The New Insight into the Effects of Different Fixing Technology on Flavor and Bioactivities of Orange Dark Tea
Abstract
:1. Introduction
2. Results
2.1. Effects of Different Fixing Methods on Sensory Quality
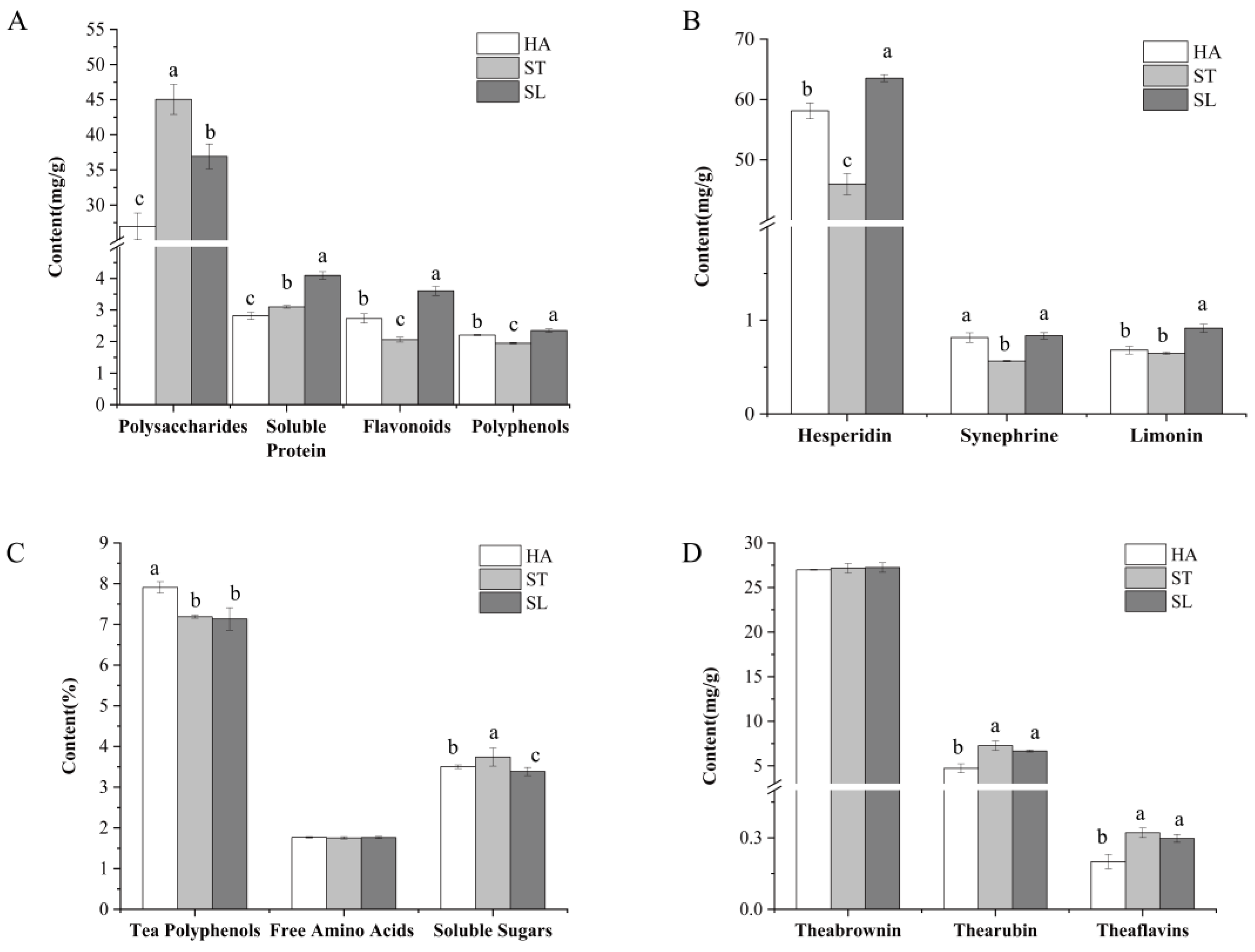
2.2. Effects of Different Fixing Methods on Chemical Composition
2.3. Effects of Different Fixing Methods on Volatile Component
2.4. Effects of Different Fixing Methods on Bioactivities
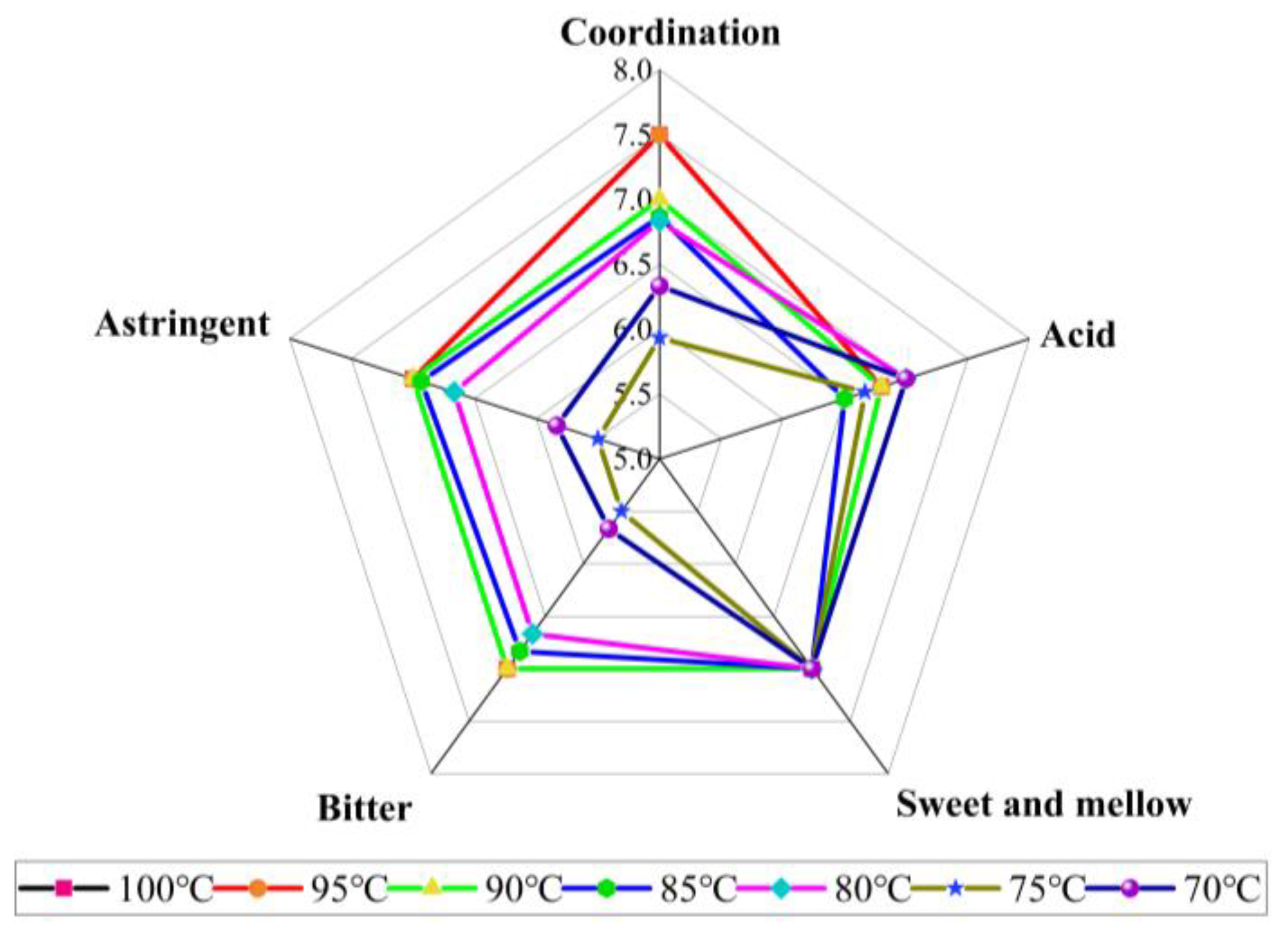
2.5. Effects of Different Temperatures of HA Fixing on Sensory Quality
2.6. Effects of Different Temperatures of HA Fixing on Chemical Composition
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
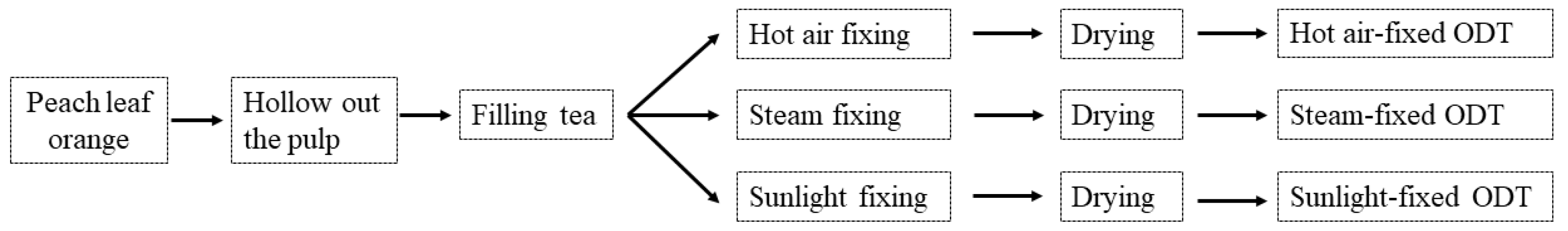
4.2. Sample Preparation
4.3. Sensory Evaluation
4.4. Chemical Composition Determination
4.5. Determination of Volatile Components in Citrus Tea
4.6. Antioxidant Activity and Enzyme Activity Inhibition Assay
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- He, M.R.; Lyu, X.H. Application of BRAFO-tiered approach for health benefit-risk assessment of dark tea consumption in China. Food Chem. Toxicol. 2021, 158, 112615. [Google Scholar] [CrossRef] [PubMed]
- Shuyuan, L.; Zeyi, A.; Yang, M.; Yuqiong, C.; Dejiang, N. Comparative studies on the physicochemical profile and potential hypoglycemic activity of different tea extracts: Effect on sucrase-isomaltase activity and glucose transport in Caco-2 cells. Food Res. Int. 2021, 148, 110604. [Google Scholar] [CrossRef]
- Tripoli, E.; La Guardia, M.; Giammanco, S.; Di Majo, D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Ting, Z.; Wei, G.; Sheng Chao, R.; Yu Ming, L.; Jin Ming, W.; Bao, Y. Flavonoid glycosides and other bioactive compounds in Citrus reticulate “Chachi” peel analysed by tandem mass spectrometry and their changes during storage. Carbohydr. Res. 2021, 510, 108462. [Google Scholar] [CrossRef]
- Farag, M.A.; Abib, B.; Ayad, L.; Khattab, A.R. Sweet and bitter oranges: An updated comparative review of their bioactives, nutrition, food quality, therapeutic merits and biowaste valorization practices. Food Chemistry 2020, 331, 127306. [Google Scholar] [CrossRef]
- Shi, Y.S.; Zhang, Y.; Li, H.T.; Wu, C.H.; El-Seedi, H.R.; Ye, W.K.; Wang, Z.W.; Li, C.B.; Zhang, X.F.; Kai, G.Y. Limonoids from Citrus: Chemistry, anti-tumor potential, and other bioactivities. J. Funct. Foods 2020, 75, 104213. [Google Scholar] [CrossRef]
- Wang, J.T.; Zhu, Y.; Shi, J.; Yan, H.; Wang, M.Q.; Ma, W.J.; Zhang, Y.; Peng, Q.H.; Chen, Y.Q.; Lin, Z. Discrimination and Identification of Aroma Profiles and Characterized Odorants in Citrus Blend Black Tea with Different Citrus Species. Molecules 2020, 25, 4208. [Google Scholar] [CrossRef]
- Ping, L.; Si-yi, P. Synergistic antioxidant effects of total flavonoids from tangerine peel and Pu’er tea. Food Res. Dev. 2020, 41, 59–64. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Zeng, X.; Chen, T.T.; Peng, W.; Su, W.W. Chemical Profile, Antioxidative, and Gut Microbiota Modulatory Properties of Ganpu Tea: A Derivative of Pu-erh Tea. Nutrients 2020, 12, 224. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.J.; Hua, J.J.; Jiang, Y.W.; Yang, Y.Q.; Wang, J.J.; Yuan, H.B. Influence of fixation methods on the chestnut-like aroma of green tea and dynamics of key aroma substances. Food Res. Int. 2020, 136, 109479. [Google Scholar] [CrossRef]
- Feng, L.; Zhou, J.; Zhang, L.; Liu, P.; Zheng, P.; Gao, S.; Song, C.; Yu, Y.; Gong, Z.; Wan, X. Gut microbiota-mediated improvement of metabolic disorders by Qingzhuan tea in high fat diet-fed mice. J. Funct. Foods 2021, 78, 104366. [Google Scholar] [CrossRef]
- An, H.M.; Ou, X.C.; Zhang, Y.B.; Li, S.; Xiong, Y.F.; Li, Q.; Huang, J.A.; Liu, Z.H. Study on the key volatile compounds and aroma quality of jasmine tea with different scenting technology. Food Chem. 2022, 385, 132718. [Google Scholar] [CrossRef] [PubMed]
- Qing-Guo, H.; Min, Z.; Mujumdar, A.S.; Gong-Nian, X.; Jin-Cai, S. Drying of edamames by hot air and vacuum microwave combination. J. Food Eng. 2006, 77, 977–982. [Google Scholar] [CrossRef]
- Xiangyang, G.; Schwab, W.; Chi-Tang, H.; Chuankui, S.; Xiaochun, W. Characterization of the aroma profiles of oolong tea made from three tea cultivars by both GC-MS and GC-IMS. Food Chem. 2022, 376, 131933. [Google Scholar] [CrossRef]
- Wang, J.T.; Shi, J.; Zhu, Y.; Ma, W.J.; Yan, H.; Shao, C.Y.; Wang, M.Q.; Zhang, Y.; Peng, Q.H.; Chen, Y.Q.; et al. Insights into crucial odourants dominating the characteristic flavour of citrus-white teas prepared from citrus reticulata Blanco ‘Chachiensis’ and Camellia sinensis ‘Fudingdabai’. Food Chem. 2022, 377, 132048. [Google Scholar] [CrossRef]
- Baptista, J.A.B.; Tavares, J.F.D.; Carvalho, R.C.B. Comparison of catechins and aromas among different green teas using HPLC/SPME-GC. Food Res. Int. 1998, 31, 729–736. [Google Scholar] [CrossRef]
- Chi-Tang, H.; Xin, Z.; Shiming, L. Tea aroma formation. Food Sci. Hum. Wellness 2015, 4, 9–27. [Google Scholar] [CrossRef] [Green Version]
- Huajie, W.; Jinjie, H.; Qinyan, Y.; Yongwen, J.; Jinjin, W.; Yanqin, Y.; Yuliang, D.; Haibo, Y. Effects of fixation methods on the formation of the chestnut-like aroma of green tea analyzed by infrared-assisted extraction coupled to headspace solid-phase microextraction combined with gas chromatography-mass spectrometry. Food Sci. China 2021, 42, 209–217. [Google Scholar] [CrossRef]
- Qi, H.T.; Ding, S.H.; Pan, Z.P.; Li, X.; Fu, F.H. Characteristic Volatile Fingerprints and Odor Activity Values in Different Citrus-Tea by HS-GC-IMS and HS-SPME-GC-MS. Molecules 2020, 25, 6027. [Google Scholar] [CrossRef]
- Jie-Qiong, W.; Yan-Qing, F.; Jian-Xin, C.; Fang, W.; Zhi-Hui, F.; Jun-Feng, Y.; Liang, Z.; Yong-Quan, X. Effects of baking treatment on the sensory quality and physicochemical properties of green tea with different processing methods. Food Chem. 2022, 380, 132217. [Google Scholar] [CrossRef]
- Seeram, N.P.; Aviram, M.; Zhang, Y.; Henning, S.M.; Feng, L.; Dreher, M.; Heber, D. Comparison of antioxidant potency of commonly consumed Polyphenol-Rich beverages in the united states. J. Agric. Food Chem. 2008, 56, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.T.; Yu, X.L.; He, C.; Qiu, A.D.; Li, Y.C.; Shu, Q.N.; Chen, Y.Q.; Ni, D.J. Withering degree affects flavor and biological activity of black tea: A non-targeted metabolomics approach. LWT Food Sci. Technol. 2020, 130, 109535. [Google Scholar] [CrossRef]
- Hee Chul, K.; Mi Gyeong, J.; Jung Min, O.; Jeong Yong, P.; Jung Eun, K.; Jae-Won, K.; Songyee, B.; Sang Heon, H.; Se-Jae, K. Changes in chemical composition and antioxidant activity of dried Citrus unshiu peel after roasting. LWT Food Sci. Technol. 2020, 131, 109612. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, Y.; Long, P.P.; Ho, C.T.; Wang, Y.J.; Kan, Z.P.; Cao, L.T.; Zhang, L.; Wan, X.C. LC-MS-Based Metabolomics Reveals the Chemical Changes of Polyphenols during High-Temperature Roasting of Large-Leaf Yellow Tea. J. Agric. Food Chem. 2019, 67, 5405–5412. [Google Scholar] [CrossRef]
- Thitima, K.; Sakamon, D.; Naphaporn, C. Evolution of antioxidant compounds in lime residues during drying. Food Chem. 2009, 113, 944–949. [Google Scholar] [CrossRef]
- Ren, G.; Wang, X.; Cheng, Z.; Huang, W.; Yang, H. Evaluation of the Quality of Dried Wolfberry at Different Temperatures Based on the Coefficient of Variation Method. Mod. Food Sci. Technol. 2022, 38, 3675. [Google Scholar] [CrossRef]
- Wenjiang, D.; Rongsuo, H.; Yuzhou, L.; Hehe, L.; Yanjun, Z.; Kexue, Z.; Zhong, C. Comparative evaluation of the volatile profiles and taste properties of roasted coffee beans as affected by drying method and detected by electronic nose, electronic tongue, and HS-SPME-GC-MS. Food Chem. 2019, 272, 723–731. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Dong, J.J.; Jin, J.; Liu, J.H.; Zheng, X.Q.; Lu, J.L.; Liang, Y.R.; Ye, J.H. Roasting process shaping the chemical profile of roasted green tea and the association with aroma features. Food Chem. 2021, 353, 129428. [Google Scholar] [CrossRef]
- Poonam, S.; Santosh, S.; Naik, S.N. Effect of different drying techniques on the nutritional, antioxidant and cyanogenic profile of bamboo shoots. Appl. Food Res. 2022, 2, 100036. [Google Scholar] [CrossRef]
- Ozcan, M.M.; Al Juhaimi, F.; Uslu, N. The effect of heat treatment on phenolic compounds and fatty acid composition of Brazilian nut and hazelnut. J. Food Sci. Technol. Mysore 2018, 55, 376–380. [Google Scholar] [CrossRef]
- Xiu-Min, C.; Tait, A.R.; Kitts, D.D. Flavonoid composition of orange peel and its association with antioxidant and anti-inflammatory activities. Food Chem. 2017, 218, 15–21. [Google Scholar] [CrossRef]
- Gong, T.; Yang, X.; Bai, F.; Li, D.; Zhao, T.; Zhang, J.; Sun, L.; Guo, Y. Young apple polyphenols as natural α-glucosidase inhibitors: In vitro and in silico studies. Bioorg. Chem. 2020, 96, 103625. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Moslehishad, M.; Salami, M. Antioxidant and alpha-glucosidase enzyme inhibitory properties of hydrolyzed protein and bioactive peptides of quinoa. Int. J. Biol. Macromol. 2022, 213, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, W.; Wang, R.; Li, C.; Lin, X.; Wang, L. Screening and identification of natural α-glucosidase and α-amylase inhibitors from partridge tea (Mallotus furetianus Muell-Arg) and in silico analysis. Food Chem. 2022, 388, 133004. [Google Scholar] [CrossRef] [PubMed]
- Yuchuan, L.; Wei, R.; Chang, H.; Jingtao, Z.; Yuqiong, C.; Zhi, Y.; Dejiang, N. Effects of different tea tree varieties on the color, aroma, and taste of Chinese Enshi green tea. Food Chem. X 2022, 14, 100289. [Google Scholar] [CrossRef]
- Liu, S.; Ai, Z.; Qu, F.; Chen, Y.; Ni, D. Effect of steeping temperature on antioxidant and inhibitory activities of green tea extracts against α-amylase, α-glucosidase and intestinal glucose uptake. Food Chem. 2017, 234, 168–173. [Google Scholar] [CrossRef]
- Chen, Q.C.; Zhu, Y.; Dai, W.D.; Lv, H.P.; Mu, B.; Li, P.L.; Tan, J.F.; Ni, D.J.; Lin, Z. Aroma formation and dynamic changes during white tea processing. Food Chem. 2019, 274, 915–924. [Google Scholar] [CrossRef]
- Qu, F.F.; Zeng, W.C.; Tong, X.; Feng, W.; Chen, Y.Q.; Ni, D.J. The new insight into the influence of fermentation temperature on quality and bioactivities of black tea. LWT Food Sci. Technol. 2020, 117, 108646. [Google Scholar] [CrossRef]
- Xiu-min, L.; Ya, L.; Chun-hui, S.; Xin-quan, Y.; Qin, Z.; Na, X.; Li-ying, X.; Wen, S. Effects of five extraction methods on total content, composition, and stability of flavonoids in jujube. Food Chem. X 2022, 14, 100287. [Google Scholar] [CrossRef]
- Yana, Z.; Hongyan, S.; Ling, M.; Anjun, L. Polysaccharides from the peels of Citrus aurantifolia induce apoptosis in transplanted H22 cells in mice. Int. J. Biol. Macromol. 2017, 101, 680–689. [Google Scholar] [CrossRef]
- Ding, L.; Luo, X.; Tang, F.; Yuan, J.; Liu, Q.; Yao, S. Simultaneous determination of flavonoid and alkaloid compounds in Citrus herbs by high-performance liquid chromatography—photodiode array detection—electro spray mass spectrometry. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2007, 857, 202–209. [Google Scholar] [CrossRef] [PubMed]
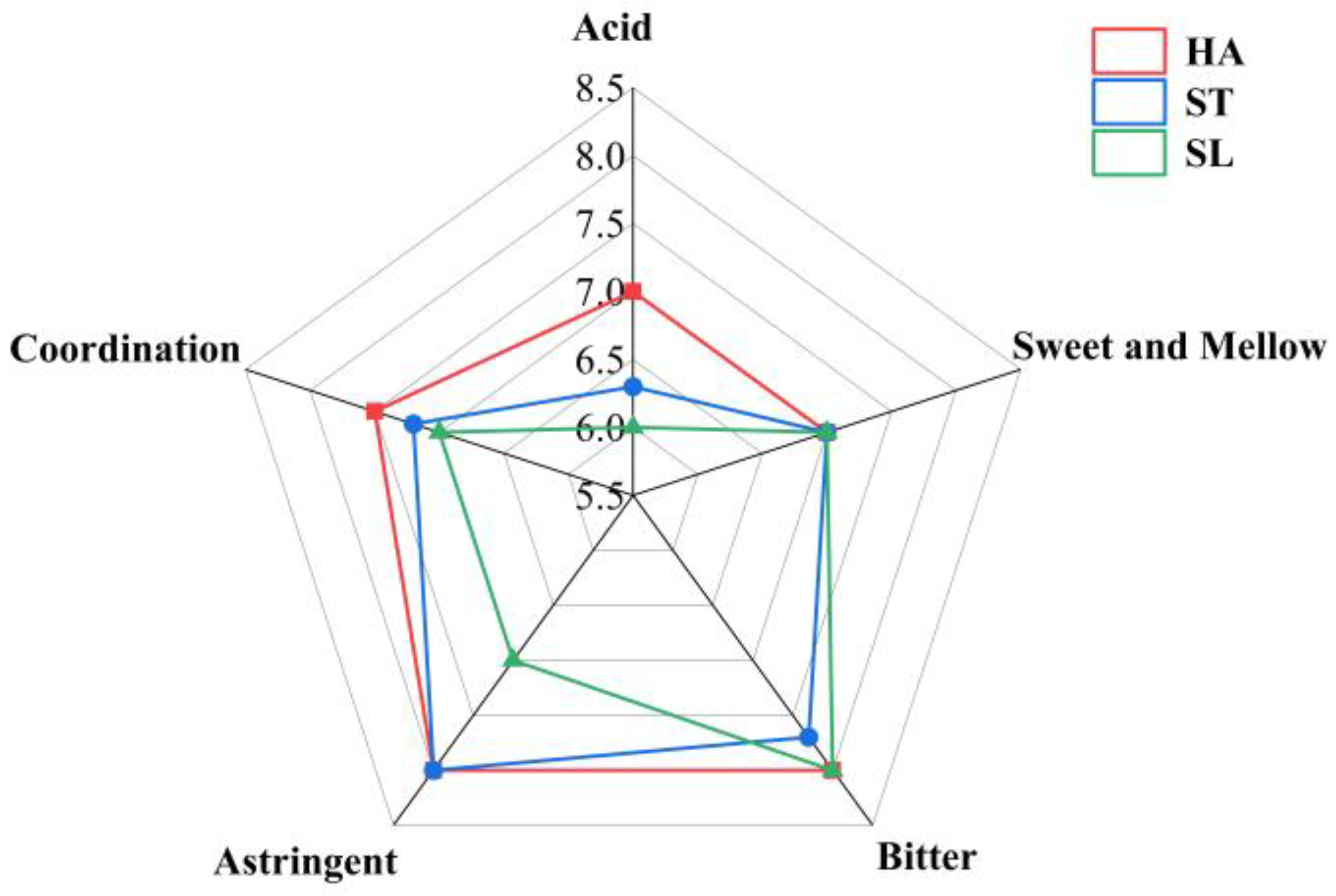


| Sample | Appearance (10 Points) | Soup Color (10 Points) | Aroma (30 Points) | Taste (50 Points) | Total Score | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aroma | Coordination | Sour | Sweet | Bitter | Astringency | Coordination | ||||
| HA | 6.20 ± 0.30 ab | 9.10 ± 0.10 | 15.30 ± 0.10 | 9.0 ± 0.00 | 7.00 ± 0.00 a | 7.00 ± 0.00 | 8.00 ± 0.00 a | 8.00 ± 0.00 a | 7.50 ± 0.00 a | 77.00 ± 0.50 a |
| ST | 5.80 ± 0.30 b | 9.00 ± 0.00 | 15.00 ± 0.00 | 9.0 ± 0.00 | 6.50 ± 0.60 b | 7.00 ± 0.00 | 7.70 ± 0.30 a | 7.80 ± 0.20 a | 7.20 ± 0.30 b | 75.00 ± 0.90 b |
| SL | 6.50 ± 0.30 a | 9.10 ± 0.10 | 15.00 ± 0.10 | 9.0 ± 0.00 | 6.00 ± 0.00 b | 7.00 ± 0.00 | 7.80 ± 0.00 ab | 7.00 ± 0.00 b | 7.20 ± 0.00 b | 74.60 ± 0.40 b |
| RT | RI | Compound Name | Odor Description | HA | ST | SL |
|---|---|---|---|---|---|---|
| Alcohols | ||||||
| 6.35 | 969 | 1-Heptanol | sweet, woody | 0.57 ± 0.08 | 0.36 ± 0.01 | - |
| 9.27 | 1070 | 1-Octanol | waxy, green, fruity | 24.70 ± 0.72 b | 7.52 ± 1.30 c | 29.35 ± 0.11 a |
| 9.83 | 1086 | Terpinolene | fresh, woody, floral | 4.24 ± 0.19 a | 3.65 ± 0.22 b | 3.11 ± 0.16 c |
| 10.51 | 1102 | Linalool | floral, sweet | 173.54 ± 5.41 | 150.14 ± 17.27 | 168.25 ± 10.53 |
| 10.87 | 1113 | Phenylethyl alcohol | floral, sweet, rosy | 13.35 ± 0.28 a | 4.33 ± 0.34 b | 4.36 ± 0.26 b |
| 11.31 | 1123 | (E)-para-2,8-1-menthadienol | fresh, minty | 23.5 ± 1.62 a | 15.83 ± 1.45 c | 19.91 ± 1.06 b |
| 11.99 | 1137 | (Z)-para-2,8-menthadien-1-ol | null | 26.14 ± 1.51 a | 19.57 ± 3.01 b | 20.89 ± 0.88 b |
| 12.55 | 1150 | Isopulegol | minty, cooling, woody | 1.20 ± 0.14 a | 1.33 ± 0.10 a | 0.90 ± 0.12 b |
| 13.65 | 1172 | 1-Nonanol | fresh, fatty, floral | 8.37 ± 0.22 a | 4.07 ± 0.07 c | 7.40 ± 0.45 b |
| 13.82 | 1176 | Linalool oxide (pyranoid) | floral, honey | 4.86 ± 0.40 a | 3.39 ± 0.09 b | 2.65 ± 0.20 c |
| 14.09 | 1181 | 4-Terpineol | pepper, woody, musty | 26.57 ± 1.45 | 22.59 ± 1.48 | 24.09 ± 2.66 |
| 14.42 | 1188 | Trans-isocarveol | null | 32.48 ± 1.08 a | 20.22 ± 1.14 c | 25.07 ± 2.33 b |
| 14.64 | 1191 | 2,6-dimethyl-3,7-Octadiene-2,6-diol, | null | 0.89 ± 0.10 b | 0.73 ± 0.02 c | 1.41 ± 0.08 a |
| 14.9 | 1196 | L-α-Terpineol | floral, terpenic | 76.31 ± 0.80 a | 55.44 ± 8.19 c | 65.3 ± 3.11 b |
| 14.96 | 1197 | Dihydrocarveol | minty, herbal | - | 2.28 ± 0.19 | - |
| 15.07 | 1198 | Isopiperitenol | null | 17.18 ± 0.41 a | 9.61 ± 1.94 c | 12.47 ± 0.65 b |
| 15.96 | 1216 | Carveol | green, weedy, herbal | 12.30 ± 0.24 b | 8.59 ± 0.55 c | 19.03 ± 0.79 a |
| 16.06 | 1218 | Trans-carveol | caraway, spearmint | 48.54 ± 1.36 a | 26.21 ± 2.55 b | 25.65 ± 2.50 b |
| 16.37 | 1224 | Nerol | sweet, citrus, green | 22.95 ± 0.64 a | 7.50 ± 0.32 c | 18.15 ± 1.23 b |
| 16.62 | 1229 | Cis-p-mentha-1(7),8-dien-2-ol | null | 34.75 ± 1.52 a | 21.96 ± 0.32 c | 27.94 ± 2.93 b |
| 16.76 | 1231 | Cis-carveol | caraway | 21.66 ± 1.47 a | 17.38 ± 0.87 b | 17.12 ± 2.27 b |
| 17.82 | 1251 | Geraniol | floral, rosy, waxy | 15.70 ± 0.26 a | 8.60 ± 0.04 c | 12.14 ± 0.48 b |
| 18 | 1254 | 2-Methoxybenzylalcohol | anisic | 1.13 ± 0.05 b | 3.08 ± 0.13 a | 2.48 ± 0.41 a |
| 20.02 | 1287 | 2-(4-Methylenecyclohexyl)-2-propen-1-ol | null | 18.22 ± 0.98 a | 16.11 ± 1.09 b | 15.91 ± 1.23 b |
| 20.5 | 1295 | Perilla alcohol | woody, spicy, floral | 7.40 ± 0.57 a | 6.34 ± 0.23 b | 6.74 ± 0.26 a |
| 32.97 | 1537 | α-elemol | green, woody, spicy | 9.01 ± 0.67 b | 8.92 ± 0.81 b | 10.85 ± 0.42 a |
| 33.75 | 1553 | Nerolidol | green, floral, woody | 4.13 ± 0.50 b | 3.53 ± 0.27 c | 4.86 ± 0.43 a |
| 34.19 | 1562 | Spathulenol | earthy, herbal, fruity | 8.04 ± 0.65 a | 3.41 ± 0.39 b | 3.35 ± 0.15 b |
| 37.04 | 1635 | Cubenol | spicy, herbal, green tea | 2.79 ± 0.12 a | 1.42 ± 0.05 b | 0.94 ± 0.13 c |
| 37.38 | 1646 | β-Eudesmol | woody, green | 7.00 ± 0.62 a | 5.80 ± 0.12 b | 5.65 ± 0.47 b |
| Aldehydes and Ketones | ||||||
| 4.16 | 854 | (E)-2-Hexenal | green, banana, fatty | 0.38 ± 0.06 | - | 1.01 ± 0.03 |
| 6.03 | 956 | (E)-2-Heptenal | green, sweet, fruity | 0.55 ± 0.04 | - | 0.23 ± 0.01 |
| 6.17 | 961 | Benzaldehyde | fruity, cherry, oily | 1.23 ± 0.16 a | 0.20 ± 0.03 c | 0.57 ± 0.03 b |
| 6.67 | 983 | Methylhepten | citrus, green, musty | 8.57 ± 0.67 | - | - |
| 7.18 | 1003 | Octanal | waxy, fatty, citrus | 58.28 ± 5.16 a | 51.67 ± 3.58 ab | 47.46 ± 2.67 b |
| 8.38 | 1044 | Benzeneacetaldehyde | honey, sweet, floral | 2.00 ± 0.17 a | 0.64 ± 0.07 c | 1.00 ± 0.05 b |
| 10.61 | 1106 | Nonanal | waxy, aldehydic, rose | 23.12 ± 2.18 a | 20.69 ± 0.59 a | 18.88 ± 0.94 b |
| 12.7 | 1153 | Citronellal | floral, green, rosy | 13.71 ± 0.73 | 12.46 ± 0.73 | 13.57 ± 0.79 |
| 13.06 | 1161 | (E)-2-Nonenal | green, soapy, cucumber | 0.84 ± 0.05 b | 0.73 ± 0.09 b | 1.02 ± 0.07 a |
| 15.29 | 1207 | Decanal | waxy, fatty, citrus | 95.24 ± 4.32 a | 78.84 ± 2.38 b | 77.62 ± 3.93 b |
| 15.67 | 1211 | Berbenone | camphor, menthol, celery | 2.91 ± 0.14 | 2.84 ± 0.13 | 2.77 ± 0.14 |
| 17.12 | 1238 | (Z)-citral | sweet, citral, lemon peel | 28.15 ± 0.46 a | 26.23 ± 0.59 a | 16.54 ± 0.46 b |
| 17.42 | 1243 | (+)-carvone | spice, mint, caraway | 36.63 ± 2.27 | 33.11 ± 1.66 | 35.75 ± 1.67 |
| 18.5 | 1263 | Trans-2-Decenal | waxy, fatty, earthy | 3.33 ± 0.12 a | 1.79 ± 0.21 b | 2.83 ± 0.41 a |
| 18.89 | 1269 | Citral | citrus, juicy, green | 50.95 ± 0.96 a | 44.63 ± 4.03 b | 33.9 ± 3.15 c |
| 19.16 | 1274 | Perillaldehyde | fresh, green, herbal | 35.96 ± 2.06 | 32.57 ± 1.43 | 34.04 ± 3.61 |
| 20.28 | 1291 | 2-Undecanone | waxy, fruity, creamy | 1.63 ± 0.02 a | 1.29 ± 0.15 b | 1.48 ± 0.12 ab |
| 21.12 | 1306 | Undecanal | waxy, soapy, floral | 19.03 ± 0.60 a | 14.83 ± 0.93 b | 15.32 ± 1.40 b |
| 22.44 | 1333 | Piperitenone | minty, phenolic | 4.7 ± 0.16 b | 6.73 ± 0.29 a | 6.19 ± 0.41 a |
| 26.27 | 1404 | Dodecanal | soapy, waxy, citrus | 34.65 ± 1.78 a | 27.01 ± 0.64 b | 25.87 ± 1.26 b |
| 26.64 | 1412 | α-Ionone | sweet, woody, floral | 3.22 ± 0.15 a | 2.44 ± 0.09 b | 2.32 ± 0.19 b |
| 28 | 1439 | Nerylacetone | fatty, metallic | 10.72 ± 0.31 a | 7.68 ± 0.27 b | 10.56 ± 0.42 a |
| 29.46 | 1467 | β-ionone | woody, floral, berry | 12.04 ± 0.41 a | 11.73 ± 0.79 ab | 10.84 ± 0.55 b |
| 29.61 | 1469 | β-Ionone epoxide | fruity, sweet, berry | 2.41 ± 0.13 a | 2.13 ± 0.12 b | 2.32 ± 0.01 a |
| 33.36 | 1545 | 4-Isopropyl-2-methyl-2-cyclohexen-1-one | null | 3.45 ± 0.49 a | 3.35 ± 0.17 ab | 3.03 ± 0.16 b |
| 36.17 | 1604 | Tetradecanal | fatty, lactonic, coconut | 1.70 ± 0.14 a | 1.32 ± 0.06 b | 0.85 ± 0.10 c |
| 38.5 | 1685 | β-sinensal | orange, sweet, fresh | 11.73 ± 0.74 a | 9.89 ± 0.41 c | 10.47 ± 0.40 b |
| 39.69 | 1741 | α-Sinensal | citrus, juicy, waxy | 2.37 ± 0.22 b | 5.89 ± 0.30 a | 5.83 ± 0.47 a |
| 41.27 | 1837 | Hexahydrofarnesyl acetone | oily, herbal, jasmin | 2.84 ± 0.25 c | 4.93 ± 0.18 a | 3.02 ± 0.23 b |
| 42.22 | 1904 | Farnesyl acetone | flower, ether | 0.33 ± 0.01 | 0.35 ± 0.04 | - |
| Alkenes | ||||||
| 5.58 | 934 | α-Pinene | fresh, camphor, sweet | 1.98 ± 0.01 b | 1.45 ± 0.11 c | 4.79 ± 0.08 a |
| 6.47 | 973 | β-Pinene | fresh, piney, woody | 1.94 ± 0.15 c | 5.12 ± 0.16 a | 3.43 ± 0.2 b |
| 6.82 | 989 | β-Myrcene | woody, vegetative, citrus | 10.81 ± 0.61 b | 12.94 ± 2.13 a | 12.73 ± 0.74 a |
| 7.61 | 1018 | α-Terpinene | terpy, woody, piney | 2.28 ± 0.13 b | 1.86 ± 0.31 b | 3.00 ± 0.19 a |
| 8.06 | 1033 | Limonene | citrus, herbal, camphor | 213.4 ± 16.56 b | 271.32 ± 11.51 a | 196.02 ± 0.16 a |
| 8.46 | 1046 | Trans-β-Ocimene | fruity, floral | - | 2.44 ± 0.34 | - |
| 8.87 | 1059 | γ-Terpinene | terpy, citrus, oily | 5.10 ± 0.43 c | 6.18 ± 0.17 b | 7.71 ± 0.65 a |
| 10.88 | 1113 | 1,3,8-p-Menthatriene | oily, terpy, camphorous | 4.37 ± 0.48 b | 5.07 ± 0.19 a | 5.32 ± 0.33 a |
| 11.66 | 1137 | 4-Acetyl-1-methylcyclohexene | null | 1.20 ± 0.21 c | 1.80 ± 0.08 b | 2.04 ± 0.09 a |
| 20.75 | 1298 | Dipentene dioxide | mentholic | 2.04 ± 0.08 a | 1.59 ± 0.06 b | 1.16 ± 0.09 c |
| 22.99 | 1344 | (-)-α-Cubebene | herbal, waxy | 17.46 ± 0.39 | 18.12 ± 1.42 | 16.90 ± 0.67 |
| 24.36 | 1370 | α-Copaene | woody, spicy, honey | 18.52 ± 2.17 | 18.42 ± 0.78 | 17.38 ± 0.61 |
| 25 | 1381 | β-cubebene | citrus, fruity, radish | 5.06 ± 0.15 a | 3.84 ± 0.23 b | 4.83 ± 0.60 a |
| 25.08 | 1383 | β-Elemen | sweet | 9.65 ± 0.88 | 10.40 ± 0.64 | 9.69 ± 0.17 |
| 26.42 | 1406 | β-Longipinene | null | 5.34 ± 0.45 | 5.42 ± 0.30 | 6.02 ± 0.31 |
| 26.5 | 1409 | Alloocimenal | null | 4.01 ± 0.18 a | 3.75 ± 0.17 a | 3.41 ± 0.2 b |
| 26.92 | 1417 | (-)-β-Copaene | null | 8.84 ± 0.33 | 9.38 ± 0.73 | 9.11 ± 0.59 |
| 27.82 | 1435 | β-copaene | null | 2.11 ± 0.11 | 1.99 ± 0.04 | 1.92 ± 0.16 |
| 28.1 | 1441 | α-Humulene | woody | 5.20 ± 0.59 a | 4.97 ± 0.25 a | 5.26 ± 0.27 b |
| 28.27 | 1444 | Cis-β-Farnesene | vitrus, green | 8.62 ± 0.59 | 8.27 ± 0.43 | 8.90 ± 0.17 |
| 29.04 | 1459 | γ-Gurjunene | null | 3.97 ± 0.13 a | 3.52 ± 0.38 ab | 3.24 ± 0.11 b |
| 29.22 | 1462 | (-)-α-muurolene | null | 4.61 ± 0.17 b | 5.08 ± 0.12 a | 4.78 ± 0.15 ab |
| 30.01 | 1477 | γ-Muurolene | woody, spice | 1.16 ± 0.07 b | 1.48 ± 0.06 a | 1.21 ± 0.07 b |
| 30.25 | 1481 | β-Selinene | null | 3.40 ± 0.08 b | 3.98 ± 0.21 a | 3.46 ± 0.15 b |
| 30.49 | 1485 | α-Muurolene | null | 5.87 ± 0.23 a | 5.89 ± 0.16 a | 5.33 ± 0.27 b |
| 31.02 | 1495 | α-Farnesene | citrus, herbal, lavender | 7.77 ± 0.13 b | 8.05 ± 0.03 a | 8.01 ± 0.36 a |
| 31.52 | 1505 | β-cadinene | green, woody | 33.64 ± 1.17 a | 31.85 ± 0.84 a | 29.20 ± 1.07 b |
| 31.86 | 1512 | β-Bisabolene | balsamic, woody | 2.23 ± 0.10 a | 1.92 ± 0.15 b | 2.30 ± 0.09 a |
| 32.16 | 1519 | Cubenene | null | 7.12 ± 0.20 a | 6.4 ± 0.24 b | 5.81 ± 0.26 c |
| 32.54 | 1527 | α-Calacorene | woody | 2.72 ± 0.19 a | 1.63 ± 0.04 b | 1.54 ± 0.12 b |
| 34.37 | 1566 | Caryophyllene oxide | sweet, fresh, woody | 3.41 ± 0.26 a | 2.58 ± 0.14 b | 2.73 ± 0.23 b |
| 35.41 | 1587 | Trans-Z-α-Bisabolene epoxide | null | - | 0.63 ± 0.08 | - |
| Acid and Esters | ||||||
| 9.56 | 1078 | Heptanoic acid | waxy, cheesy, fruity | 2.06 ± 0.10 a | 1.35 ± 0.09 b | 0.92 ± 0.07 c |
| 14.23 | 1183 | Octanoic acid | fatty, waxy, rancid | 5.01 ± 0.23 | - | 3.72 ± 0.06 |
| 21.77 | 1319 | Methyl geranate | waxy, green, fruity | 8.97 ± 0.37 b | 9.35 ± 0.74 a | 9.32 ± 0.61 a |
| 21.97 | 1324 | Methyl decanoate | oily, fruity, floral | 1.95 ± 0.16 | - | - |
| 23.29 | 1350 | Citronellyl acetate | floral, waxy, aldehydic | 5.47 ± 0.13 a | 5.21 ± 0.43 a | 4.42 ± 0.34 b |
| 23.73 | 1358 | Neryl acetate | floral, rosy, soapy | 7.94 ± 0.40 b | 9.58 ± 0.50 a | 8.17 ± 0.64 b |
| 23.98 | 1363 | 2-(2-Butoxyethoxy)ethyl acetate | null | 6.16 ± 0.19 a | 5.69 ± 0.28 b | 5.23 ± 0.29 b |
| 24.23 | 1367 | Decanoic acid | soapy, waxy, fruity | 1.00 ± 0.06 c | 1.55 ± 0.08 b | 2.25 ± 0.04 a |
| 24.75 | 1377 | Geranyl acetate | waxy, green, floral | 14.66 ± 0.20 b | 18.68 ± 0.82 a | 14.59 ± 0.57 b |
| 25.8 | 1395 | Methyl methanthranilate | fruity, woody, floral | 10.25 ± 0.36 b | 11.03 ± 0.10 a | 9.40 ± 0.46 c |
| 31.68 | 1508 | Dihydroactinidiolide | musk, coumarin | 14.86 ± 1.90 | 16.94 ± 1.39 | 15.29 ± 0.98 |
| 34.94 | 1578 | Pentanoicacid,2,2,4-trimethyl-3-Carboxyisopropyl,isobutylester | null | 4.04 ± 0.26 a | 1.85 ± 0.15 b | 2.07 ± 0.16 b |
| 42.43 | 1922 | Methyl palmitate | oily, waxy, fatty, orris | 6.89 ± 0.37 a | 1.52 ± 0.18 c | 4.15 ± 0.24 b |
| 44.35 | 2089 | Methyl linolenate | null | 0.38 ± 0.05 | - | 0.21 ± 0.01 |
| Others | ||||||
| 7.86 | 1026 | o-Cymene | null | 6.89 ± 0.46 a | 3.06 ± 0.05 b | 6.50 ± 0.69 a |
| 8.5 | 1047 | 1-ethylpyrrole-2-carbaldehyde | burnt, roasted, smoky | - | - | 4.25 ± 0.26 |
| 9.98 | 1090 | α,P-Dimethylstyrene | spicy, balsamic, musty | 5.24 ± 0.38 a | 3.91 ± 0.06 b | 3.48 ± 0.16 c |
| 12.89 | 1157 | (+)-β-Pinene oxide | rosemary, sage, herbal | 11.67 ± 0.24 a | 6.55 ± 0.99 c | 9.21 ± 0.41 b |
| 19.59 | 1281 | 3,4-Diethylphenol | null | - | 2.44 ± 0.08 a | 1.43 ± 0.08 b |
| 21 | 1303 | 1,2,3-Trimethoxybenzene | null | 10.39 ± 0.86 | 9.51 ± 0.36 | 9.09 ± 0.42 |
| 24.16 | 1366 | 1,2,4-Trimethoxybenzene | null | 13.90 ± 1.16 | 13.55 ± 1.02 | 12.6 ± 0.77 |
| 40.17 | 1768 | 3-methylheptadecane | null | 0.56 ± 0.05 a | 0.36 ± 0.01 b | 0.37 ± 0.02 b |
| Samples | HA | ST | SL |
|---|---|---|---|
| Alcohols | 647.48 ± 24.08 a | 459.93 ± 44.57 c | 555.94 ± 36.30 b |
| Aldehydes and Ketones | 472.68 ± 24.95 a | 405.97 ± 19.99 b | 385.31 ± 23.68 b |
| Alkenes | 403.84 ± 27.23 b | 467.34 ± 22.51 a | 387.24 ± 9.17 b |
| Acid and Asters | 89.65 ± 4.77 a | 82.74 ± 4.74 ab | 79.73 ± 4.48 b |
| Others | 48.64 ± 3.15 a | 39.38 ± 2.57 b | 46.92 ± 2.80 a |
| Total | 1662.29 ± 84.20 a | 1455.36 ± 94.40 b | 1455.14 ± 76.45 b |
| Samples | μmol TE/g | APC Index | Comprehensive APC Index | ||||
|---|---|---|---|---|---|---|---|
| FRAP | DPPH | ABTS | FRAP | DPPH | ABTS | ||
| HA | 254.49 ± 1.84 a | 351.11 ± 3.21 a | 324.04 ± 1.07 ab | 100.00 | 100.00 | 99.39 | 99.8 |
| ST | 224.91 ± 1.12 b | 334.33 ± 1.94 b | 322.29 ± 1.23 b | 88.37 | 95.22 | 98.85 | 94.15 |
| SL | 212.61 ± 8.67 c | 344.41 ± 5.07 a | 326.04 ± 2.32 a | 83.54 | 98.09 | 100.00 | 93.88 |
| α-Glucosidase | α-Amylase | |
|---|---|---|
| HA | 387.59 ± 7.50 c | 780.43 ± 24.99 a |
| ST | 401.67 ± 4.22 b | 538.17 ± 16.25 b |
| SL | 438.11 ± 6.99 a | 514.16 ± 13.42 b |
| Substances | FRAP | DPPH | ABTS | α-Amylase | α-Glucosidase |
|---|---|---|---|---|---|
| Amino acid | 0.202 | 0.235 | 0.162 | 0.201 | −0.139 |
| Soluble sugar | 0.025 | −0.574 | −0.679 * | −0.218 | −0.435 |
| Tea polyphenols | 0.848 ** | 0.698 * | −0.028 | 0.886 ** | −0.642 |
| Theaflavins | −0.792 * | −0.752 * | −0.061 | −0.943 ** | 0.507 |
| Thearubin | −0.785 * | −0.802 ** | −0.197 | −0.957 ** | 0.470 |
| Theabrownin | −0.350 | −0.273 | 0.022 | −0.246 | 0.240 |
| Soluble protein | −0.818 ** | −0.129 | 0.576 | −0.557 | 0.943 ** |
| Flavonoids | −0.346 | 0.492 | 0.754 * | 0.069 | 0.720 * |
| Polyphenols | −0.152 | 0.618 | 0.792 * | 0.233 | 0.589 |
| Polysaccharide | −0.683 * | −0.926 ** | −0.314 | −0.932 ** | 0.278 |
| Hesperidin | −0.071 | 0.657 | 0.691 * | 0.336 | 0.492 |
| Synephrine | 0.199 | 0.779 * | 0.743 * | 0.528 | 0.250 |
| Limonin | −0.600 | 0.228 | 0.763 * | −0.249 | 0.886 ** |
| Sample | Appearance (10 Points) | Soup Color (10 Points) | Aroma (30 Points) | Taste (50 Points) | Total Score | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aroma | Coordination | Sour | Sweet | Bitter | Astringency | Coordination | ||||
| 70 °C | 7.60 ± 0.400 a | 9.10 ± 0.10 | 15. 00 ± 0.00 | 9.00 ± 0.00 | 7.00 ± 0.00 a | 7.00 ± 0.00 | 5.50 ± 0.30 c | 5.50 ± 0.30 cd | 5.90 ± 0.30 c | 71.60 ± 0.90 c |
| 75 °C | 6.50 ± 0.00 b | 9.00 ± 0.10 | 14.90 ± 0.00 | 9.00 ± 0.00 | 6.70 ± 0.60 ab | 7.00 ± 0.00 | 5.70 ± 0.00 c | 5.80 ± 0. 00 d | 6.30 ± 0.10 d | 70.90 ± 0.70 c |
| 80 °C | 6.30 ± 0.30 bc | 9.10 ± 0.10 | 14.90 ± 0.00 | 9.00 ± 0.00 | 7.00 ± 0.00 a | 7.00 ± 0.00 | 6.70 ± 0.60 ab | 6.70 ± 0.60 ab | 6.80 ± 0.30 bc | 73.50 ± 1.80 ab |
| 85 °C | 6.40 ± 0.20 bc | 9.10 ± 0.10 | 15.10 ± 0.10 | 9.00 ± 0.00 | 6.50 ± 0.00 c | 7.00 ± 0.00 | 6.80 ± 0.30 ab | 6.90 ± 0.10 a | 6.90 ± 0.10 b | 73.70 ± 0.60 ab |
| 90 °C | 6.5 ± 0.3 b | 9.1 ± 0.1 | 15 ± 0.0 | 9.0 ± 0.0 | 6.8 ± 0.0 b | 7.0 ± 0.0 | 7.0 ± 0.0 a | 7.0 ± 0.0 a | 7.0 ± 0.0 b | 74.5 ± 0.2 a |
| 95 °C | 6.3 ± 0.3 bc | 9.1 ± 0.1 | 15 ± 0.0 | 9.0 ± 0.0 | 6.8 ± 0.0 b | 7.0 ± 0.0 | 7.0 ± 0.0 a | 7.0 ± 0.0 a | 7.5 ± 0.0 a | 74.7 ± 0.4 a |
| 100 °C | 6.1 ± 0.1 c | 9.1 ± 0.2 | 14.9 ± 0.1 | 9.0 ± 0.0 | 6.8 ± 0.0 b | 7.0 ± 0.0 | 7.0 ± 0.0 a | 7.0 ± 0.0 a | 7.5 ± 0.0 a | 74.4 ± 0.2 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, Y.; Song, Y.; Yan, Z.; Wu, Z.; Yu, Z.; Zhang, D.; Ni, D.; Chen, Y. The New Insight into the Effects of Different Fixing Technology on Flavor and Bioactivities of Orange Dark Tea. Molecules 2023, 28, 1079. https://doi.org/10.3390/molecules28031079
Jiao Y, Song Y, Yan Z, Wu Z, Yu Z, Zhang D, Ni D, Chen Y. The New Insight into the Effects of Different Fixing Technology on Flavor and Bioactivities of Orange Dark Tea. Molecules. 2023; 28(3):1079. https://doi.org/10.3390/molecules28031079
Chicago/Turabian StyleJiao, Yuanfang, Yulin Song, Zhi Yan, Zhuanrong Wu, Zhi Yu, De Zhang, Dejiang Ni, and Yuqiong Chen. 2023. "The New Insight into the Effects of Different Fixing Technology on Flavor and Bioactivities of Orange Dark Tea" Molecules 28, no. 3: 1079. https://doi.org/10.3390/molecules28031079
APA StyleJiao, Y., Song, Y., Yan, Z., Wu, Z., Yu, Z., Zhang, D., Ni, D., & Chen, Y. (2023). The New Insight into the Effects of Different Fixing Technology on Flavor and Bioactivities of Orange Dark Tea. Molecules, 28(3), 1079. https://doi.org/10.3390/molecules28031079






