The Role of Magnetic Resonance Imaging (MRI) in the Diagnosis of Pancreatic Cystic Lesions (PCLs)
Abstract
:1. Introduction
2. Literature Search
3. Evolution of MRI on Assessment of PCLs
4. Prevalence of PCLs in MRI
5. MRI Features of PCLs
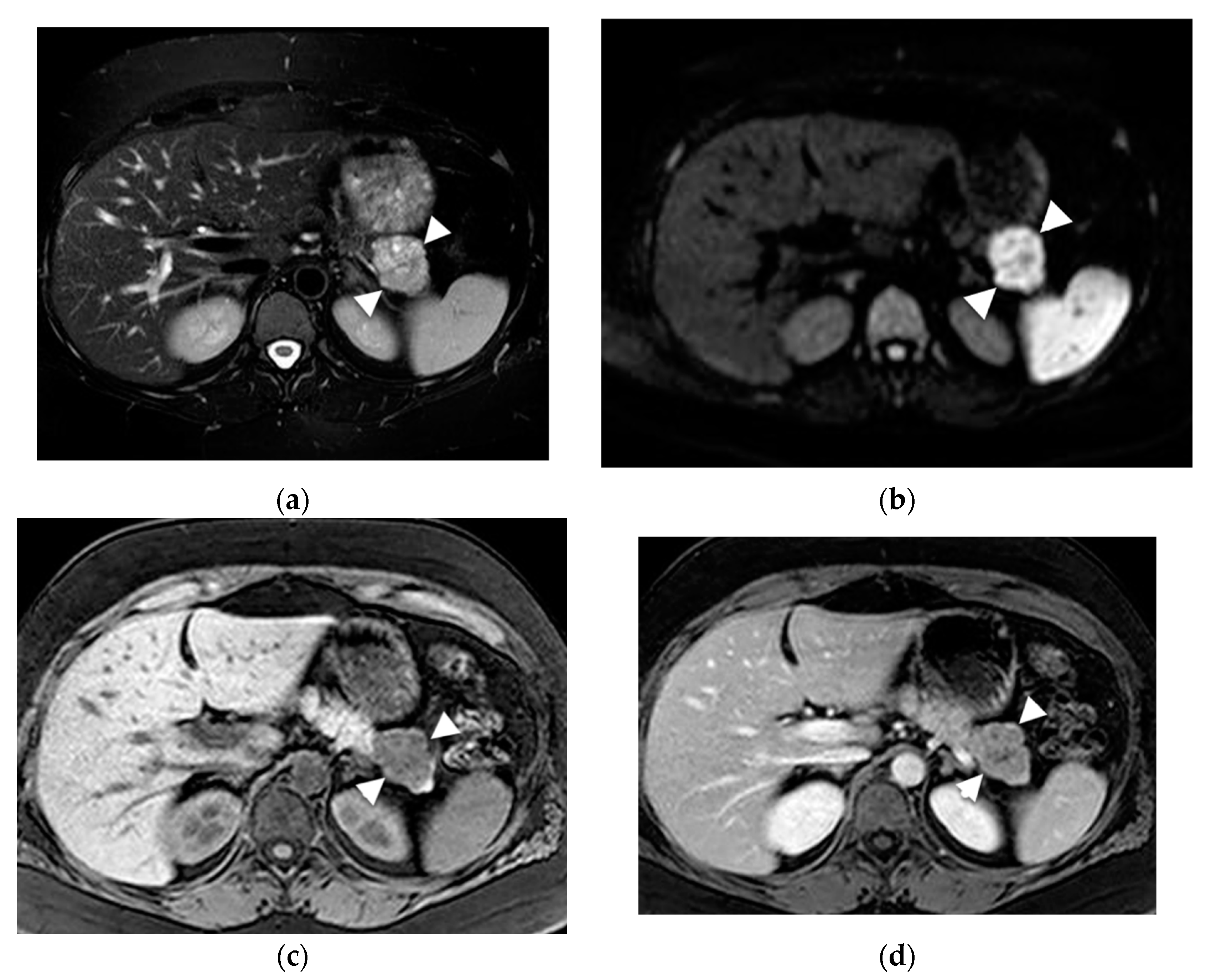
6. Role of MRI to Diagnose Specific Cyst Types
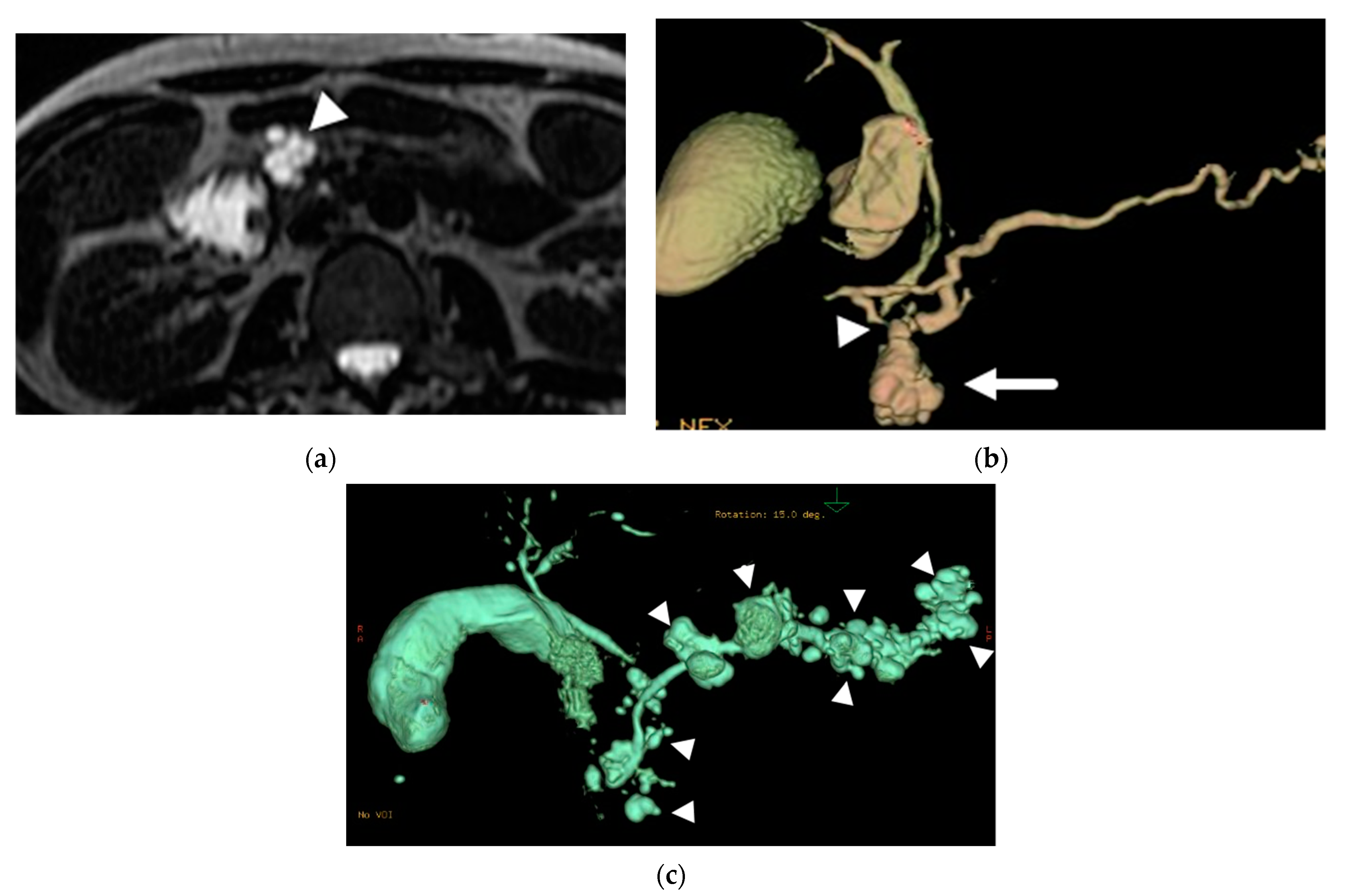
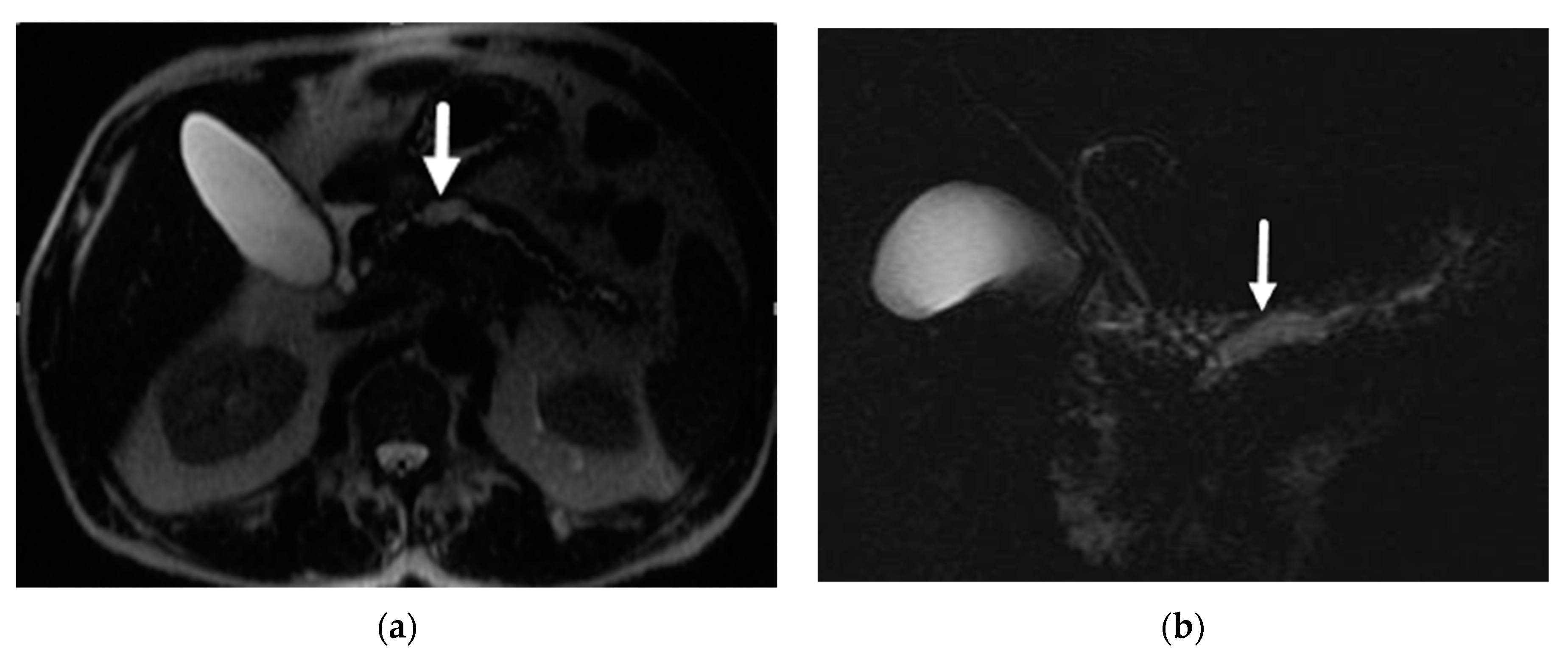
6.1. Intraductal Papillary Mucinous Neoplasm (IPMN)
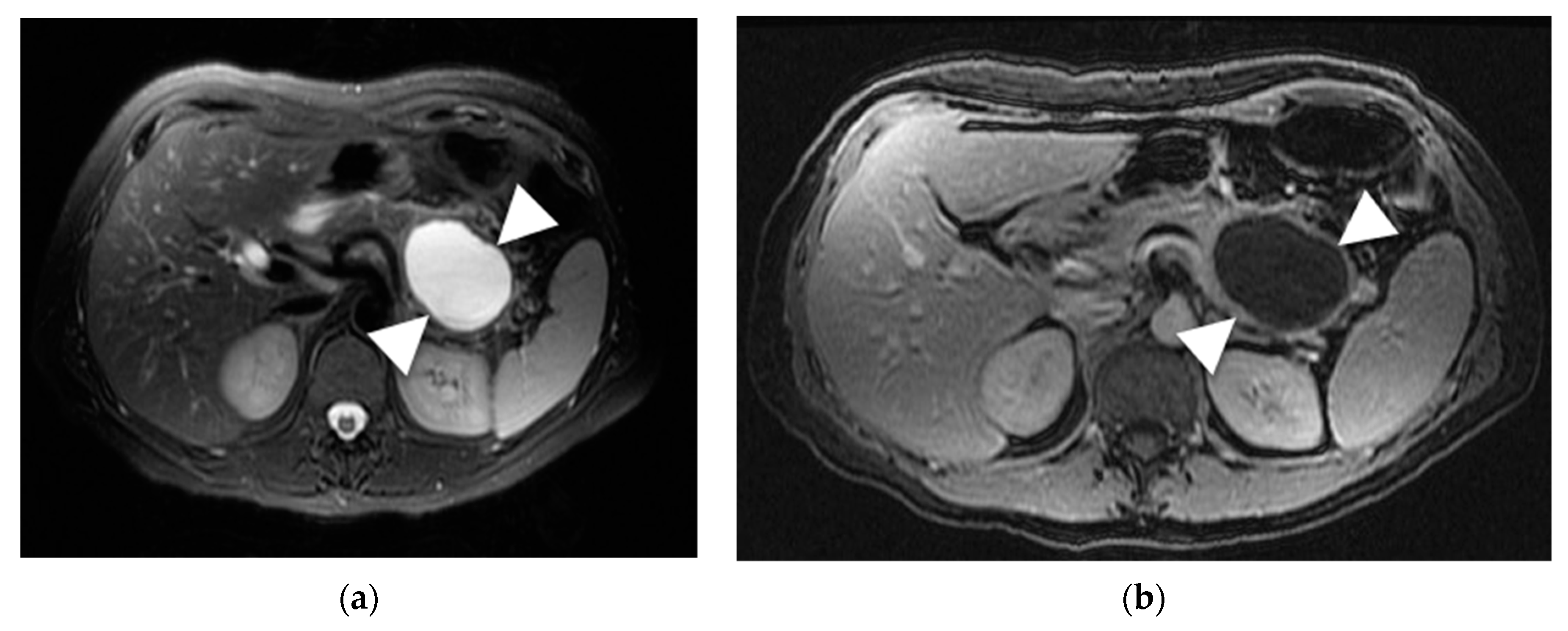
6.2. Mucinous Cystic Neoplasm (MCN)
6.3. Serous Cystic Adenoma (SCA)
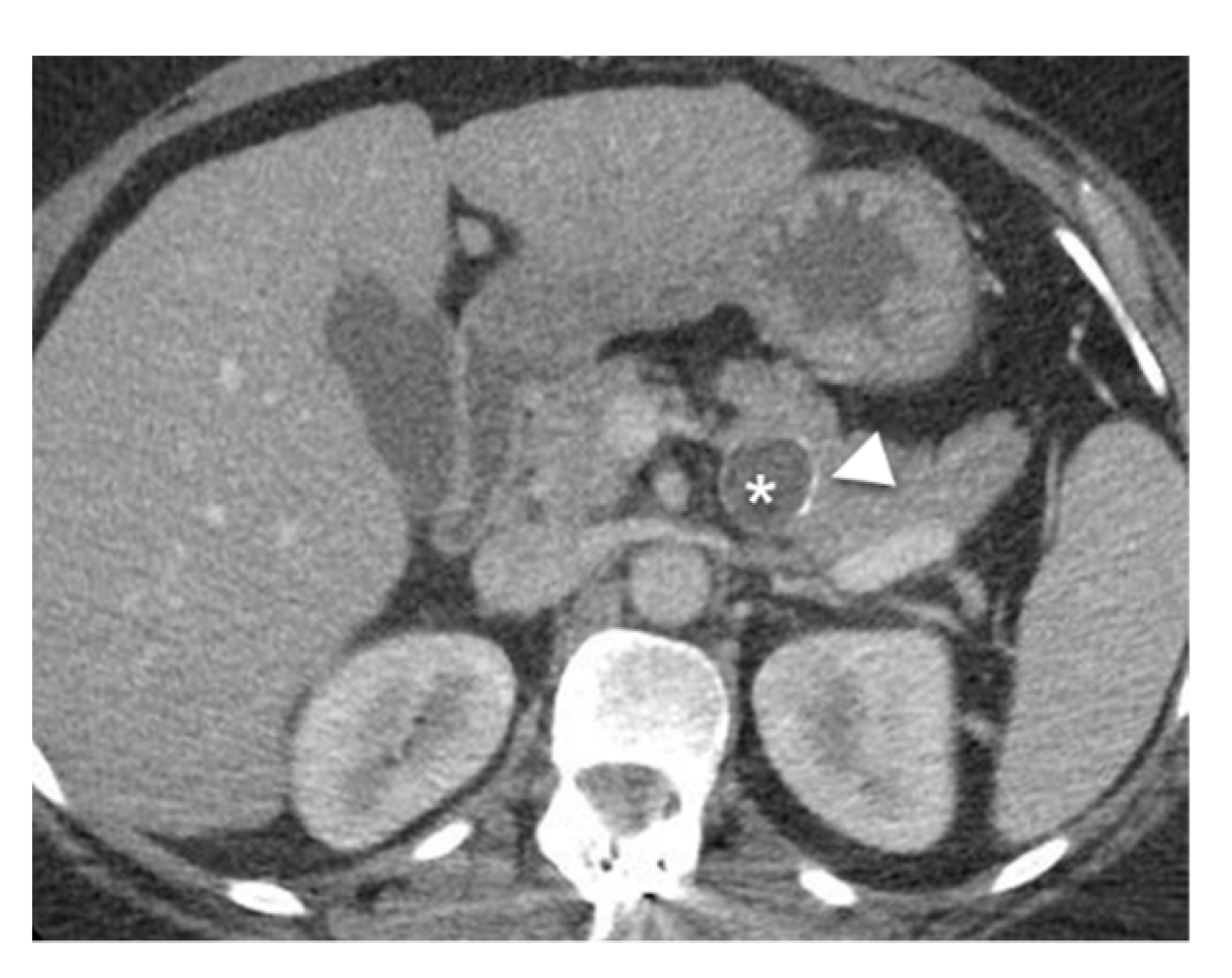
6.4. Pseudocyst
6.5. Lymphoepithelial Cysts
6.6. Cystic Neuroendocrine Tumor (Cystic-NET)
6.7. Solid Pseudopapillary Neoplasm (SPN)
6.8. Retention Cysts
7. Role of MRI in Detecting Advanced Neoplasia
8. Methods to Improve Evaluation of PCLs with MRI
8.1. Use of Secretin
8.2. Use of Gadolinium
8.3. Radiomics
8.4. Artificial Intelligence (AI)
9. Limitations of MRI
10. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Tanaka, M.; Fernandez-Del Castillo, C.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.L. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017, 17, 738–753. [Google Scholar] [CrossRef] [PubMed]
- Hecht, E.M.; Khatri, G.; Morgan, D.; Kang, S.; Bhosale, P.R.; Francis, I.R.; Gandhi, N.S.; Hough, D.M.; Huang, C.; Luk, L.; et al. Intraductal papillary mucinous neoplasm (IPMN) of the pancreas: Recommendations for Standardized Imaging and Reporting from the Society of Abdominal Radiology IPMN disease focused panel. Abdom. Radiol. 2021, 46, 1586–1606. [Google Scholar] [CrossRef]
- Berland, L.L.; Silverman, S.G.; Gore, R.M.; Mayo-Smith, W.W.; Megibow, A.J.; Yee, J.; Brink, J.A.; Baker, M.E.; Federle, M.P.; Foley, W.D.; et al. Managing incidental findings on abdominal CT: White paper of the ACR incidental findings committee. J. Am. Coll. Radiol. 2010, 7, 754–773. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, R.B. Imaging Pancreatic Cysts with CT and MRI. Dig. Dis. Sci. 2017, 62, 1787–1795. [Google Scholar] [CrossRef]
- Moris, M.; Bridges, M.D.; Pooley, R.A.; Raimondo, M.; Woodward, T.A.; Stauffer, J.A.; Asbun, H.J.; Wallace, M.B. Association Between Advances in High-Resolution Cross-Section Imaging Technologies and Increase in Prevalence of Pancreatic Cysts From 2005 to 2014. Clin. Gastroenterol. Hepatol. 2016, 14, 585–593.e583. [Google Scholar] [CrossRef] [PubMed]
- Stark, D.D.; Moss, A.A.; Goldberg, H.I.; Davis, P.L.; Federle, M.P. Magnetic resonance and CT of the normal and diseased pancreas: A comparative study. Radiology 1984, 150, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Tscholakoff, D.; Hricak, H.; Thoeni, R.; Winkler, M.L.; Margulis, A.R. MR imaging in the diagnosis of pancreatic disease. AJR Am. J. Roentgenol. 1987, 148, 703–709. [Google Scholar] [CrossRef]
- Laubenberger, J.; Buchert, M.; Schneider, B.; Blum, U.; Hennig, J.; Langer, M. Breath-hold projection magnetic resonance-cholangio-pancreaticography (MRCP): A new method for the examination of the bile and pancreatic ducts. Magn. Reson. Med. 1995, 33, 18–23. [Google Scholar] [CrossRef]
- Yoon, L.S.; Catalano, O.A.; Fritz, S.; Ferrone, C.R.; Hahn, P.F.; Sahani, D.V. Another dimension in magnetic resonance cholangiopancreatography: Comparison of 2- and 3-dimensional magnetic resonance cholangiopancreatography for the evaluation of intraductal papillary mucinous neoplasm of the pancreas. J. Comput. Assist. Tomogr. 2009, 33, 363–368. [Google Scholar] [CrossRef]
- Harisinghani, M.G.; O’Shea, A.; Weissleder, R. Advances in clinical MRI technology. Sci. Transl. Med. 2019, 11, eaba2591. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, S.; Chatterjee, A. Recent Advances in Pancreatic MR Imaging: A Guide on How, When, and Why to Perform. J. Gastrointest. Abdom. Radiol. 2020, 3, 002–013. [Google Scholar] [CrossRef]
- Zerboni, G.; Signoretti, M.; Crippa, S.; Falconi, M.; Arcidiacono, P.G.; Capurso, G. Systematic review and meta-analysis: Prevalence of incidentally detected pancreatic cystic lesions in asymptomatic individuals. Pancreatology 2019, 19, 2–9. [Google Scholar] [CrossRef] [PubMed]
- De Jong, K.; Nio, C.Y.; Hermans, J.J.; Dijkgraaf, M.G.; Gouma, D.J.; van Eijck, C.H.; van Heel, E.; Klass, G.; Fockens, P.; Bruno, M.J. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin. Gastroenterol. Hepatol. 2010, 8, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Choi, S.-Y.; Min, J.H.; Yi, B.H.; Lee, M.H.; Kim, S.S.; Hwang, J.A.; Kim, J.H. Determining Malignant Potential of Intraductal Papillary Mucinous Neoplasm of the Pancreas: CT versus MRI by Using Revised 2017 International Consensus Guidelines. Radiology 2019, 293, 134–143. [Google Scholar] [CrossRef]
- Girometti, R.; Intini, S.; Brondani, G.; Como, G.; Londero, F.; Bresadola, F.; Zuiani, C.; Bazzocchi, M. Incidental pancreatic cysts on 3D turbo spin echo magnetic resonance cholangiopancreatography: Prevalence and relation with clinical and imaging features. Abdom. Imaging 2011, 36, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, S.; Tada, M.; Akahane, M.; Yagioka, H.; Kogure, H.; Sasaki, T.; Arizumi, T.; Togawa, O.; Nakai, Y.; Sasahira, N.; et al. Incidental pancreatic cysts found by magnetic resonance imaging and their relationship with pancreatic cancer. Pancreas 2012, 41, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, P.B.; Puchnick, A.; Szejnfeld, J.; Goldman, S.M. Prevalence of incidental pancreatic cysts on 3 tesla magnetic resonance. PLoS ONE 2015, 10, e0121317. [Google Scholar] [CrossRef]
- Ulus, S.; Suleyman, E.; Ozcan, U.A.; Karaarslan, E. Whole-Body MRI Screening in Asymptomatic Subjects; Preliminary Experience and Long-Term Follow-Up Findings. Pol. J. Radiol. 2016, 81, 407–414. [Google Scholar] [CrossRef]
- Kim, J.A.; Blumenfeld, J.D.; Chhabra, S.; Dutruel, S.P.; Thimmappa, N.D.; Bobb, W.O.; Donahue, S.; Rennert, H.E.; Tan, A.Y.; Giambrone, A.E.; et al. Pancreatic Cysts in Autosomal Dominant Polycystic Kidney Disease: Prevalence and Association with PKD2 Gene Mutations. Radiology 2016, 280, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Isayama, H.; Nakai, Y.; Yoshikawa, T.; Ishigaki, K.; Matsubara, S.; Yamamoto, N.; Ijichi, H.; Tateishi, K.; Tada, M.; et al. Prevalence of Pancreatic Cystic Lesions Is Associated With Diabetes Mellitus and Obesity: An Analysis of 5296 Individuals Who Underwent a Preventive Medical Examination. Pancreas 2017, 46, 801–805. [Google Scholar] [CrossRef]
- Kromrey, M.L.; Bülow, R.; Hübner, J.; Paperlein, C.; Lerch, M.M.; Ittermann, T.; Völzke, H.; Mayerle, J.; Kühn, J.P. Prospective study on the incidence, prevalence and 5-year pancreatic-related mortality of pancreatic cysts in a population-based study. Gut 2018, 67, 138–145. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, W.T.; Shang, X.S.; Ni, T.; Wu, W.C.; Lou, W.H.; Zeng, M.S.; Rao, S.X. Difference analysis in prevalence of incidental pancreatic cystic lesions between computed tomography and magnetic resonance imaging. BMC Med. Imaging 2019, 19, 43. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, Y.; Jiang, F.; Qian, W.; Shao, C.; Jin, Z. Prevalence of pancreatic cystic lesions detected by magnetic resonance imaging in the Chinese population. J. Gastroenterol. Hepatol. 2019, 34, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Dunn, D.P.; Brook, O.R.; Brook, A.; Revah, G.; Jawadi, S.; Sun, M.; Lee, K.S.; Mortele, K.J. Measurement of pancreatic cystic lesions on magnetic resonance imaging: Efficacy of standards in reducing inter-observer variability. Abdom. Radiol. 2016, 41, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Singhi, A.D.; Koay, E.J.; Chari, S.T.; Maitra, A. Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology 2019, 156, 2024–2040. [Google Scholar] [CrossRef]
- Scheiman, J.M.; Hwang, J.H.; Moayyedi, P. American gastroenterological association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015, 148, 824–848.e822. [Google Scholar] [CrossRef]
- Burk, K.S.; Knipp, D.; Sahani, D.V. Cystic Pancreatic Tumors. Magn. Reson. Imaging Clin. N. Am. 2018, 26, 405–420. [Google Scholar] [CrossRef]
- Sahani, D.V.; Kadavigere, R.; Blake, M.; Fernandez-Del Castillo, C.; Lauwers, G.Y.; Hahn, P.F. Intraductal papillary mucinous neoplasm of pancreas: Multi-detector row CT with 2D curved reformations--correlation with MRCP. Radiology 2006, 238, 560–569. [Google Scholar] [CrossRef]
- Song, S.J.; Lee, J.M.; Kim, Y.J.; Kim, S.H.; Lee, J.Y.; Han, J.K.; Choi, B.I. Differentiation of intraductal papillary mucinous neoplasms from other pancreatic cystic masses: Comparison of multirow-detector CT and MR imaging using ROC analysis. J Magn. Reson. Imaging 2007, 26, 86–93. [Google Scholar] [CrossRef]
- Choi, J.Y.; Lee, J.M.; Lee, M.W.; Kim, S.J.; Choi, S.Y.; Kim, J.Y.; Han, J.K.; Choi, B.I. Magnetic resonance pancreatography: Comparison of two- and three-dimensional sequences for assessment of intraductal papillary mucinous neoplasm of the pancreas. Eur. Radiol. 2009, 19, 2163–2170. [Google Scholar] [CrossRef]
- Rodriguez, J.R.; Salvia, R.; Crippa, S.; Warshaw, A.L.; Bassi, C.; Falconi, M.; Thayer, S.P.; Lauwers, G.Y.; Capelli, P.; Mino-Kenudson, M.; et al. Branch-duct intraductal papillary mucinous neoplasms: Observations in 145 patients who underwent resection. Gastroenterology 2007, 133, 72–79. [Google Scholar] [CrossRef]
- Mamone, G.; Barresi, L.; Tropea, A.; Di Piazza, A.; Miraglia, R. MRI of mucinous pancreatic cystic lesions: A new updated morphological approach for the differential diagnosis. Updates Surg. 2020, 72, 617–637. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Raijman, I.; Machicado, J.D.; Edmundowicz, S.A.; Shah, R.J. Per Oral Pancreatoscopy Identification of Main-duct Intraductal Papillary Mucinous Neoplasms and Concomitant Pancreatic Duct Stones: Not Mutually Exclusive. Pancreas 2019, 48, 792–794. [Google Scholar] [CrossRef]
- Naveed, S.; Qari, H.; Banday, T.; Altaf, A.; Para, M. Mucinous Cystic Neoplasms of Pancreas. Gastroenterol. Res. 2014, 7, 44–50. [Google Scholar] [CrossRef]
- Nilsson, L.N.; Keane, M.G.; Shamali, A.; Millastre Bocos, J.; Marijinissen van Zanten, M.; Antila, A.; Verdejo Gil, C.; Del Chiaro, M.; Laukkarinen, J. Nature and management of pancreatic mucinous cystic neoplasm (MCN): A systematic review of the literature. Pancreatology 2016, 16, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Garces-Descovich, A.; Beker, K.; Castillo-Angeles, M.; Brook, A.; Resnick, E.; Shinagare, S.; Najarian, R.M.; Mortele, K.J. Mucinous cystic neoplasms of the pancreas: High-resolution cross-sectional imaging features with clinico-pathologic correlation. Abdom. Radiol. 2018, 43, 1413–1422. [Google Scholar] [CrossRef]
- Dababneh, Y.; Mousa, O.Y. Pancreatic Serous Cystadenoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Malleo, G.; Bassi, C.; Rossini, R.; Manfredi, R.; Butturini, G.; Massignani, M.; Paini, M.; Pederzoli, P.; Salvia, R. Growth pattern of serous cystic neoplasms of the pancreas: Observational study with long-term magnetic resonance surveillance and recommendations for treatment. Gut 2012, 61, 746–751. [Google Scholar] [CrossRef]
- Charlesworth, M.; Verbeke, C.S.; Falk, G.A.; Walsh, M.; Smith, A.M.; Morris-Stiff, G. Pancreatic lesions in von Hippel-Lindau disease? A systematic review and meta-synthesis of the literature. J. Gastrointest. Surg. 2012, 16, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Kim, M.J.; Lee, J.Y.; Lim, J.S.; Chung, J.J.; Kim, K.W.; Yoo, H.S. Typical and atypical manifestations of serous cystadenoma of the pancreas: Imaging findings with pathologic correlation. AJR Am. J. Roentgenol. 2009, 193, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Giambelluca, D.; Bruno, A.; Picone, D.; Midiri, M. The honeycomb pattern of pancreatic serous cystadenoma. Abdom. Radiol. 2019, 44, 1191–1192. [Google Scholar] [CrossRef]
- Macari, M.; Finn, M.E.; Bennett, G.L.; Cho, K.C.; Newman, E.; Hajdu, C.H.; Babb, J.S. Differentiating pancreatic cystic neoplasms from pancreatic pseudocysts at MR imaging: Value of perceived internal debris. Radiology 2009, 251, 77–84. [Google Scholar] [CrossRef]
- Tirkes, T.; Aisen, A.M.; Cramer, H.M.; Zyromski, N.J.; Sandrasegaran, K.; Akisik, F. Cystic neoplasms of the pancreas; findings on magnetic resonance imaging with pathological, surgical, and clinical correlation. Abdom. Imaging 2014, 39, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Kavuturu, S.; Sarwani, N.E.; Ruggeiro, F.M.; Deshaies, I.; Kimchi, E.T.; Kaifi, J.T.; Staveley-O’Carroll, K.F.; Gusani, N.J. Lymphoepithelial cysts of the pancreas. Can preoperative imaging distinguish this benign lesion from malignant or pre-malignant cystic pancreatic lesions? JOP 2013, 14, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Dalal, K.S.; DeWitt, J.M.; Sherman, S.; Cramer, H.M.; Tirkes, T.; Al-Haddad, M.A. Endoscopic ultrasound characteristics of pancreatic lymphoepithelial cysts: A case series from a large referral center. Endosc. Ultrasound 2016, 5, 248–253. [Google Scholar] [CrossRef]
- Bordeianou, L.; Vagefi, P.A.; Sahani, D.; Deshpande, V.; Rakhlin, E.; Warshaw, A.L.; Fernandez-del Castillo, C. Cystic pancreatic endocrine neoplasms: A distinct tumor type? J. Am. Coll. Surg. 2008, 206, 1154–1158. [Google Scholar] [CrossRef]
- Law, J.K.; Ahmed, A.; Singh, V.K.; Akshintala, V.S.; Olson, M.T.; Raman, S.P.; Ali, S.Z.; Fishman, E.K.; Kamel, I.; Canto, M.I.; et al. A systematic review of solid-pseudopapillary neoplasms: Are these rare lesions? Pancreas 2014, 43, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Cantisani, V.; Mortele, K.J.; Levy, A.; Glickman, J.N.; Ricci, P.; Passariello, R.; Ros, P.R.; Silverman, S.G. MR imaging features of solid pseudopapillary tumor of the pancreas in adult and pediatric patients. AJR Am. J. Roentgenol. 2003, 181, 395–401. [Google Scholar] [CrossRef]
- Yu, M.H.; Lee, J.Y.; Kim, M.A.; Kim, S.H.; Lee, J.M.; Han, J.K.; Choi, B.I. MR imaging features of small solid pseudopapillary tumors: Retrospective differentiation from other small solid pancreatic tumors. AJR Am. J. Roentgenol. 2010, 195, 1324–1332. [Google Scholar] [CrossRef]
- Ren, F.; Zuo, C.; Chen, G.; Wang, J.; Lu, J.; Shao, C.; Hao, X. Pancreatic retention cyst: Multi-modality imaging findings and review of the literature. Abdom. Imaging 2013, 38, 818–826. [Google Scholar] [CrossRef]
- Megibow, A.J.; Baker, M.E.; Morgan, D.E.; Kamel, I.R.; Sahani, D.V.; Newman, E.; Brugge, W.R.; Berland, L.L.; Pandharipande, P.V. Management of Incidental Pancreatic Cysts: A White Paper of the ACR Incidental Findings Committee. J. Am. Coll. Radiol. 2017, 14, 911–923. [Google Scholar] [CrossRef]
- European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018, 67, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Elta, G.H.; Enestvedt, B.K.; Sauer, B.G.; Lennon, A.M. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am. J. Gastroenterol. 2018, 113, 464–479. [Google Scholar] [CrossRef]
- Vege, S.S.; Ziring, B.; Jain, R.; Moayyedi, P.; Clinical Guidelines, C.; American Gastroenterology, A. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015, 148, 819–822. [Google Scholar] [CrossRef]
- Kim, K.W.; Park, S.H.; Pyo, J.; Yoon, S.H.; Byun, J.H.; Lee, M.G.; Krajewski, K.M.; Ramaiya, N.H. Imaging features to distinguish malignant and benign branch-duct type intraductal papillary mucinous neoplasms of the pancreas: A meta-analysis. Ann. Surg. 2014, 259, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Seo, N.; Byun, J.H.; Kim, J.H.; Kim, H.J.; Lee, S.S.; Song, K.B.; Kim, S.C.; Han, D.J.; Hong, S.M.; Lee, M.G. Validation of the 2012 International Consensus Guidelines Using Computed Tomography and Magnetic Resonance Imaging: Branch Duct and Main Duct Intraductal Papillary Mucinous Neoplasms of the Pancreas. Ann. Surg. 2016, 263, 557–564. [Google Scholar] [CrossRef]
- Swensson, J.; Zaheer, A.; Conwell, D.; Sandrasegaran, K.; Manfredi, R.; Tirkes, T. Secretin-Enhanced MRCP: How and Why-AJR Expert Panel Narrative Review. AJR Am. J. Roentgenol. 2021, 216, 1139–1149. [Google Scholar] [CrossRef]
- Rastegar, N.; Matteoni-Athayde, L.G.; Eng, J.; Takahashi, N.; Tamm, E.P.; Mortele, K.J.; Syngal, S.; Margolis, D.; Lennon, A.M.; Wolfgang, C.L.; et al. Incremental value of secretin-enhanced magnetic resonance cholangiopancreatography in detecting ductal communication in a population with high prevalence of small pancreatic cysts. Eur. J. Radiol. 2015, 84, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Gorris, M.; van Huijgevoort, N.; Besselink, M.; Struik, F.; Engelbrecht, M.; van Hooft, J. Added value of secretin during magnetic resonance imaging to identify ductal communication in pancreatic cystic neoplasms (IMAGE-S): Prospective study. Endoscopy 2022, 54, OP271. [Google Scholar]
- Macari, M.; Lee, T.; Kim, S.; Jacobs, S.; Megibow, A.J.; Hajdu, C.; Babb, J. Is gadolinium necessary for MRI follow-up evaluation of cystic lesions in the pancreas? Preliminary results. AJR Am. J. Roentgenol. 2009, 192, 159–164. [Google Scholar] [CrossRef]
- Kierans, A.S.; Gavlin, A.; Wehrli, N.; Flisnik, L.M.; Eliades, S.; Pittman, M.E. Utility of gadolinium for identifying the malignant potential of pancreatic cystic lesions. Abdom. Radiol. 2022, 47, 1351–1359. [Google Scholar] [CrossRef]
- Pozzi-Mucelli, R.M.; Rinta-Kiikka, I.; Wunsche, K.; Laukkarinen, J.; Labori, K.J.; Anonsen, K.; Verbeke, C.; Del Chiaro, M.; Kartalis, N. Pancreatic MRI for the surveillance of cystic neoplasms: Comparison of a short with a comprehensive imaging protocol. Eur. Radiol. 2017, 27, 41–50. [Google Scholar] [CrossRef]
- Nougaret, S.; Reinhold, C.; Chong, J.; Escal, L.; Mercier, G.; Fabre, J.M.; Guiu, B.; Molinari, N. Incidental pancreatic cysts: Natural history and diagnostic accuracy of a limited serial pancreatic cyst MRI protocol. Eur. Radiol. 2014, 24, 1020–1029. [Google Scholar] [CrossRef]
- Machicado, J.D.; Koay, E.J.; Krishna, S.G. Radiomics for the Diagnosis and Differentiation of Pancreatic Cystic Lesions. Diagnostics 2020, 10, 505. [Google Scholar] [CrossRef]
- Stefan, P.A.; Lupean, R.A.; Lebovici, A.; Csutak, C.; Crivii, C.B.; Opincariu, I.; Caraiani, C. Quantitative MRI of Pancreatic Cystic Lesions: A New Diagnostic Approach. Healthcare 2022, 10, 1039. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.K.; Kim, J.H.; Yoo, J.; Kim, J.-E.; Park, S.J.; Han, J.K. Assessment of malignant potential in intraductal papillary mucinous neoplasms of the pancreas using MR findings and texture analysis. Eur. Radiol. 2021, 31, 3394–3404. [Google Scholar] [CrossRef] [PubMed]
- Rangwani, S.; Ardeshna, D.R.; Rodgers, B.; Melnychuk, J.; Turner, R.; Culp, S.; Chao, W.L.; Krishna, S.G. Application of Artificial Intelligence in the Management of Pancreatic Cystic Lesions. Biomimetics 2022, 7, 79. [Google Scholar] [CrossRef]
- Corral, J.E.; Hussein, S.; Kandel, P.; Bolan, C.W.; Bagci, U.; Wallace, M.B. Deep Learning to Classify Intraductal Papillary Mucinous Neoplasms Using Magnetic Resonance Imaging. Pancreas 2019, 48, 805–810. [Google Scholar] [CrossRef]
- Sadigh, G.; Applegate, K.E.; Saindane, A.M. Prevalence of Unanticipated Events Associated with MRI Examinations: A Benchmark for MRI Quality, Safety, and Patient Experience. J. Am. Coll. Radiol. 2017, 14, 765–772. [Google Scholar] [CrossRef]
- Hudson, D.M.; Heales, C.; Meertens, R. Review of claustrophobia incidence in MRI: A service evaluation of current rates across a multi-centre service. Radiography 2022, 28, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Dill, T. Contraindications to magnetic resonance imaging: Non-invasive imaging. Heart 2008, 94, 943–948. [Google Scholar] [CrossRef]
- Blaszczak, A.M.; Krishna, S.G. Endoscopic diagnosis of pancreatic cysts. Curr. Opin. Gastroenterol. 2019, 35, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Machicado, J.D.; Napoleon, B.; Lennon, A.M.; El-Dika, S.; Pereira, S.P.; Tan, D.; Pannala, R.; Girotra, M.; Kongkam, P.; Bertani, H.; et al. Accuracy and agreement of a large panel of endosonographers for endomicroscopy-guided virtual biopsy of pancreatic cystic lesions. Pancreatology 2022, 22, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Paniccia, A.; Polanco, P.M.; Boone, B.A.; Wald, A.I.; McGrath, K.; Brand, R.E.; Khalid, A.; Kubiliun, N.; O’Broin-Lennon, A.M.; Park, W.G.; et al. Prospective, Multi-Institutional, Real-Time Next-Generation Sequencing of Pancreatic Cyst Fluid Reveals Diverse Genomic Alterations That Improve the Clinical Management of Pancreatic Cysts. Gastroenterology 2022, 164, 117–133.e7. [Google Scholar] [CrossRef] [PubMed]
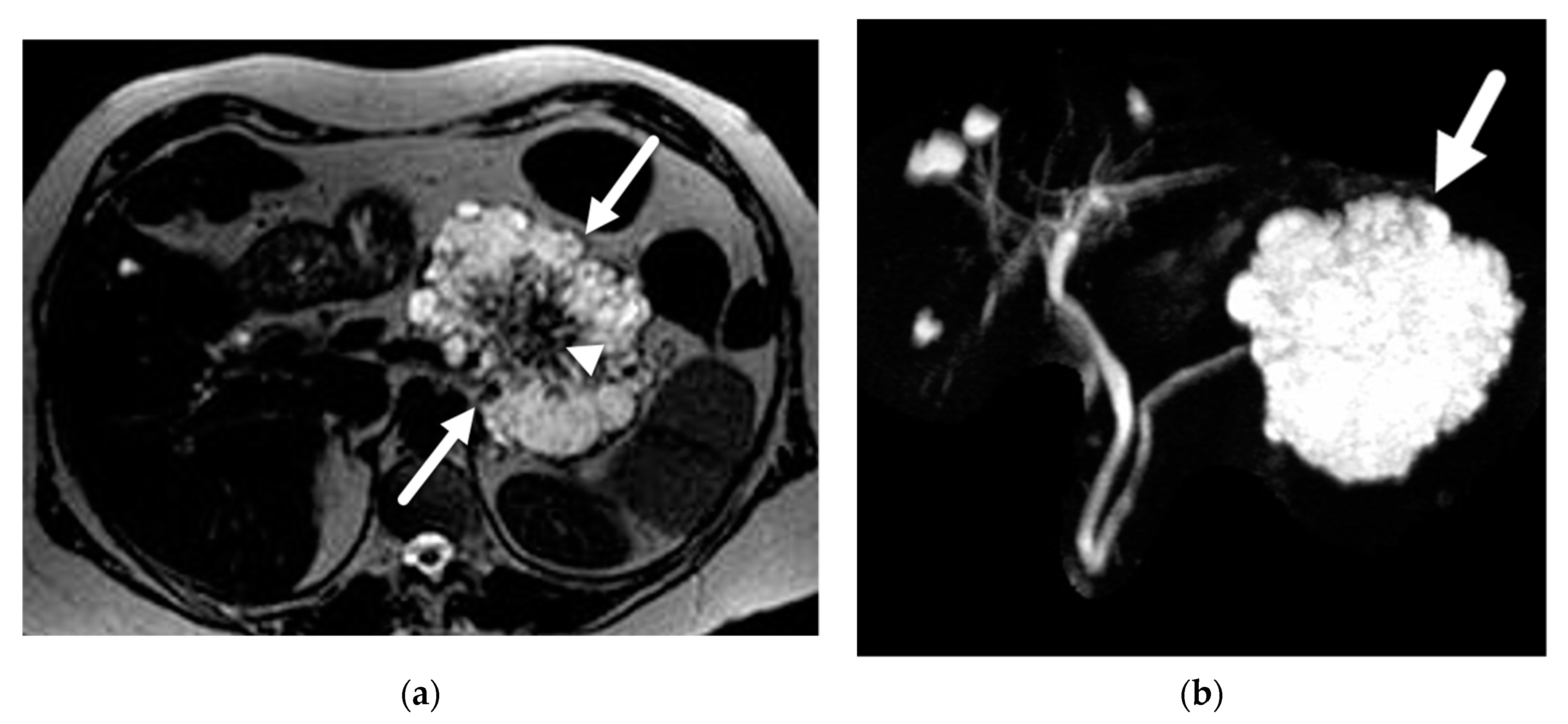
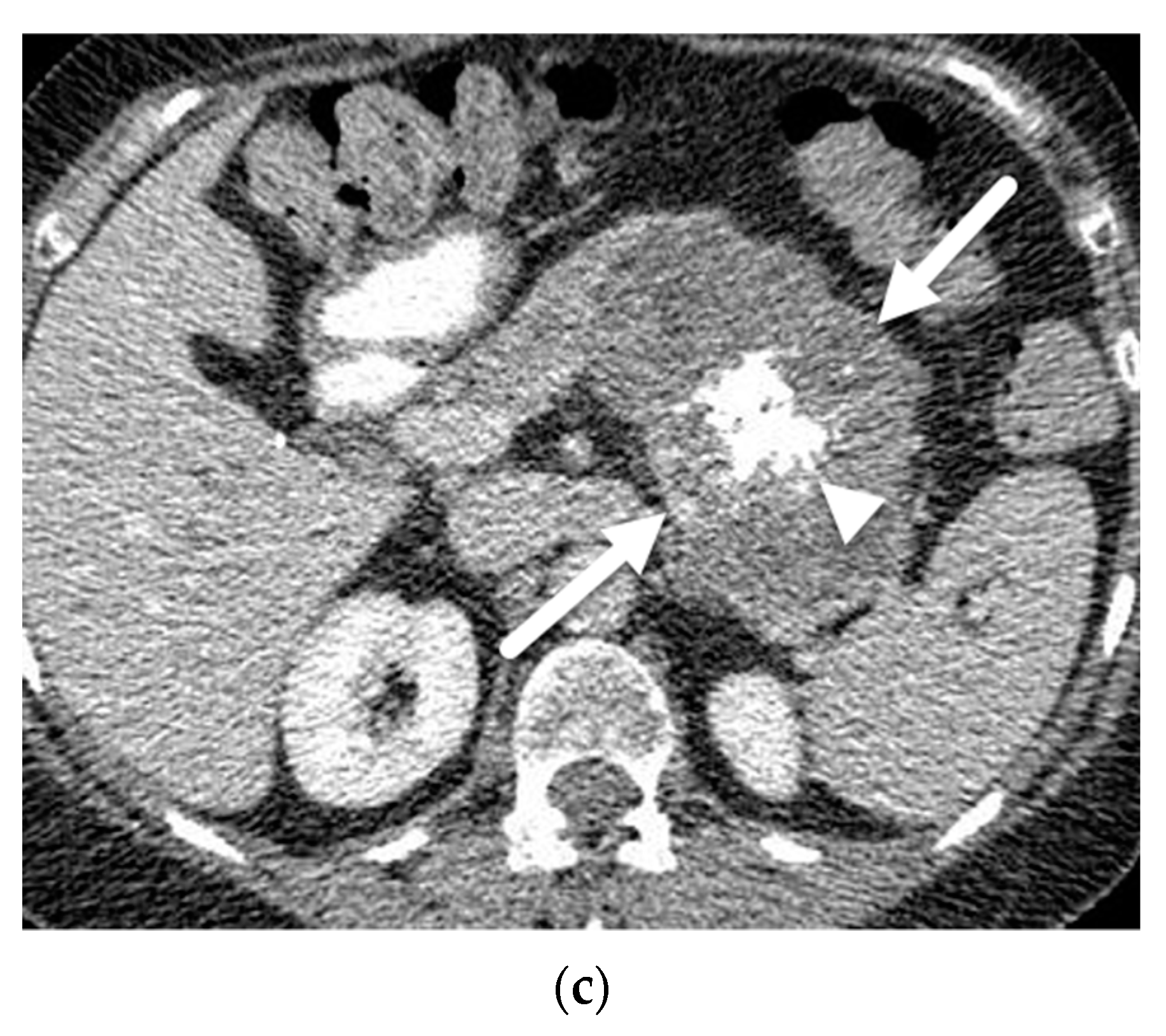
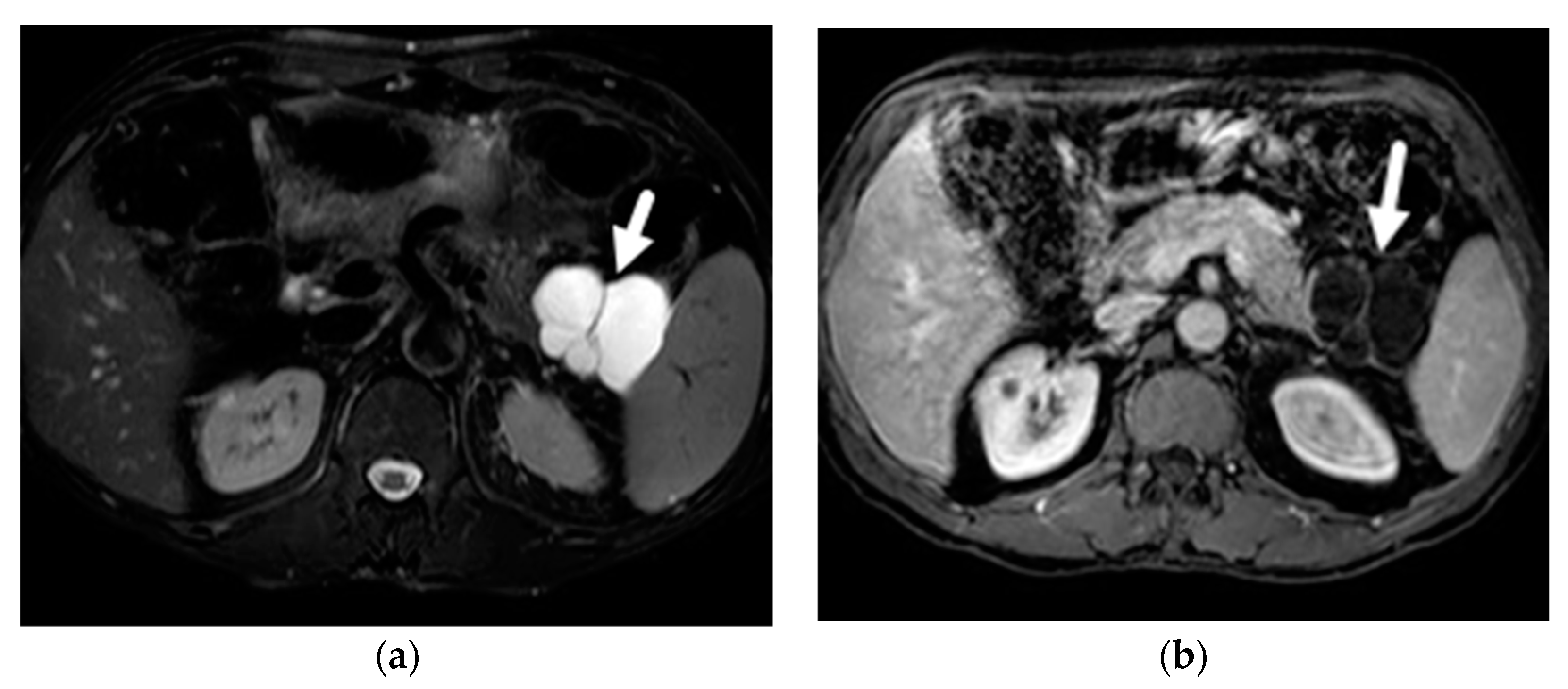
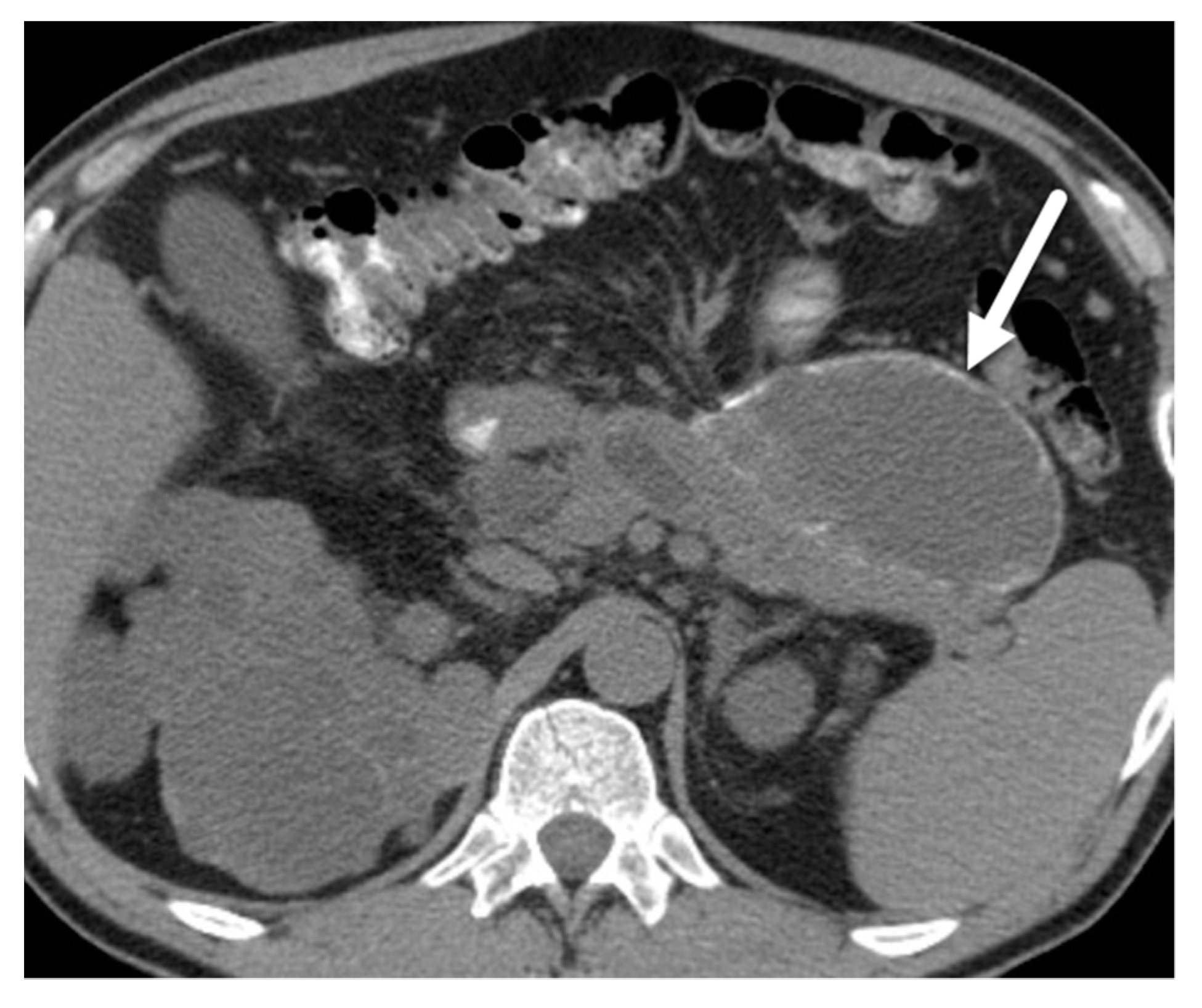
| Author | Year | Country | Design | Magnet | N | PCLs | Prevalence |
|---|---|---|---|---|---|---|---|
| De Jong [13] | 2010 | Holland | Retrospective | 1.5 T, MRI | 2803 | 66 | 2.4% |
| Lee [14] | 2010 | USA | Retrospective | 1.5 T, MRI | 616 | 83 | 13.5% |
| Girometti [15] | 2011 | Italy | Retrospective | 1.5 T MRI/MRCP | 152 | 68 | 44.7% |
| Matsubara [16] | 2012 | Japan | Retrospective | 1.5 T, MRI/MRCP | 1226 | 123 | 10% |
| De Oliveira [17] | 2015 | Brazil | Retrospective | 3 T, MRI | 2583 | 239 | 9.3% |
| Ulus [18] | 2016 | Turkey | Prospective | 1.5 T, MRI | 118 | 1 | 0.8% |
| Moris [5] | 2016 | USA | Retrospective | 1.5T/3 T, MRI/MRCP | 500 | 208 | 41.6% |
| Kim [19] | 2016 | USA | Retrospective | 1.5 T, MRI | 110 | 25 | 22.7% |
| Mizuno [20] | 2017 | Japan | Retrospective | 3 T, MRI/MRCP | 5296 | 724 | 13.7% |
| Kromrey [21] | 2018 | Germany | Retrospective | 1.5 T, MRI/MRCP | 1077 | 494 | 45.9% |
| Zhu [22] | 2019 | China | Retrospective | 1.5 T, MRI/MRCP | 38,099 | 1282 | 3.4% |
| Sun [23] | 2019 | China | Retrospective | 1.5 T/3 T, MRI | 10,987 | 212 | 1.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quingalahua, E.; Al-Hawary, M.M.; Machicado, J.D. The Role of Magnetic Resonance Imaging (MRI) in the Diagnosis of Pancreatic Cystic Lesions (PCLs). Diagnostics 2023, 13, 585. https://doi.org/10.3390/diagnostics13040585
Quingalahua E, Al-Hawary MM, Machicado JD. The Role of Magnetic Resonance Imaging (MRI) in the Diagnosis of Pancreatic Cystic Lesions (PCLs). Diagnostics. 2023; 13(4):585. https://doi.org/10.3390/diagnostics13040585
Chicago/Turabian StyleQuingalahua, Elit, Mahmoud M. Al-Hawary, and Jorge D. Machicado. 2023. "The Role of Magnetic Resonance Imaging (MRI) in the Diagnosis of Pancreatic Cystic Lesions (PCLs)" Diagnostics 13, no. 4: 585. https://doi.org/10.3390/diagnostics13040585




