Optimization of Mixed Fermentation Conditions of Dietary Fiber from Soybean Residue and the Effect on Structure, Properties and Potential Biological Activity of Dietary Fiber from Soybean Residue
Abstract
:1. Introduction
2. Results
2.1. Optimization of Fermentation Conditions
2.2. Structural Properties of Soybean Residue
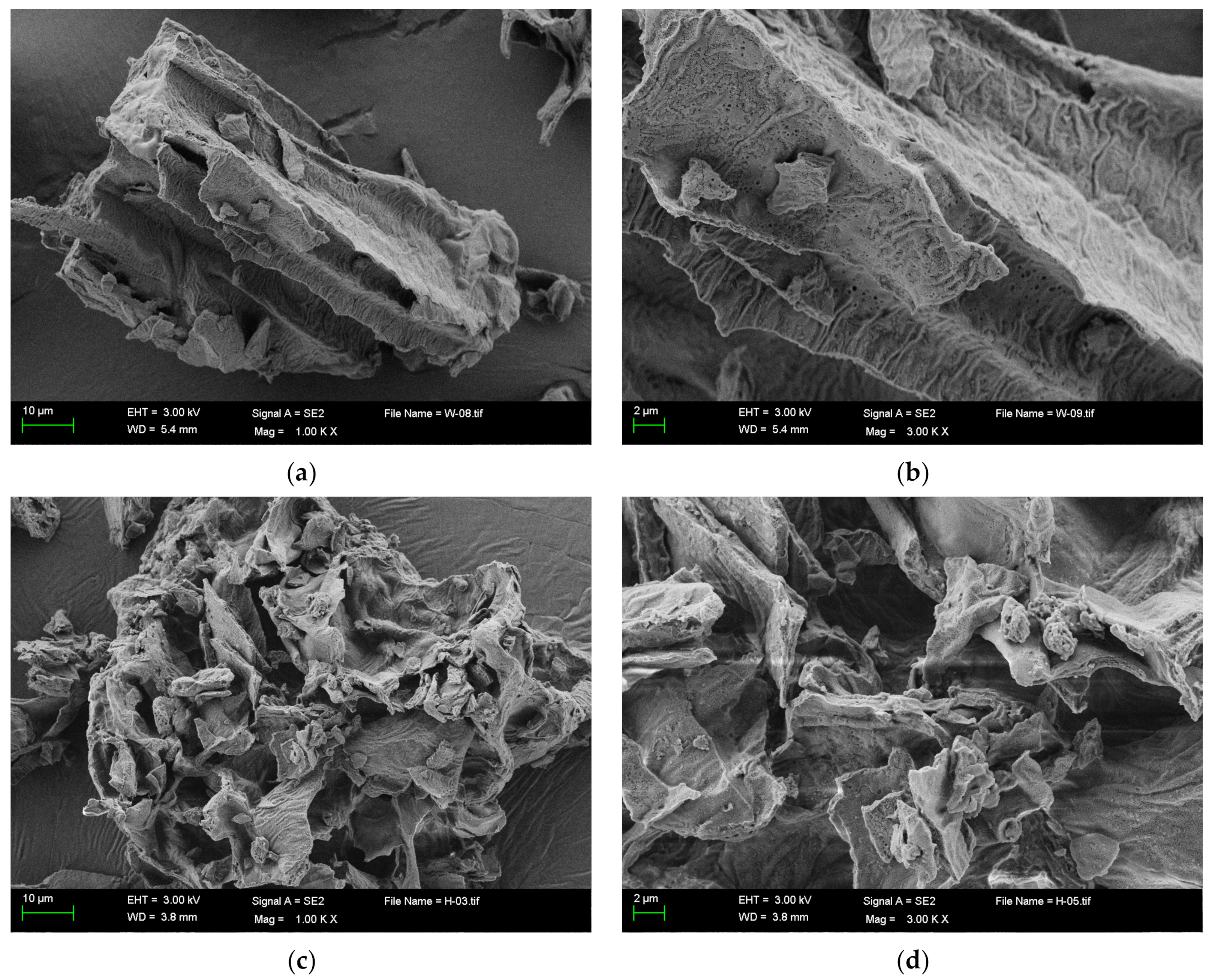
2.2.1. Scanning Electron Microscope Determination
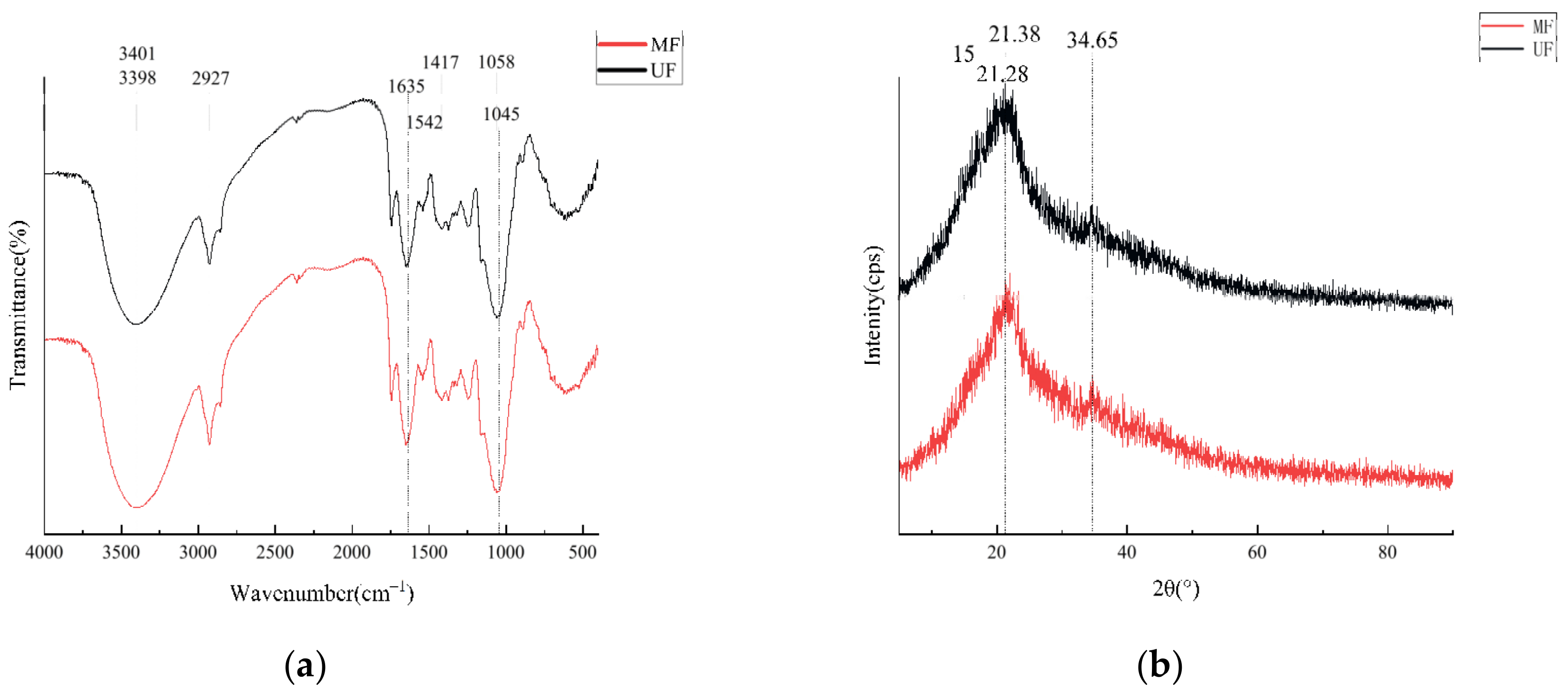
2.2.2. Fourier Transform Infrared Spectroscopy Assay
2.2.3. X-ray Diffraction Determination
2.2.4. Physicochemical Properties
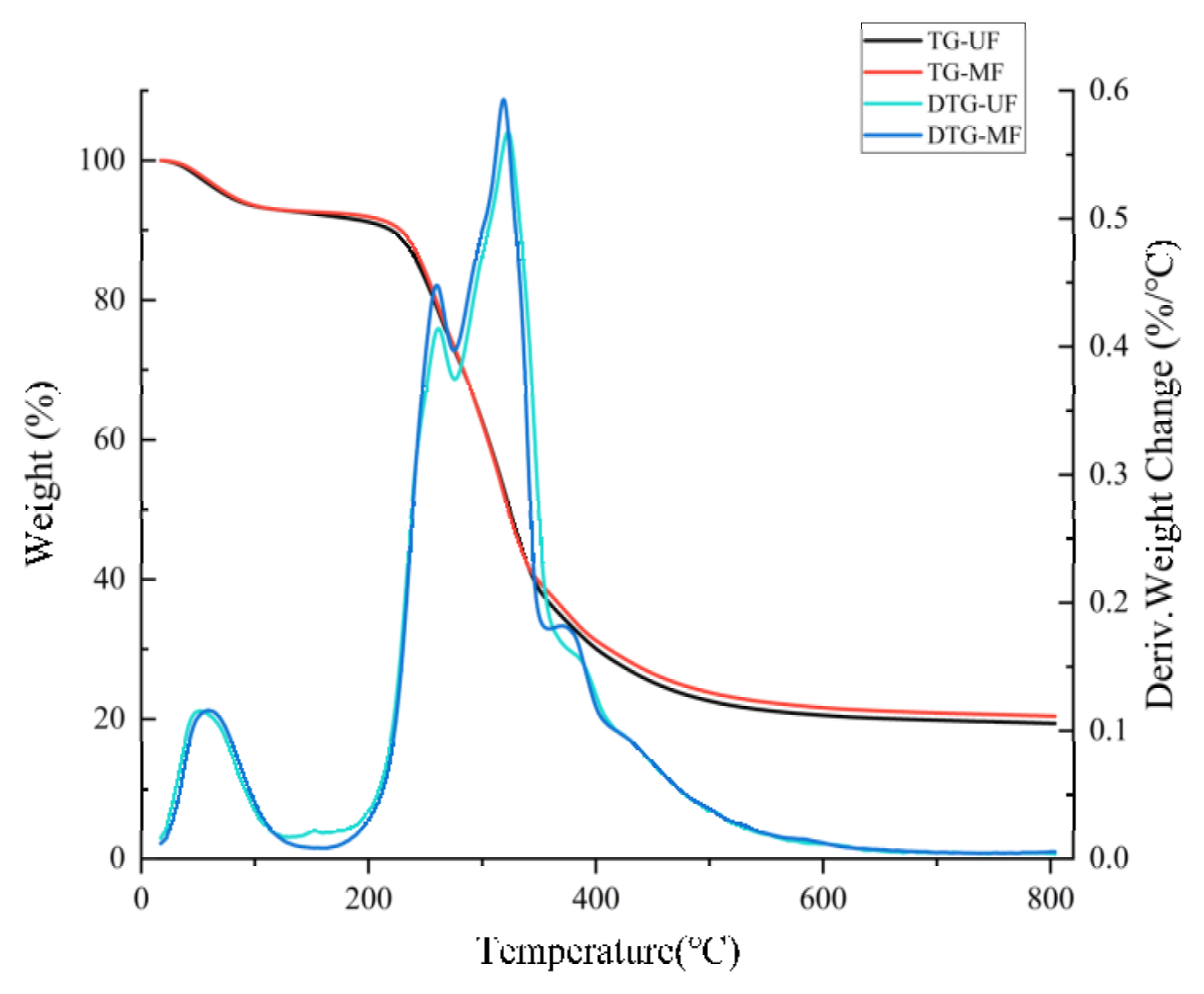
2.2.5. Effect of Mixed Fermentation Modification on the Thermal Stability of Soybean Residue
2.3. Adsorption Capacity Analysis
2.3.1. Oil Holding Capacity Determination
2.3.2. Water Holding Capacity Determination
2.3.3. Water Swelling Capacity Determination
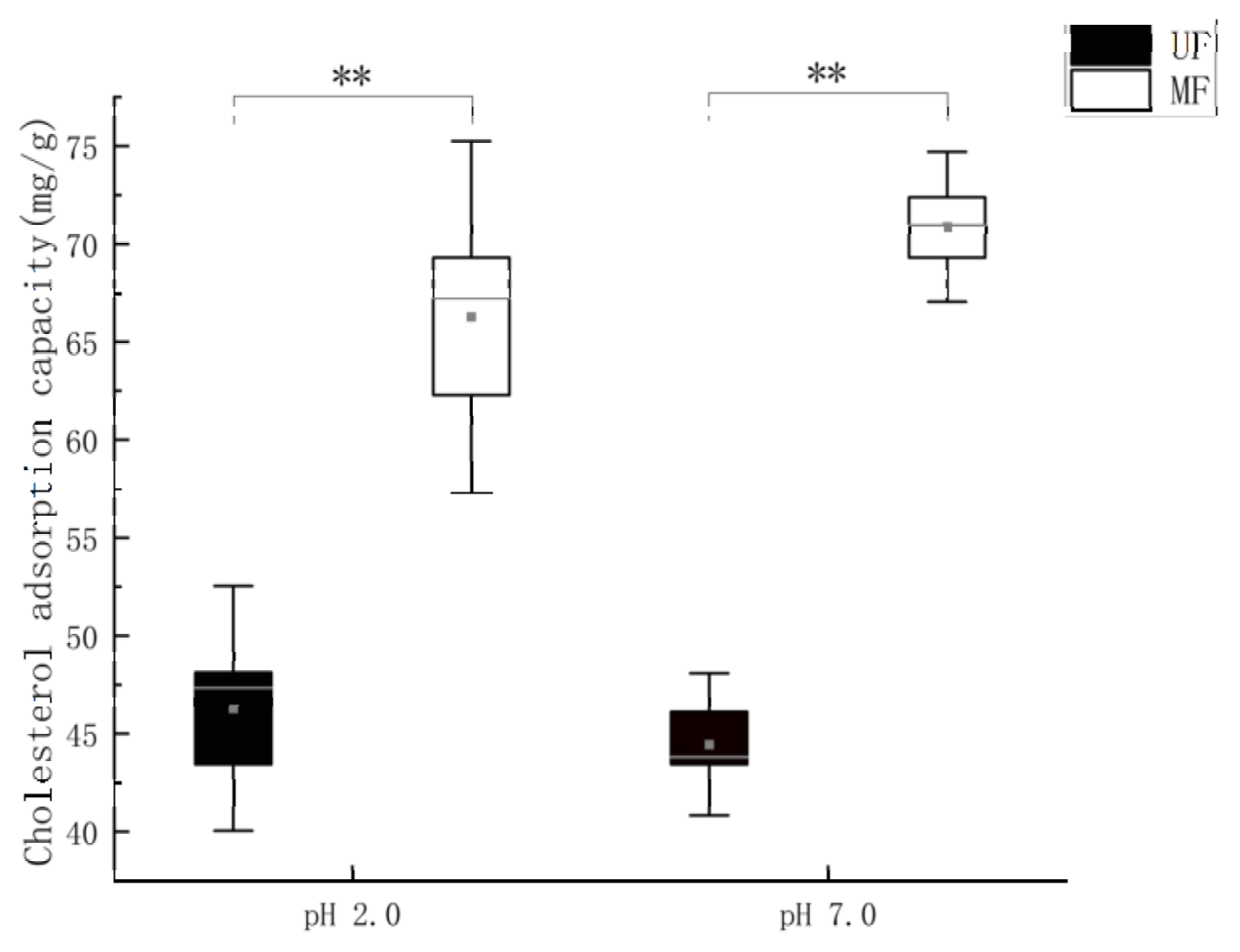
2.3.4. Cholesterol Adsorption Capacity Determination
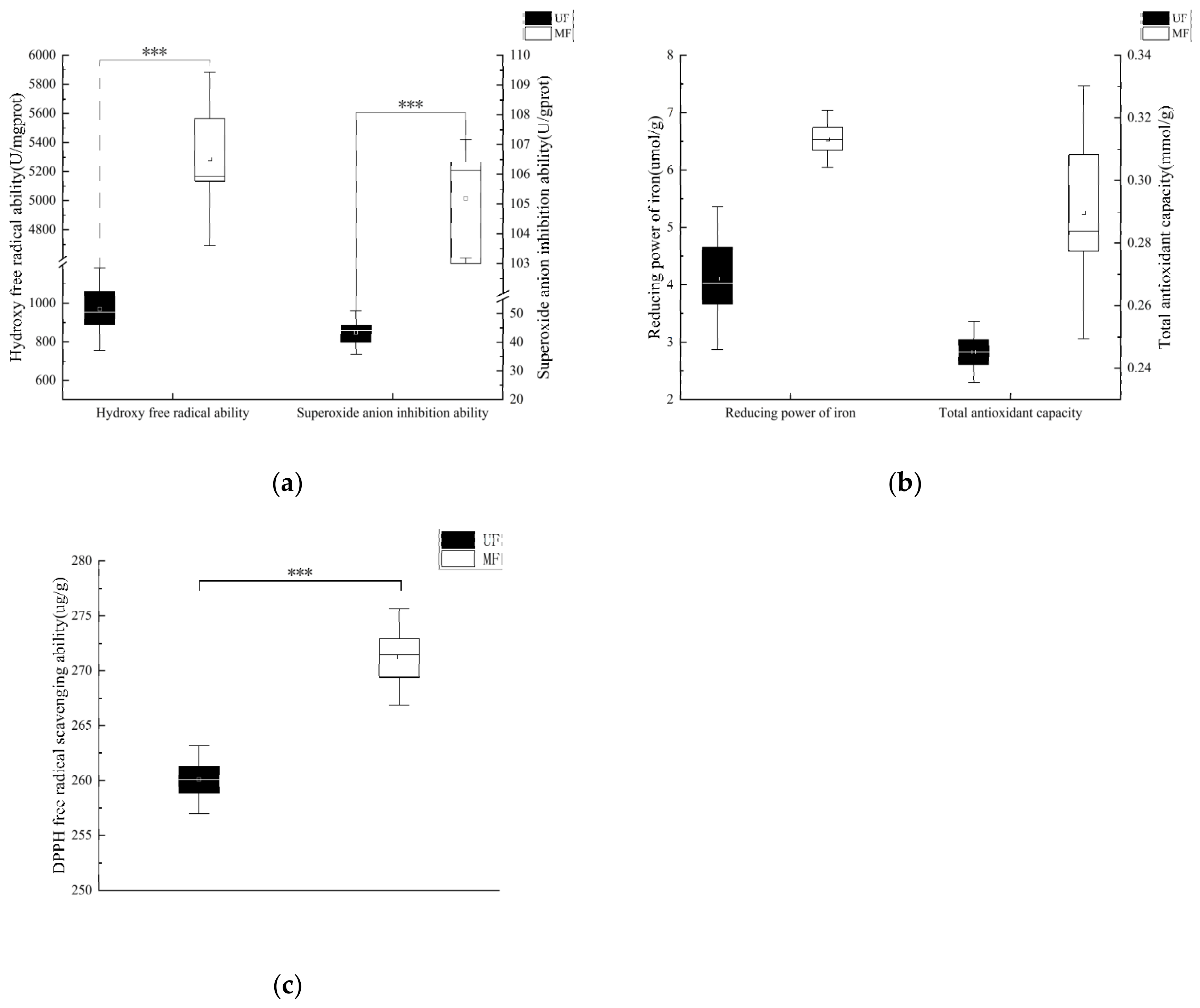
2.4. Analysis of Antioxidant Capacity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of the Culture Medium
4.3. Preparation of Fermented Soybean Residue
4.3.1. Strain Culture Method
4.3.2. Response Surface Methodology (RSM)
4.3.3. Mixed Fermentation
4.4. Structural Analysis
4.4.1. Scanning Electron Microscope (SEM) Analysis
4.4.2. Fourier Transform Infrared Spectroscopy Assay
4.4.3. Crystallinity Determination
4.4.4. Determination of Basic Physicochemical Composition of DF from Soybean Residue
4.4.5. Thermal Performance
4.5. Functional Features
4.5.1. Oil Holding Capacity
4.5.2. Water Holding Capacity
4.5.3. Water Swelling Capacity
4.5.4. Cholesterol Adsorption Capacity
4.6. Determination of Antioxidant Capacity
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mateos-Aparicio, I.; Mateos-Peinado, C.; Jiménez-Escrig, A.; Rupérez, P. Multifunctional antioxidant activity of polysaccharide fractions from the soybean byproduct okara. Carbohydr. Polym. 2010, 82, 245–250. [Google Scholar] [CrossRef] [Green Version]
- Jankowiak, L.; Kantzas, N.; Boom, R.; Goot, A.J. Isoflavone extraction from okara using water as extractant. Food Chem. 2014, 160, 371–378. [Google Scholar] [CrossRef]
- Swann, O.G.; Kilpatrick, M.; Breslin, M.; Oddy, W.H. Dietary fiber and its associations with depression and inflammation. Nutr. Rev. 2020, 78, 394–411. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wang, L.; Lü, J.; Zhu, Y.; Shen, R. Structural, antioxidant and adsorption properties of dietary fiber from foxtail millet (Setaria italica) bran. J. Sci. Food Agric. 2019, 99, 3886–3894. [Google Scholar] [CrossRef] [PubMed]
- Surampudi, P.; Enkhmaa, B.; Anuurad, E.; Berglund, L. Lipid Lowering with Soluble Dietary Fiber. Curr. Atheroscler. Rep. 2016, 18, 75. [Google Scholar] [CrossRef]
- Bao, Y.; Xue, H.; Yue, Y.; Wang, X.; Yu, H.; Piao, C. Preparation and Characterization of Pickering Emulsions with Modified Okara Insoluble Dietary Fiber. Foods 2021, 10, 2982. [Google Scholar] [CrossRef]
- Furukawa, S.; Watanabe, T.; Toyama, H.; Morinaga, Y. Significance of microbial symbiotic coexistence in traditional fermentation. J. Biosci. Bioeng. 2013, 116, 533–539. [Google Scholar] [CrossRef]
- Zhao, L.; Wu, J.; Liu, Y.; Wang, H.; Cao, C. Effect of Lactobacillus rhamnosus GG fermentation on the structural and functional properties of dietary fiber in bamboo shoot and its application in bread. J. Food Biochem. 2022, 46, e14231. [Google Scholar] [CrossRef]
- Nikinmaa, M.; Kajala, I.; Liu, X.; Nordlund, E.; Sozer, N. The role of rye bran acidification and in situ dextran formation on structure and texture of high fibre extrudates. Food Res. Int. 2020, 137, 109438. [Google Scholar] [CrossRef]
- Mohamed, S.H.; Magdy, A.S.; Eman, Y.M.; Mohamed, A.E.; Ehab, R.E.; Simon, J.D. Growth and physiological responses of Nile tilapia, Oreochromis niloticus fed dietary fermented sunflower meal inoculated with Saccharomyces cerevisiae and Bacillus subtilis. Aquaculture 2018, 495, 592–601. [Google Scholar] [CrossRef]
- Ma, S.; Wang, Z.; Tian, X.; Sun, B.; Huang, J.; Yan, J.; Bao, Q.; Wang, X. Effect of synergistic fermentation of Lactobacillus plantarum and Saccharomyces cerevisiae on thermal properties of wheat bran dietary fiber-wheat starch system. Food Chem. 2022, 373, 131417. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, S.; Li, L.; Huang, J. Synergistic fermentation of Lactobacillus plantarum and Saccharomyces cerevisiae to improve the quality of wheat bran dietary fiber-steamed bread. Food Chem. X 2022, 16, 100528. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, S.; Li, L.; Huang, J. Effect of wheat bran dietary fiber on structural properties and hydrolysis behavior of gluten after synergistic fermentation of Lactobacillus plantarum and Saccharomyces cerevisiae. Front. Nutr. 2022, 9, 982878. [Google Scholar] [CrossRef]
- Si, J.; Yang, C.; Ma, W.; Chen, Y.; Xie, J.; Qin, X.; Hu, X.; Yu, Q. Screen of high efficiency cellulose degrading strains and effects on tea residues dietary fiber modification: Structural properties and adsorption capacities. Int. J. Biol. Macromol. 2022, 1, 220, 337–347. [Google Scholar] [CrossRef]
- Qi, J.; Li, Y.; Masamba, K.G.; Shoemaker, C.F.; Zhong, F.; Majeed, H.; Ma, J. The effect of chemical treatment on the in vitro hypoglycemic properties of rice bran insoluble dietary fiber. Food Hydrocoll. 2016, 52, 699–706. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Li, Y.; Yu, H.; Wang, Y.; Piao, C. Insoluble dietary fibre from okara (soybean residue) modified by yeast kluyveromyces marxianus. LWT Food Sci. Technol. 2020, 134, 110252. [Google Scholar] [CrossRef]
- Huang, H.; Chen, J.; Hu, X.; Chen, Y.; Xie, J.; Ao, T.; Wang, H.; Xie, J.; Yu, Q. Elucidation of the interaction effect between dietary fiber and bound polyphenol components on the anti-hyperglycemic activity of tea residue dietary fiber. Food Funct. 2022, 13, 2710–2728. [Google Scholar] [CrossRef]
- Ren, F.; Feng, Y.; Zhang, H.; Wang, J. Effects of modification methods on microstructural and physicochemical characteristics of defatted rice bran dietary fiber. LWT Food Sci. Technol. 2021, 151, 112161. [Google Scholar] [CrossRef]
- Blaiotta, G.; Capua, M.D.; Coppola, R.; Aponte, M. Production of fermented chestnut purees by lactic acid bacteria. Int. J. Food Microbiol. 2012, 158, 195–202. [Google Scholar] [CrossRef]
- Chu, J.; Zhao, H.; Lu, Z.; Lu, F.; Bie, X.; Zhang, C. Improved physicochemical and functional properties of dietary fiber from millet bran fermented by Bacillus natto. Food Chem. 2019, 294, 79–86. [Google Scholar] [CrossRef]
- Ma, M.; Mu, T. Modification of deoiled cumin dietary fiber with laccase and cellulase under high hydrostatic pressure. Carbohydr. Polym. 2016, 136, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Ai, N.; Jiang, Y.; Omar, S.; Wang, J.; Xia, L.; Ren, J. Rapid Measurement of Cellulose, Hemicellulose, and Lignin Content in Sargassum horneri by Near-Infrared Spectroscopy and Characteristic Variables Selection Methods. Molecules 2022, 27, 335. [Google Scholar] [CrossRef] [PubMed]
- Chylińska, M.; Szymańska-Chargot, M.; Zdunek, A. FT-IR and FT-Raman characterization of non-cellulosic polysaccharides fractions isolated from plant cell wall. Carbohydr. Polym. 2016, 154, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Yin, T.; Xiong, S.; Zhang, J.; Din, Z.U.; Zhang, M. Structural characteristics and physicochemical properties of okara (soybean residue) insoluble dietary fiber modified by high-energy wet media milling. LWT Food Sci. Technol. 2017, 82, 15–22. [Google Scholar] [CrossRef]
- Chylińska, M.; Szymańska-Chargot, M.; Kruk, B.; Zdunek, A. Study on dietary fibre by Fourier transform-infrared spectroscopy and chemometric methods. Food Chem. 2016, 196, 114–122. [Google Scholar] [CrossRef]
- Niu, Y.; Li, N.; Xia, Q.; Hou, Y.; Xu, G. Comparisons of three modifications on structural, rheological and functional properties of soluble dietary fibers from tomato peels. LWT 2018, 88, 56–63. [Google Scholar] [CrossRef]
- Li, Y.; Niu, L.; Guo, Q.; Shi, L.; Deng, X.; Liu, X.; Xiao, C. Effects of fermentation with lactic bacteria on the structural characteristics and physicochemical and functional properties of soluble dietary fiber from prosomillet bran. LWT Food Sci. Technol. 2022, 154, 112609. [Google Scholar] [CrossRef]
- Sang, J.; Li, L.; Wen, J.; Liu, H.; Wu, J.; Yu, Y.; Xu, Y.; Gu, Q.; Fu, M.; Lin, X. Chemical composition, structural and functional properties of insoluble dietary fiber obtained from the Shatian pomelo peel sponge layer using different modification methods. LWT 2022, 165, 113737. [Google Scholar] [CrossRef]
- Yang, C.; Si, J.; Chen, Y.; Xie, J.; Tian, S.; Cheng, Y.; Hu, X.; Yu, Q. Physicochemical structure and functional properties of soluble dietary fibers obtained by different modification methods from Mesona chinensis Benth. residue. Food Res. Int. 2022, 157, 111489. [Google Scholar] [CrossRef]
- Zhang, M.; Liao, A.; Thakur, K.; Huang, J.; Zhang, J.; Wei, Z. Modification of wheat bran insoluble dietary fiber with carboxymethylation, complex enzymatic hydrolysis and ultrafine comminution. Food Chem. 2019, 297, 124983. [Google Scholar] [CrossRef]
- Lim, E.; Lim, J.Y.; Shin, J.H.; Seok, P.R.; Jung, S.; Yoo, S.H.; Kim, Y. d-Xylose suppresses adipogenesis and regulates lipid metabolism genes in high-fat diet–induced obese mice. Nutr. Res. 2015, 35, 626–636. [Google Scholar] [CrossRef]
- Bonnin, E.; Garnier, C.; Ralet, M. Pectin-modifying enzymes and pectin-derived materials: Applications and impacts. Appl. Microbiol. Biotechnol. 2014, 98, 519–532. [Google Scholar] [CrossRef]
- Navarro-González, I.; García-Valverde, V.; García-Alonso, J.; Periago, M. Chemical profile, functional and antioxidant properties of tomato peel fiber. Food Res. Int. 2011, 44, 1528–1535. [Google Scholar] [CrossRef]
- Deepak, M.; Kartikey, C.; Arushdeep, S.; Shivhare, U.S.; Sudesh, K.Y. Processing treatment of atmospheric- and vacuum-cold plasma improved physical properties, glucose diffusion and fermentability of dietary fibers extracted from de-oiled rice and corn bran. Bioact. Carbohydr. Diet. Fibre 2022, 28, 100326. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, S.; Li, Y.; Tian, J.; Zhang, C. Structure, physicochemical properties and effects on nutrients digestion of modified soluble dietary fiber extracted from sweet potato residue. Food Res. Int. 2021, 150, 110761. [Google Scholar] [CrossRef]
- Lyu, B.; Wang, H.; Swallah, M.S.; Fu, H.; Shen, Y.; Guo, Z.; Tong, X.; Li, Y.; Jiang, L. Structure, properties and potential bioactivities of high-purity insoluble fibre from soybean dregs (Okara). Food Chem. 2021, 364, 130402. [Google Scholar] [CrossRef]
- Yao, S.; Xu, Y.; Zhang, Y.; Lu, Y. Black rice and anthocyanins induce inhibition of cholesterol absorption in vitro. Food Funct. 2013, 4, 1602–1608. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Yi, C.; Quan, K.; Lin, B. Chemical composition, structure, physicochemical and functional properties of rice bran dietary fiber modified by cellulase treatment. Food Chem. 2021, 342, 128352. [Google Scholar] [CrossRef]
- Li, S.; Hu, N.; Zhu, J.; Zheng, M.; Liu, H.; Liu, J. Influence of modification methods on physicochemical and structural properties of soluble dietary fiber from corn bran. Food Chem. X 2022, 14, 100298. [Google Scholar] [CrossRef]
- Pei, F.; Sun, L.; Fang, Y.; Yang, W.; Ma, G.; Ma, N.; Hu, Q. Behavioral changes in glutenin macropolymer fermented by lactobacillus plantarum lb-1 to promote the rheological and gas production properties of dough. J. Agric. Food Chem. 2020, 68, 3585–3593. [Google Scholar] [CrossRef]
- Lin, D.; Long, X.; Huang, Y.; Yang, Y.; Wu, Z.; Chen, H.; Zhang, Q.; Wu, D.; Qin, W.; Tu, Z. Effects of microbial fermentation and microwave treatment on the composition, structural characteristics, and functional properties of modified okara dietary fiber. LWT 2020, 123, 109059. [Google Scholar] [CrossRef]
- Ma, Q.; Ma, Z.; Wang, W.; Mu, J.; Liu, Y.; Wang, J.; Stipkovits, L.; Hui, X.; Wu, G.; Sun, J. The effects of enzymatic modification on the functional ingredient-Dietary fiber extracted from potato residue. LWT 2022, 153, 112511. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, W.; Wu, B.; Wu, P.; Duan, Y.; Yang, Q.; Ma, H. Modification of garlic skin dietary fiber with twin-screw extrusion process and in vivo evaluation of Pb binding. Food Chem. 2018, 268, 550–557. [Google Scholar] [CrossRef] [PubMed]
- GB/T 5009.88-2014; Method for Determination of Dietary Fiber in Foods. National Health and Family Planning Commission of PRC: Beijing, China, 2014.
- Yang, T.; Chiu, C.; Lu, T.; Liu, S.; Chiang, M. The Anti-Obesity Effect of Polysaccharide-Rich Red Algae (Gelidium amansii) Hot-Water Extracts in High-Fat Diet-Induced Obese Hamsters. Mar. Drugs 2019, 17, 532. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.J.; Shin, N.H.; Jeong, J.S.; Kim, E.T.; Lee, S.K.; Lee, I.D.; Lee, S.S. Effects of Gelidium amansii extracts on in vitro ruminal fermentation characteristics, methanogenesis, and microbial populations. Asian-Australas J. Anim. Sci. 2018, 31, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Jabastin, J.; Palanisamy, B.D.; Digambar, K.; Venkatesan, B.P.; Prathapkumar, H.S. Prebiotic potential of water extractable polysaccharide from red kidney bean (Phaseolus vulgaris L.). LWT 2019, 101, 703–710. [Google Scholar] [CrossRef]
- Sun, C.; Wu, X.; Chen, X.; Li, X.; Zheng, Z.; Jiang, S. Production and characterization of okara dietary fiber produced by fermentation with Monascus anka. Food Chem. 2020, 316, 126243. [Google Scholar] [CrossRef]
- Tang, C.; Wang, Y.; Long, Y.; An, X.; Shen, J.; Ni, Y. Anchoring 20 (R)-ginsenoside Rg3 onto cellulose nanocrystals to increase the hydroxyl radical scavenging activity. ACS Sustain. Chem. Eng. 2017, 5, 7507–7513. [Google Scholar] [CrossRef]
- Qiao, C.C.; Zeng, F.K.; Wu, N.N.; Tan, B. Functional, physicochemical and structural properties of soluble dietary fiber from rice bran with extrusion cooking treatment. Food Hydrocoll. 2021, 121, 107057. [Google Scholar] [CrossRef]

| Levels | Water Content (%) | Inoculation Proportion (L:S) | Inoculation Amount (%) | Time (h) |
|---|---|---|---|---|
| −1 | 70 | 1:2 | 60 | 48 |
| 0 | 80 | 1:1 | 80 | 72 |
| 1 | 90 | 2:1 | 100 | 96 |
| Runs | Water Content (%) | Inoculation Proportion (L:S) | Inoculation Amount (%) | Time(h) | Soluble Dietary Fiber Content (g/100 g) |
|---|---|---|---|---|---|
| 1 | 90 | 1:1 | 60 | 72 | 2.8695 |
| 2 | 80 | 1:1 | 80 | 72 | 3.6770 |
| 3 | 80 | 1:1 | 80 | 72 | 3.6305 |
| 4 | 80 | 1:1 | 80 | 72 | 3.5278 |
| 5 | 70 | 2:1 | 80 | 72 | 3.0139 |
| 6 | 80 | 1:1 | 60 | 96 | 2.5454 |
| 7 | 80 | 2:1 | 100 | 72 | 3.0155 |
| 8 | 80 | 2:1 | 80 | 48 | 2.4095 |
| 9 | 80 | 1:1 | 100 | 96 | 2.8960 |
| 10 | 70 | 1:1 | 60 | 72 | 3.2147 |
| 11 | 80 | 1:1 | 60 | 48 | 2.8762 |
| 12 | 90 | 1:1 | 80 | 96 | 2.4063 |
| 13 | 70 | 1:1 | 80 | 96 | 2.7680 |
| 14 | 70 | 1:1 | 80 | 48 | 2.7270 |
| 15 | 80 | 1:1 | 100 | 48 | 2.5941 |
| 16 | 80 | 1:2 | 100 | 72 | 3.1628 |
| 17 | 70 | 1:2 | 80 | 72 | 3.1569 |
| 18 | 80 | 1:1 | 80 | 72 | 3.4516 |
| 19 | 80 | 1:2 | 80 | 48 | 2.6605 |
| 20 | 90 | 1:1 | 100 | 72 | 2.8524 |
| 21 | 90 | 1:1 | 80 | 48 | 2.3874 |
| 22 | 90 | 1:2 | 80 | 72 | 2.7265 |
| 23 | 70 | 1:1 | 100 | 72 | 3.2867 |
| 24 | 80 | 2:1 | 80 | 96 | 2.6494 |
| 25 | 80 | 2:1 | 60 | 72 | 3.0354 |
| 26 | 90 | 2:1 | 80 | 72 | 2.7045 |
| 27 | 80 | 1:1 | 80 | 72 | 3.6325 |
| 28 | 80 | 1:2 | 60 | 72 | 3.0258 |
| 29 | 80 | 1:2 | 80 | 96 | 2.5094 |
| Source | Sum of Squares | Df | Mean Square | F value | p Value | Significance a |
|---|---|---|---|---|---|---|
| Model | 4.08 | 14 | 0.29 | 107.87 | <0.0001 | *** |
| A -Water content | 0.41 | 1 | 0.41 | 152.18 | <0.0001 | *** |
| B -Inoculation proportion | 0.014 | 1 | 0.014 | 5.28 | 0.0375 | * |
| C -Inoculation amount | 0.000482 | 1 | 0.00482 | 1.79 | 0.2028 | |
| D -Time | 0.001196 | 1 | 0.001196 | 0.44 | 0.5165 | |
| AB | 0.00366 | 1 | 0.00366 | 1.36 | 0.2638 | |
| AC | 0.001985 | 1 | 0.001985 | 0.74 | 0.4057 | |
| AD | 0.0001221 | 1 | 0.0001221 | 0.045 | 0.8347 | |
| BC | 0.006154 | 1 | 0.006154 | 2.28 | 0.10534 | |
| BD | 0.038 | 1 | 0.038 | 14.15 | 0.0021 | ** |
| CD | 0.1 | 1 | 0.1 | 37.06 | <0.0001 | *** |
| A2 | 0.75 | 1 | 0.75 | 277.62 | <0.0001 | *** |
| B2 | 0.77 | 1 | 0.77 | 286.61 | <0.0001 | *** |
| C2 | 0.22 | 1 | 0.22 | 79.89 | <0.0001 | *** |
| D2 | 2.96 | 1 | 2.96 | 1096.21 | <0.0001 | *** |
| Residual | 0.038 | 14 | 0.0027 | |||
| Lack of fit | 0.003952 | 10 | 0.003952 | 0.047 | 0.9999 | Not significant |
| Pure error | 0.034 | 4 | 0.008463 | |||
| Cor total | 4.12 | 28 | ||||
| R-squared | 0.9908 | |||||
| Adj. R-squared | 0.9816 | |||||
| Adeq. precision | 32.243 | |||||
| C.V.% | 1.76 |
| Sample | SDF (g/100 g) | IDF (g/100 g) | TDF (g/100 g) | OHC (g/g) | WHC (g/g) | WSC (g/g) |
|---|---|---|---|---|---|---|
| UF | 1.37 ± 0.28 | 88.15 ± 4.15 | 89.52 ± 5.14 | 2.466 ± 0.34 | 11.336 ± 0.63 | 4.0 ± 0.5 |
| MF | 3.62 ± 0.03 *** | 84.96 ± 5.24 | 88.67 ± 4.19 | 10.699 ± 0.53 *** | 16.856 ± 0.97 *** | 7.0 ± 0.25 *** |
| Sample | Crude Fat (%) | Protein (g/100 g) | Water (%) | Ash content (%) |
|---|---|---|---|---|
| UF | 6.7 ± 1.2 | 16.2 ± 2.5 | 7.8 ± 1.6 | 4.08 ± 0.71 |
| MF | 6.9 ± 0.9 | 16.9 ± 2.1 | 4.9 ± 0.3 * | 3.31 ± 1.08 |
| Name | UF (g/kg) | MF (g/kg) |
|---|---|---|
| Mannose | 17.011 ± 4.329 | 10.397 ± 0.981 |
| Rhamnose | 25.355 ± 7.457 | 22.953 ± 5.064 |
| Glucuronic acid | 3.143 ± 1.047 | 2.844 ± 0.976 |
| Galacturonic acid | 33.178 ± 3.115 | 30.483 ± 4.853 |
| Glucose | 18.959 ± 5.752 | 17.589 ± 2.611 |
| Galactose | 168.372 ± 31.623 | 157.096 ± 27.352 |
| Xylose | 44.563 ± 8.391 | 45.911 ± 9.407 |
| Arabinose | 93.193 ± 14.067 | 81.567 ± 9.225 |
| Fucose | 16.663 ± 5.584 | 14.538 ± 3.428 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Zhang, X.; Sun, M.; Li, D.; Hua, M.; Miao, X.; Su, Y.; Chi, Y.; Wang, J.; Niu, H. Optimization of Mixed Fermentation Conditions of Dietary Fiber from Soybean Residue and the Effect on Structure, Properties and Potential Biological Activity of Dietary Fiber from Soybean Residue. Molecules 2023, 28, 1322. https://doi.org/10.3390/molecules28031322
Xu X, Zhang X, Sun M, Li D, Hua M, Miao X, Su Y, Chi Y, Wang J, Niu H. Optimization of Mixed Fermentation Conditions of Dietary Fiber from Soybean Residue and the Effect on Structure, Properties and Potential Biological Activity of Dietary Fiber from Soybean Residue. Molecules. 2023; 28(3):1322. https://doi.org/10.3390/molecules28031322
Chicago/Turabian StyleXu, Xifei, Xuejing Zhang, Mubai Sun, Da Li, Mei Hua, Xinyu Miao, Ying Su, Yanping Chi, Jinghui Wang, and Honghong Niu. 2023. "Optimization of Mixed Fermentation Conditions of Dietary Fiber from Soybean Residue and the Effect on Structure, Properties and Potential Biological Activity of Dietary Fiber from Soybean Residue" Molecules 28, no. 3: 1322. https://doi.org/10.3390/molecules28031322
APA StyleXu, X., Zhang, X., Sun, M., Li, D., Hua, M., Miao, X., Su, Y., Chi, Y., Wang, J., & Niu, H. (2023). Optimization of Mixed Fermentation Conditions of Dietary Fiber from Soybean Residue and the Effect on Structure, Properties and Potential Biological Activity of Dietary Fiber from Soybean Residue. Molecules, 28(3), 1322. https://doi.org/10.3390/molecules28031322





