Synthesis of 6-(4-Chlorophenyl)-N-aryl-4-(trichloromethyl)-4H-1,3,5-oxadiazin-2-amines: A Comparative Evaluation of Dehydrosulfurization Methods of Starting 4-Chloro-N-(2,2,2 -trichloro-1-(3-arylthioureido)ethyl)benzamides †
Abstract
:1. Introduction
2. Materials and Methods
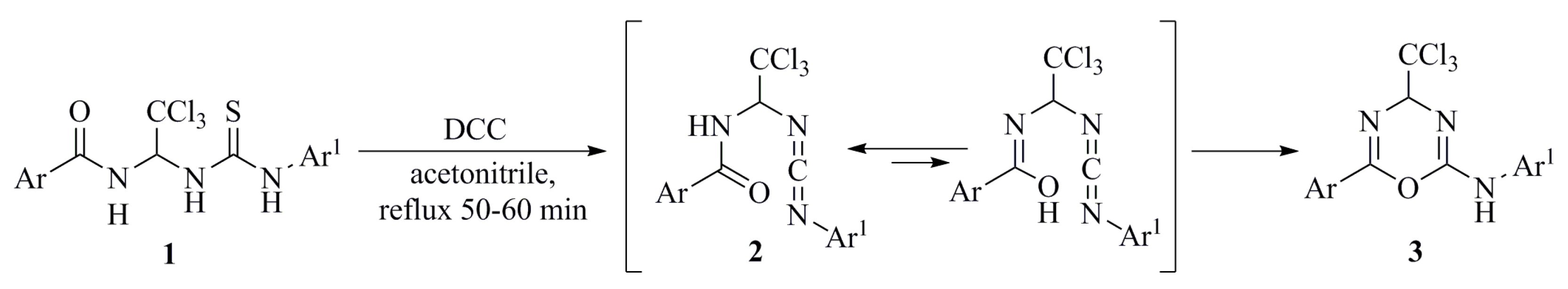
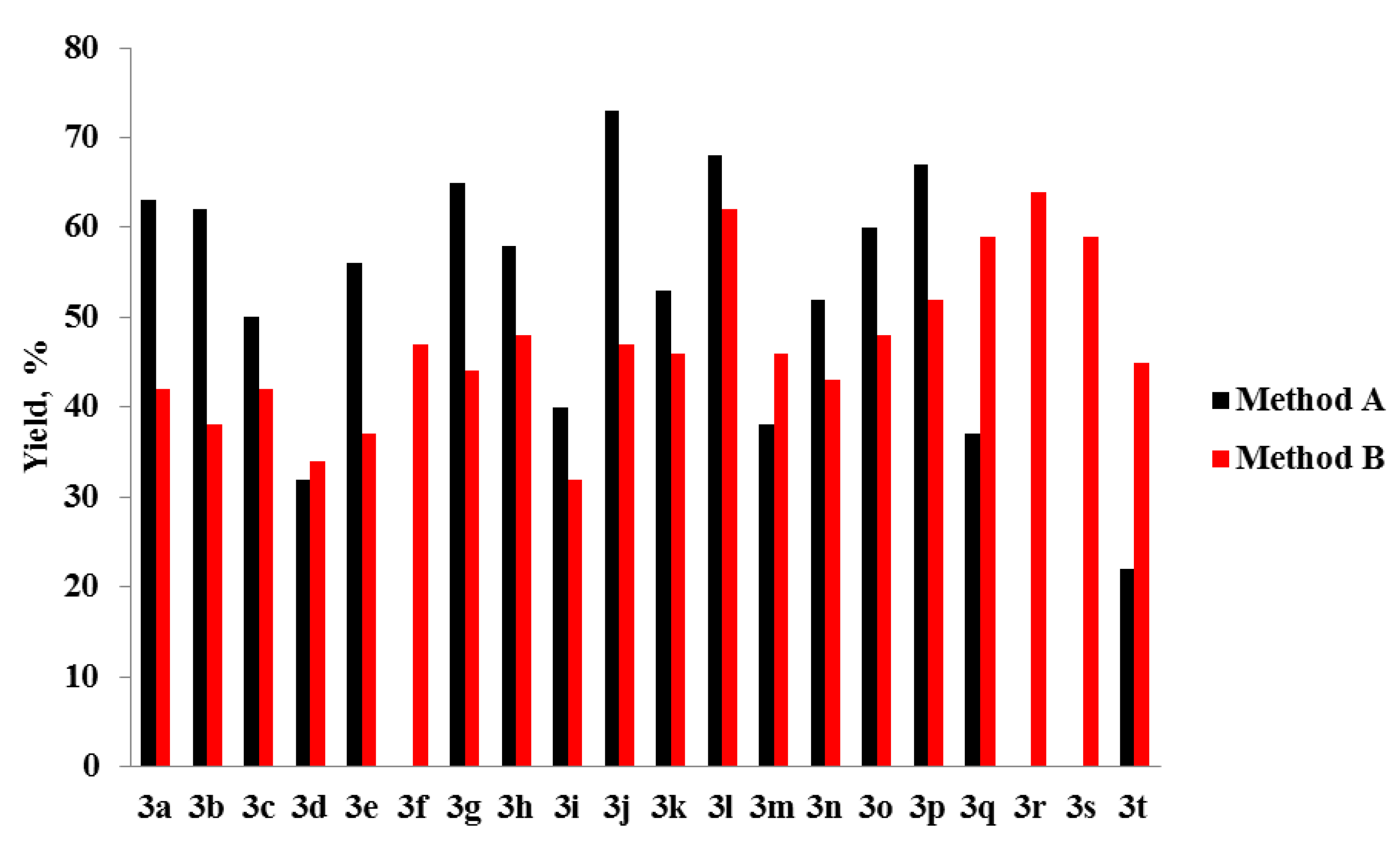
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zadorozhnii, P.V.; Kiselev, V.V.; Kharchenko, A.V. 1,3,5-Oxadiazines and 1,3,5-Thiadiazines. In Comprehensive Heterocyclic Chemistry, 4th ed.; Black, D.S.C., Cossy, J., Stevens, C.V., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 9, pp. 456–506. [Google Scholar] [CrossRef]
- Shobana, N.; Farid, P. 1,3,5-Oxadiazines and 1,3,5-Thiadiazines. In Comprehensive Heterocyclic Chemistry, 3rd ed.; Katritzky, A.R., Scriven, E.F.V., Ramsden, C.A., Taylor, R.J.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 9, pp. 457–521. [Google Scholar] [CrossRef]
- Ke, S.; Cao, X.; Liang, Y.; Wang, K.; Yang, Z. Synthesis and Biological Properties of Dihydro-Oxadiazine-Based Heterocyclic Derivatives. Mini Rev. Med. Chem. 2011, 11, 642–657. [Google Scholar] [CrossRef] [PubMed]
- Pasha, M.A.; Mondal, S.; Panigrahi, N. Review of synthetic strategies in the development of oxadiazine scaffolds. Mediterr. J. Chem. 2019, 8, 338–364. [Google Scholar] [CrossRef]
- El-Ziaty, A.K.; Shiba, S.A. Antibacterial Activities of New (E)-2-Cyano-3-(3′,4′-dimethoxyphenyl)-2-propenoylamide Derivatives. Synth. Commun. 2007, 37, 4043–4057. [Google Scholar] [CrossRef]
- Patel, H.S.; Patel, K.B. Synthesis and Biological Activity of 3-[4H-(1,2,4)-Triazolyl]-2,6-diaryl-1,3,5-oxadiazine-4-thione. Phosphorus Sulfur Silicon Relat. Elem. 2009, 184, 2443–2452. [Google Scholar] [CrossRef]
- Modi, V.P.; Jani, D.H.; Patel, H.S. Synthesis and antimicrobial evaluation of spiro compound containing 1,2,4-triazole and isatin. Orbital Elec. J. Chem. 2011, 3, 68–79. [Google Scholar] [CrossRef]
- Rambabu, N.; Ramachandran, D.; Viral, B.M.; Kirti, J.G. Synthesis, characterrization and biological evaluation of 2,6-diphenyl-3-(4-(3-phenyl-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazol-6-yl)phenyl)-2H-1,3,5-oxadiazine-4(3H)-thione. Der Pharma Chemica 2012, 4, 639–643. [Google Scholar]
- Patel, K.H.; Mehta, A.G. Synthesis and antifungal activity of [(4-(2-naphthalenyl)thiazol-2-yl)-2-(substitutedphenyl)-6-phenyl-4-thioxo-1,3,5-oxadiazine]derivatives. Der Chemica Sinica 2012, 3, 1410–1414. [Google Scholar]
- Zadorozhnii, P.V.; Pokotylo, I.O.; Kiselev, V.V.; Kharchenko, A.V.; Okhtina, O.V. In silico analysis of 6-(4-chlorophenyl)-N-aryl-4-(trichloromethyl)-4H-1,3,5-oxadiazin-2-amines as potential antagonists of VEGFR-1. Indo Amer. J. Pharm. Sci. 2019, 6, 4196–4200. [Google Scholar] [CrossRef]
- Maienfisch, P. Synthesis and Properties of Thiamethoxam and Related Compounds. Z. Naturforsch. B 2006, 61, 353–359. [Google Scholar] [CrossRef]
- Assy, M.G.; Haiekl, A.; Moustafa, H.Y. Behavior of terephthaloyl isothiocyanate towards carbon and nitrogen reagents. Phosphorus Sulphur Silicon Relat. Elem. 1995, 106, 179–185. [Google Scholar] [CrossRef]
- Shiba, S.A. Decomposition of 2-Propenoyl Azide Derivatives. Synthesis and Larvicidal Activity of Novel Products. Arch. Pharm. Pharm. Med. Chem. 1998, 331, 91–96. [Google Scholar] [CrossRef]
- Shiba, S.A. Synthesis and insecticidal activity of novel acrylonitrile derivatives. Phosphorus Sulphur Silicon Relat. Elem. 1996, 114, 29–37. [Google Scholar] [CrossRef]
- Gao, Y.; Arritt, S.W.; Twamley, B.; Shreeve, J.M. Guanidinium-Based Ionic Liquids. Inorg. Chem. 2005, 44, 1704–1712. [Google Scholar] [CrossRef] [PubMed]
- Decostanzi, M.; Auvergne, R.; Darroman, E.; Boutevin, B.; Caillol, S. Reactivity and kinetics of HDI-iminooxadiazinedione: Application to polyurethane synthesis. Eur. Polym. J. 2017, 96, 443–451. [Google Scholar] [CrossRef]
- Gao, H.; Shreeve, J.M. The Many Faces of FOX-7: A Precursor to High-Performance Energetic Materials. Angew. Chem. Int. Ed. 2015, 54, 6335–6338. [Google Scholar] [CrossRef]
- Bauer, D.; Andrae, B.; Gaß, P.; Trenz, D.; Becker, S.; Kubik, S. Functionalisable acyclic cucurbiturils. Org. Chem. Front. 2019, 6, 1555–1560. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, L.; Yang, X.; Jin, Y.; Gao, J.; Ma, P. A study on the coordination of cyclohexanocucurbit[6]uril with copper, zinc, and magnesium ions. Green Process. Synth. 2021, 10, 835–841. [Google Scholar] [CrossRef]
- Zhou, F.; Ma, D.; Liu, Y. Preparation and recognition property of an acyclic cucurbit[n]uril dimer. J. Incl. Phenom. Macrocycl. Chem. 2022, 102, 487–491. [Google Scholar] [CrossRef]
- Meng, Y.; Jin, Y.-M.; Ma, P.-H. Synthesis of symmetric dicyclohexanocucurbit[6]uril and its interaction with glycine. Tetrahedron 2021, 97, 132409. [Google Scholar] [CrossRef]
- Wu, J.B.; Cheng, Y.D.; Kuo, S.C.; Wu, T.S.; Iitaka, Y.; Ebizuka, Y.; Sankawa, U. Fissoldhimine, a novel skeleton alkaloid from fissistigma oldhamii. Chem. Pharm. Bull. 1994, 42, 2202–2204. [Google Scholar] [CrossRef]
- Bergmann, T.; Schories, D.; Steffan, B. Alboinon, an Oxadiazinone Alkaloid from the Ascidian Dendrodoa grossularia. Tetrahedron 1997, 53, 2055–2060. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, Y.; He, S.; Chen, H.; Wu, B.; Li, S.; Zhao, Z.; Li, Zh.; Wang, X.; Zuo, J.; et al. Heterocyclic Compounds from the Mushroom Albatrellus confluens and Their Inhibitions against Lipopolysaccharides-Induced B Lymphocyte Cell Proliferation. J. Org. Chem. 2018, 83, 10158–10165. [Google Scholar] [CrossRef] [PubMed]
- Behalo, M.S.; Gad El-karim, I.A.; Issac, Y.A.; Farag, M.A. Synthesis of novel pyridazine derivatives as potential antimicrobial agents. J. Sulfur Chem. 2014, 35, 661–673. [Google Scholar] [CrossRef]
- Ran, G.-Y.; Gong, M.; Yue, J.-F.; Yang, X.-X.; Zhou, S.-L.; Du, W.; Chen, Y.-Ch. Asymmetric Cascade Assembly of 1,2-Diaza-1,3-dienes and α,β-Unsaturated Aldehydes via Dienamine Activation. Org. Lett. 2017, 19, 1874–1877. [Google Scholar] [CrossRef]
- Zhang, Y.; Kuang, J.; Xiao, X.; Wang, L.; Ma, Y. DMSO as a Dual Carbon Synthon and Water as Oxygen Donor for the Construction of 1,3,5-Oxadiazines from Amidines. Org. Lett. 2021, 23, 3960–3964. [Google Scholar] [CrossRef]
- Widemann, M.; Driest, P.J.; Orecchia, P.; Naline, F.; Golling, F.E.; Hecking, A.; Eggert, C.; Pires, R.; Danielmeier, K.; Richter, F.U. Structure−Property Relations in Oligomers of Linear Aliphatic Diisocyanates. ACS Sustain. Chem. Eng. 2018, 6, 9753–9759. [Google Scholar] [CrossRef]
- Vijayan, A.; Jumaila, C.U.; Radhakrishnan, K.V. Rhodium(III)-Catalyzed C−H Activation of O-Acetyl Ketoximes/N-Methoxybenzamides toward the Synthesis of Isoquinoline/Isoquinolone-Fused Bicycles. Asian J. Org. Chem. 2017, 6, 1561–1565. [Google Scholar] [CrossRef]
- Ni, H.; Zhang, Y.; Zhang, F.; Zhao, J.; Wu, L.; Chu, X. Synthesis, structural characterization and theoretical approach of 3-(2,6-dichlorobenzyl)-5-methyl-N-nitro-1,3,5-oxadiazinan-4-imine. Spectrochim. Acta Part A 2015, 138, 648–659. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, Z.; Dong, J.; Liu, J.; Xu, X. Chemoselective Double Annulation of Two Different Isocyanides: Rapid Access to Trifluoromethylated Indole-Fused Heterocycles. Org. Lett. 2017, 19, 5292–5295. [Google Scholar] [CrossRef]
- Zadorozhnii, P.V.; Kiselev, V.V.; Pokotylo, I.O.; Kharchenko, A.V. A new method for the synthesis of 4H-1,3,5-oxadiazine derivatives. Heterocycl. Commun. 2017, 23, 369–374. [Google Scholar] [CrossRef]
- Zadorozhnii, P.V.; Kiselev, V.V.; Pokotylo, I.O.; Okhtina, O.V.; Kharchenko, A.V. Synthesis and mass spectrometric fragmentation pattern of 6-(4-chlorophenyl)-N-aryl-4-(trichloromethyl)-4H-1,3,5-oxadiazin-2-amines. Heterocycl. Commun. 2018, 24, 273–278. [Google Scholar] [CrossRef]
- Zadorozhnii, P.V.; Pokotylo, I.O.; Kiselev, V.V.; Kharchenko, A.V.; Okhtina, O.V. Synthesis and Spectral Characteristics of Some New 4H-1,3,5-Oxadiazine Derivatives. Res. J. Pharm. Biol. Chem. Sci. 2019, 10, 1508–1515. [Google Scholar]
- Zadorozhnii, P.V.; Kiselev, V.V.; Hrek, O.O.; Kharchenko, A.V.; Okhtina, O.V. Synthesis, spectral characteristics, and molecular structure of 2-(2,4-dichlorophenyl)-6-(2-methoxybenzyl)-4-(trichloromethyl)-4H-1,3,5-oxadiazine. Struct. Chem. 2022, 33, 2127–2132. [Google Scholar] [CrossRef]
- Boyce, M.; Bryant, K.F.; Jousse, C.; Long, K.; Harding, H.P.; Scheuner, D.; Kaufman, R.J.; Ma, D.; Coen, D.M.; Ron, D.; et al. A Selective Inhibitor of eIF2α Dephosphorylation Protects Cells from ER Stress. Science 2005, 307, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, K.L.; Li, X.; Li, R.J.; Liu, C.L.; Zhong, W.; Li, S. SAR, Cardiac Myocytes Protection Activity and 3D-QSAR Studies of Salubrinal and its Potent Derivatives. Curr. Med. Chem. 2012, 19, 6072–6079. [Google Scholar] [CrossRef]
- Long, K.; Boyce, M.; Lin, H.; Yuan, J.; Ma, D. Structure-activity relationship studies of salubrinal lead to its active biotinylated derivative. Bioorg. Med. Chem. Lett. 2005, 15, 3849–3852. [Google Scholar] [CrossRef]
- Zadorozhnii, P.V.; Pokotylo, I.O.; Kiselev, V.V.; Okhtina, O.V.; Kharchenko, A.V. Molecular docking studies of salubrinal and its analogs as inhibitors of the GADD34:PP1 enzyme. ADMET DMPK 2019, 7, 140–150. [Google Scholar] [CrossRef]
- Zadorozhnii, P.V.; Kiselev, V.V.; Kharchenko, A.V. In silico toxicity evaluation of Salubrinal and its analogues. Eur. J. Pharm. Sci. 2020, 155, 105538. [Google Scholar] [CrossRef]
- Zadorozhnii, P.V.; Kiselev, V.V.; Kharchenko, A.V. In Silico ADME Profiling of Salubrinal and Its Analogues. Future Pharmacol. 2022, 2, 160–197. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lominoga, E.R.; Zadorozhnii, P.V.; Kiselev, V.V.; Kharchenko, A.V. Synthesis of 6-(4-Chlorophenyl)-N-aryl-4-(trichloromethyl)-4H-1,3,5-oxadiazin-2-amines: A Comparative Evaluation of Dehydrosulfurization Methods of Starting 4-Chloro-N-(2,2,2 -trichloro-1-(3-arylthioureido)ethyl)benzamides. Chem. Proc. 2022, 12, 58. https://doi.org/10.3390/ecsoc-26-13538
Lominoga ER, Zadorozhnii PV, Kiselev VV, Kharchenko AV. Synthesis of 6-(4-Chlorophenyl)-N-aryl-4-(trichloromethyl)-4H-1,3,5-oxadiazin-2-amines: A Comparative Evaluation of Dehydrosulfurization Methods of Starting 4-Chloro-N-(2,2,2 -trichloro-1-(3-arylthioureido)ethyl)benzamides. Chemistry Proceedings. 2022; 12(1):58. https://doi.org/10.3390/ecsoc-26-13538
Chicago/Turabian StyleLominoga, Elizaveta R., Pavlo V. Zadorozhnii, Vadym V. Kiselev, and Aleksandr V. Kharchenko. 2022. "Synthesis of 6-(4-Chlorophenyl)-N-aryl-4-(trichloromethyl)-4H-1,3,5-oxadiazin-2-amines: A Comparative Evaluation of Dehydrosulfurization Methods of Starting 4-Chloro-N-(2,2,2 -trichloro-1-(3-arylthioureido)ethyl)benzamides" Chemistry Proceedings 12, no. 1: 58. https://doi.org/10.3390/ecsoc-26-13538
APA StyleLominoga, E. R., Zadorozhnii, P. V., Kiselev, V. V., & Kharchenko, A. V. (2022). Synthesis of 6-(4-Chlorophenyl)-N-aryl-4-(trichloromethyl)-4H-1,3,5-oxadiazin-2-amines: A Comparative Evaluation of Dehydrosulfurization Methods of Starting 4-Chloro-N-(2,2,2 -trichloro-1-(3-arylthioureido)ethyl)benzamides. Chemistry Proceedings, 12(1), 58. https://doi.org/10.3390/ecsoc-26-13538





