; Jacobs, D.A.; Roh, J.; Rossi, S. Feasibility of muscle synergy outcomes in clinics, robotics, and sports: A systematic review. Appl. Bionics Biomech. 2018, 2018, 3934698. [Google Scholar] [CrossRef] [PubMed]
- Zelik, K.E.; La Scaleia, V.; Ivanenko, Y.P.; Lacquaniti, F. Can modular strategies simplify neural control of multidirectional human locomotion? J. Neurophysiol. 2014, 111, 1686–1702. [Google Scholar] [CrossRef] [PubMed]
- Lacquaniti, F.; Ivanenko, Y.P.; Zago, M. Patterned control of human locomotion. J. Physiol. 2012, 590, 2189–2199. [Google Scholar] [CrossRef] [PubMed]
- Rosevall, J.; Rusu, C.; Talavera, G.; Carrabina, J.; Garcia, J.; Carenas, C.; Breuil, F.; Reixach, E.; Torrent, M.; Burkard, S.; et al. A wireless sensor insole for collecting gait data. Stud. Health Technol. Inf. 2014, 200, 176–178. [Google Scholar]
- Taborri, J.; Palermo, E.; Del Prete, Z.; Rossi, S. On the reliability and repeatability of surface electromyography factorization by muscle synergies in daily life activities. Appl. Bionics Biomech. 2018, 2018, 5852307. [Google Scholar] [CrossRef]
- Rau, G.; Disselhorst-Klug, C.; Biomechanics, R. Movement biomechanics goes upwards: From the leg to the arm. J. Biomech. 2000, 33, 1207–1216. [Google Scholar] [CrossRef]
- Cruz Ruiz, A.L.; Pontonnier, C.; Levy, J.; Dumont, G. Motion Control via Muscle Synergies: Application to Throwing. In Proceedings of the 8th International ACM SIGGRAPH Conference on Motion in Games 2015 (MIG’15), Paris, France, 16–18 November 2015. [Google Scholar]
- Stegeman, D.F.; Hermens, H.J. Standards for Surface Electromyography: The European Project Surface EMG for Non-Invasive Assessment of Muscles (SENIAM). Available online: http//www.med.uni-jena.de/motorik/pdf/stegeman.pdf (accessed on 6 December 2019).
- Scalona, E.; Taborri, J.; Del Prete, Z.; Palermo, E.; Rossi, S. EMG factorization during walking: Does digital filtering influence the accuracy in the evaluation of the muscle synergy number? In Proceedings of the 2018 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Rome, Italy, 11–13 June 2018; pp. 1–6. [Google Scholar]
- Lee, D.D.; Seung, H.S. Learning the parts of objects by non-negative matrix factorization. Nature 2000, 401, 788–791. [Google Scholar] [CrossRef]
- Shuman, B.; Goudriaan, M.; Bar-on, L.; Schwartz, M.H.; Desloovere, K.; Steele, K.M. Repeatability of muscle synergies within and between days for typically developing children and children with cerebral palsy. Gait Posture 2016, 45, 127–132. [Google Scholar] [CrossRef]
- Field, A. Discovering Statistics Using SPSS, 3rd ed.; Sage Publications Ltd.: Newbury Park, CA, USA, 2013; ISBN 978-184-787-9066. [Google Scholar]
- Hug, F.; Turpin, N.A.; Guével, A.; Dorel, S. Is interindividual variability of EMG patterns in trained cyclists related to different muscle synergies? J. Appl. Physiol. 2010, 108, 1727–1736. [Google Scholar] [CrossRef] [Green Version]
- Spath, H. Helmuth The Cluster Dissection and Analysis Theory FORTRAN Programs Examples; Prentice-Hall, Inc.: Upper Saddle River, NJ, USA, 1985; ISBN 013-137-9852. [Google Scholar]
- D’Avella, A.; Saltiel, P.; Bizzi, E. Combinations of muscle synergies in the construction of a natural motor behavior. Nat. Neurosci. 2003, 6, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Chvatal, S.A.; Ting, L.H. Common muscle synergies for balance and walking. Front. Comput. Neurosci. 2013, 7, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz Ruiz, A.L.; Pontonnier, C.; Sorel, A.; Dumont, G. Identifying representative muscle synergies in overhead football throws. Comput. Methods Biomech. Biomed. Eng. 2015, 18, 1918–1919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padilla-Castañeda, M.A.; Sotgiu, E.; Barsotti, M.; Frisoli, A.; Orsini, P.; Martiradonna, A.; Laddaga, C.; Bergamasco, M. An Orthopaedic Robotic-Assisted Rehabilitation Method of the Forearm in Virtual Reality Physiotherapy. J. Healthc. Eng. 2018, 2018, 7438609. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.; Stanley, C.J.; Damiano, D.L.; Kim, J.; Yoon, J.; Park, H.S. Biomechanical evaluation of virtual reality-based turning on a self-paced linear treadmill. Gait Posture 2018, 65, 157–162. [Google Scholar] [CrossRef]
- Martinho, N.; Carvalho, L.C.; Iunes, D.H.; Botelho, S.; Marques, J.; Silva, V.R. The effects of training by virtual reality or gym ball on pelvic floor muscle strength in postmenopausal women: A randomized controlled trial. Braz. J. Phys. Ther. 2016, 20, 248–257. [Google Scholar] [CrossRef] [Green Version]
- Aşkın, A.; Atar, E.; Koçyiğit, H.; Tosun, A. Effects of Kinect-based virtual reality game training on upper extremity motor recovery in chronic stroke. Somatosens. Mot. Res. 2018, 35, 25–32. [Google Scholar] [CrossRef]
- McNish, R.N.; Chembrammel, P.; Speidel, N.C.; Lin, J.J.; López-Ortiz, C. Rehabilitation for children with dystonic cerebral palsy using haptic feedback in virtual reality: Protocol for a randomized controlled trial. J. Med. Internet Res. 2019, 21, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Sveistrup, H. Motor rehabilitation using virtual reality. J. Neuroeng. Rehabil. 2004, 1, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Burdea, G.C. Virtual Rehabilitation—Benefits and Challenges. Methods Inf. Med. 2003, 42, 519–523. [Google Scholar]
- Hermassi, S.; van den Tillaar, R.; Khlifa, R.; Chelly, M.S.; Chamari, K. Comparison of In-Season-Specific Resistance vs. A Regular Throwing Training Program on Throwing Velocity, Anthropometry, and Power Performance in Elite Handball Players. J. Strength Cond. Res. 2015, 29, 2105–2114. [Google Scholar] [CrossRef] [PubMed]
- Laver, K.E.; George, S.; Crotty, M.; Lange, B.; Deutsch, J.E.; Saposnik, G. Virtual reality for stroke rehabilitation. Cochrane Database Syst. Rev. 2017, 11, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, K.M.; Kelly, V.E.; Chang, T.; Weismann, M.C.; Westcott McCoy, S.; Steele, K.M. Muscle recruitment and coordination during upper-extremity functional tests. J. Electromyogr. Kinesiol. 2018, 38, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Priya, S.; Rai, M.; Joseph, D.M. Comparison between Handgrip Strength Measurement of Dominant Hand and Non Dominant Hand in Basketball Players. Indian J. Physiother. Occup. Ther. 2018, 12, 126–130. [Google Scholar] [CrossRef]
- Escamilla, R.F.; Fleisig, G.S.; Zheng, N.; Barrentine, S.W.; Andrews, J.R. Kinematic comparisons of 1996 Olympic baseball pitchers. J. Sports Sci. 2001, 19, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Sachlikidis, A.; Salter, C. A biomechanical comparison of dominant and non-dominant arm throws for speed and accuracy. Sports Biomech. 2007, 6, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Wilk, K.E.; Meister, K.; Fleisig, G.; Andrews, J.R. Biomechanics of the Overhead Throwing Motion. Sports Med. Arthrosc. 2000, 8, 124–134. [Google Scholar] [CrossRef]
- Baltaci, G.; Tunay, V.B. Isokinetic performance at diagonal pattern and shoulder mobility in elite overhead athletes. Scand. J. Med. Sci. Sports 2004, 14, 231–238. [Google Scholar] [CrossRef]


| ID | VR Task | Real Task | Mode | ||
|---|---|---|---|---|---|
| D | ND | D | ND | ||
| ID1 | 3 | 3 | 3 | 2 | 3 |
| ID2 | 3 | 3 | 4 | 3 | 3 |
| ID3 | 3 | 3 | 2 | 3 | 3 |
| ID4 | 3 | 4 | 3 | 3 | 3 |
| ID5 | 3 | 4 | 3 | 3 | 3 |
| ID6 | 3 | 3 | 3 | 3 | 3 |
| ID7 | 3 | 2 | 2 | 4 | 2 |
| ID8 | 2 | 2 | 3 | 2 | 2 |
| ID9 | 2 | 3 | 3 | 3 | 3 |
| ID10 | 4 | 3 | 2 | 3 | 3 |
| ID11 | 2 | 2 | 3 | 2 | 2 |
| ID12 | 3 | 4 | 3 | 4 | 3 |
| ID13 | 4 | 4 | 3 | 3 | 3 |
| ID14 | 3 | 3 | 3 | 3 | 3 |
| ID15 | 2 | 3 | 3 | 3 | 3 |
| ID16 | 3 | 4 | 3 | 3 | 3 |
| ID17 | 4 | 5 | 4 | 4 | 4 |
| Real Task vs. Virtual Task—Dominant Side | |||||
|---|---|---|---|---|---|
| ID | I-sy | II-sy | III-sy | IV-sy | Overall Model |
| ID1 | 0.95 | 0.91 | 0.95 | - | 0.94 |
| ID2 | 0.84 | 0.58 | 0.84 | - | 0.75 |
| ID3 | 0.91 | 0.93 | 0.98 | - | 0.94 |
| ID4 | 0.97 | 0.81 | 0.93 | - | 0.91 |
| ID5 | 0.90 | 0.85 | 0.92 | - | 0.89 |
| ID6 | 0.86 | 0.96 | 0.95 | - | 0.93 |
| ID7 | 0.84 | 0.67 | - | - | 0.75 |
| ID8 | 0.93 | 0.94 | - | - | 0.93 |
| ID9 | 0.71 | 0.71 | 0.88 | - | 0.77 |
| ID10 | 0.84 | 0.89 | 0.97 | - | 0.90 |
| ID11 | 0.98 | 0.85 | - | - | 0.91 |
| ID12 | 0.96 | 0.96 | 0.84 | - | 0.92 |
| ID13 | 0.93 | 0.85 | 0.94 | - | 0.90 |
| ID14 | 0.88 | 0.84 | 0.77 | - | 0.83 |
| ID15 | 0.72 | 0.86 | 0.56 | - | 0.71 |
| ID16 | 0.96 | 0.96 | 0.90 | - | 0.94 |
| ID17 | 0.95 | 0.97 | 0.70 | 0.57 | 0.80 |
| Real Task vs. Virtual Task—Nondominant Side | |||||
|---|---|---|---|---|---|
| ID | I-sy | II-sy | III-sy | IV-sy | Overall Model |
| ID1 | 0.96 | 0.88 | 0.84 | - | 0.89 |
| ID2 | 0.98 | 0.90 | 0.94 | - | 0.94 |
| ID3 | 0.88 | 0.80 | 0.50 | - | 0.72 |
| ID4 | 0.81 | 0.93 | 0.85 | - | 0.86 |
| ID5 | 0.87 | 0.87 | 0.60 | - | 0.78 |
| ID6 | 0.85 | 0.64 | 0.98 | - | 0.82 |
| ID7 | 0.77 | 0.80 | - | - | 0.79 |
| ID8 | 0.98 | 0.95 | - | - | 0.96 |
| ID9 | 0.88 | 0.90 | 0.93 | - | 0.90 |
| ID10 | 0.95 | 0.93 | 0.98 | - | 0.95 |
| ID11 | 0.96 | 0.84 | - | - | 0.90 |
| ID12 | 0.98 | 0.98 | 0.98 | - | 0.98 |
| ID13 | 0.61 | 0.96 | 0.85 | - | 0.80 |
| ID14 | 0.93 | 0.73 | 0.67 | - | 0.77 |
| ID15 | 0.89 | 0.99 | 0.96 | - | 0.95 |
| ID16 | 0.74 | 0.92 | 0.73 | - | 0.79 |
| ID17 | 0.65 | 0.89 | 0.99 | 0.75 | 0.82 |
| Dominant vs. Nondominant Side—Virtual Task | |||||
|---|---|---|---|---|---|
| ID | I-sy | II-sy | III-sy | IV-sy | Overall Model |
| ID1 | 0.94 | 0.90 | 0.93 | - | 0.92 |
| ID2 | 0.78 | 0.87 | 0.67 | - | 0.78 |
| ID3 | 0.76 | 0.86 | 0.88 | - | 0.83 |
| ID4 | 0.90 | 0.84 | 0.59 | - | 0.78 |
| ID5 | 0.97 | 0.95 | 0.86 | - | 0.93 |
| ID6 | 0.81 | 0.58 | 0.97 | - | 0.79 |
| ID7 | 0.79 | 0.73 | - | - | 0.76 |
| ID8 | 0.91 | 0.85 | - | - | 0.88 |
| ID9 | 0.80 | 0.41 | 0.91 | - | 0.71 |
| ID10 | 0.52 | 0.80 | 0.98 | - | 0.76 |
| ID11 | 0.99 | 0.94 | - | - | 0.96 |
| ID12 | 0.93 | 0.92 | 0.83 | - | 0.89 |
| ID13 | 0.74 | 0.84 | 0.95 | - | 0.84 |
| ID14 | 0.77 | 0.91 | 0.92 | - | 0.87 |
| ID15 | 0.73 | 0.91 | 0.89 | - | 0.84 |
| ID16 | 0.55 | 0.81 | 0.83 | - | 0.73 |
| ID17 | 0.74 | 0.82 | 0.58 | 0.94 | 0.77 |
| Dominant vs. Nondominant Side—Real Task | |||||
|---|---|---|---|---|---|
| ID | I-sy | II-sy | III-sy | IV-sy | Overall Model |
| ID1 | 0.91 | 0.82 | 0.81 | - | 0.85 |
| ID2 | 0.74 | 0.87 | 0.71 | - | 0.77 |
| ID3 | 0.85 | 0.90 | 0.69 | - | 0.81 |
| ID4 | 0.94 | 0.67 | 0.47 | - | 0.69 |
| ID5 | 0.92 | 0.82 | 0.61 | - | 0.78 |
| ID6 | 0.91 | 0.88 | 0.97 | - | 0.92 |
| ID7 | 0.78 | 0.51 | - | - | 0.64 |
| ID8 | 0.87 | 0.84 | - | - | 0.85 |
| ID9 | 0.89 | 0.53 | 0.98 | - | 0.80 |
| ID10 | 0.90 | 0.96 | 0.98 | - | 0.94 |
| ID11 | 0.96 | 0.80 | - | - | 0.88 |
| ID12 | 0.99 | 0.97 | 0.91 | - | 0.96 |
| ID13 | 0.68 | 0.74 | 0.92 | - | 0.78 |
| ID14 | 0.72 | 0.86 | 0.56 | - | 0.71 |
| ID15 | 0.85 | 0.92 | 0.94 | - | 0.90 |
| ID16 | 0.89 | 0.89 | 0.71 | - | 0.83 |
| ID17 | 0.79 | 0.92 | 0.94 | 0.75 | 0.85 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scalona, E.; Taborri, J.; Hayes, D.R.; Del Prete, Z.; Rossi, S.; Palermo, E. Is the Neuromuscular Organization of Throwing Unchanged in Virtual Reality? Implications for Upper Limb Rehabilitation. Electronics 2019, 8, 1495. https://doi.org/10.3390/electronics8121495
Scalona E, Taborri J, Hayes DR, Del Prete Z, Rossi S, Palermo E. Is the Neuromuscular Organization of Throwing Unchanged in Virtual Reality? Implications for Upper Limb Rehabilitation. Electronics. 2019; 8(12):1495. https://doi.org/10.3390/electronics8121495
Chicago/Turabian StyleScalona, Emilia, Juri Taborri, Darren Richard Hayes, Zaccaria Del Prete, Stefano Rossi, and Eduardo Palermo. 2019. "Is the Neuromuscular Organization of Throwing Unchanged in Virtual Reality? Implications for Upper Limb Rehabilitation" Electronics 8, no. 12: 1495. https://doi.org/10.3390/electronics8121495





