p-Xylene Oxidation to Terephthalic Acid: New Trends
Abstract
:1. Introduction
1.1. The Beginning of Terephthalic Acid Production
1.2. The AMACO Process
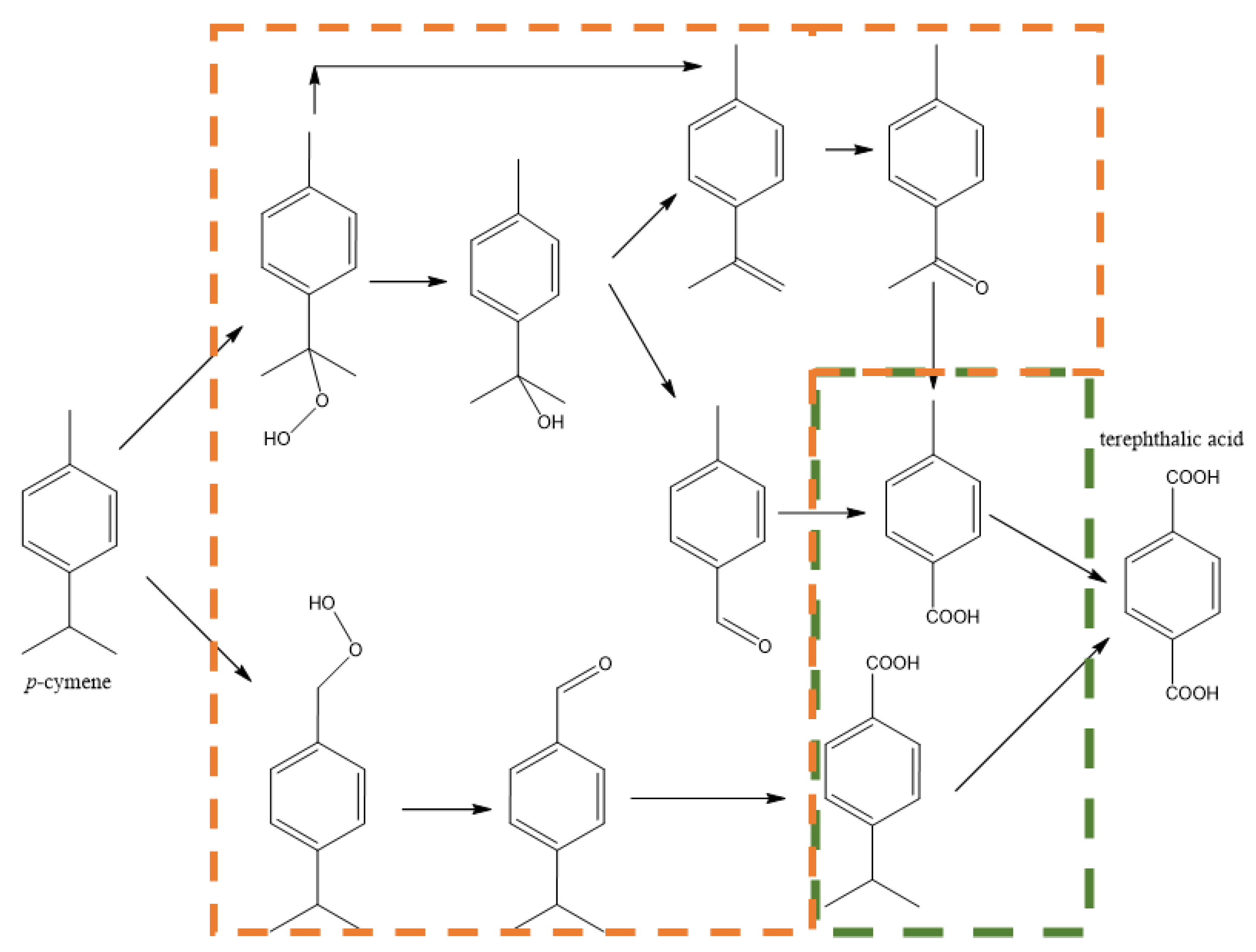
1.3. Production from Biomass and Availability of p-Xylene
2. Catalytic Advances
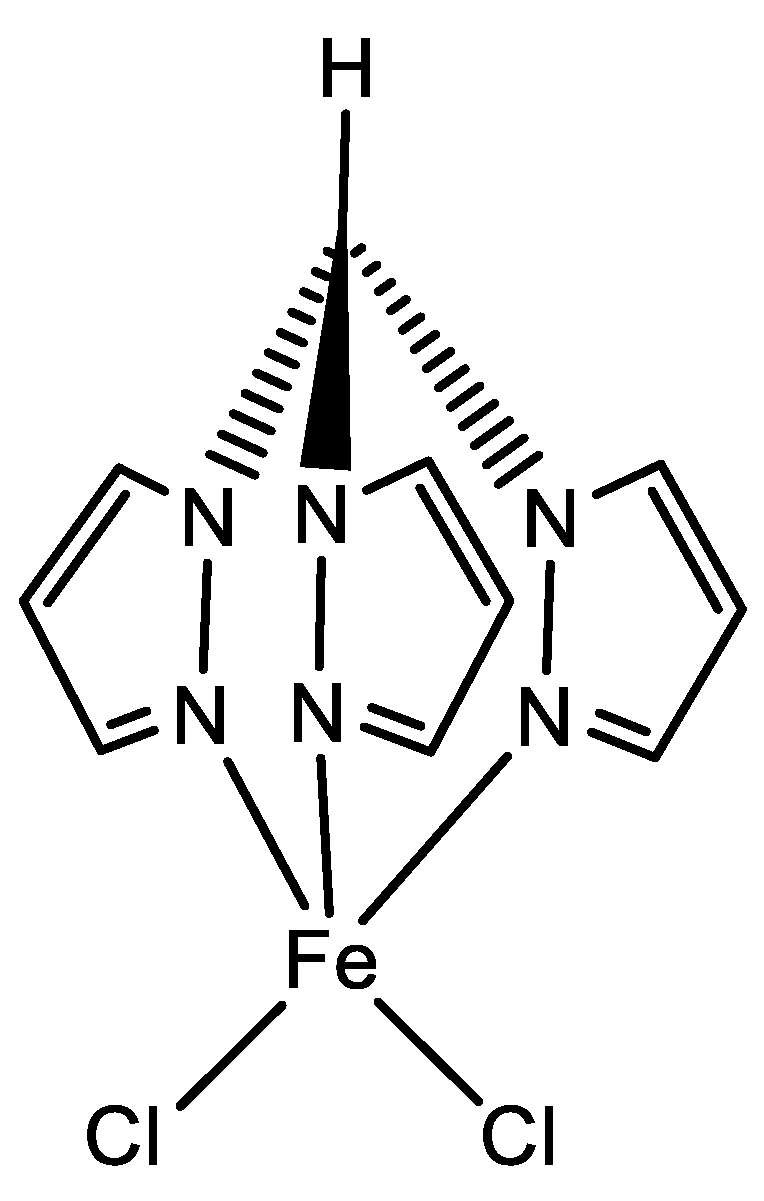
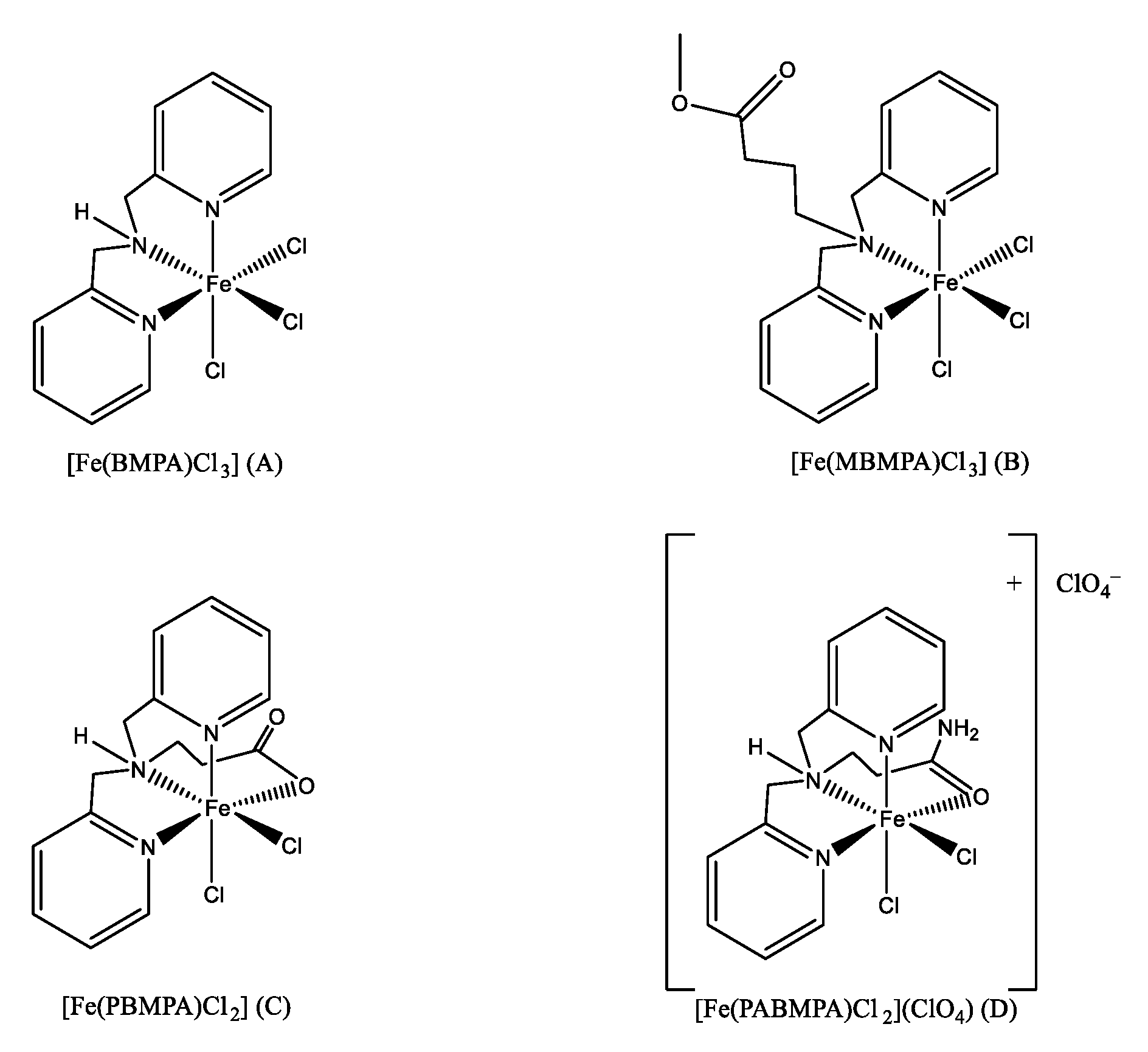
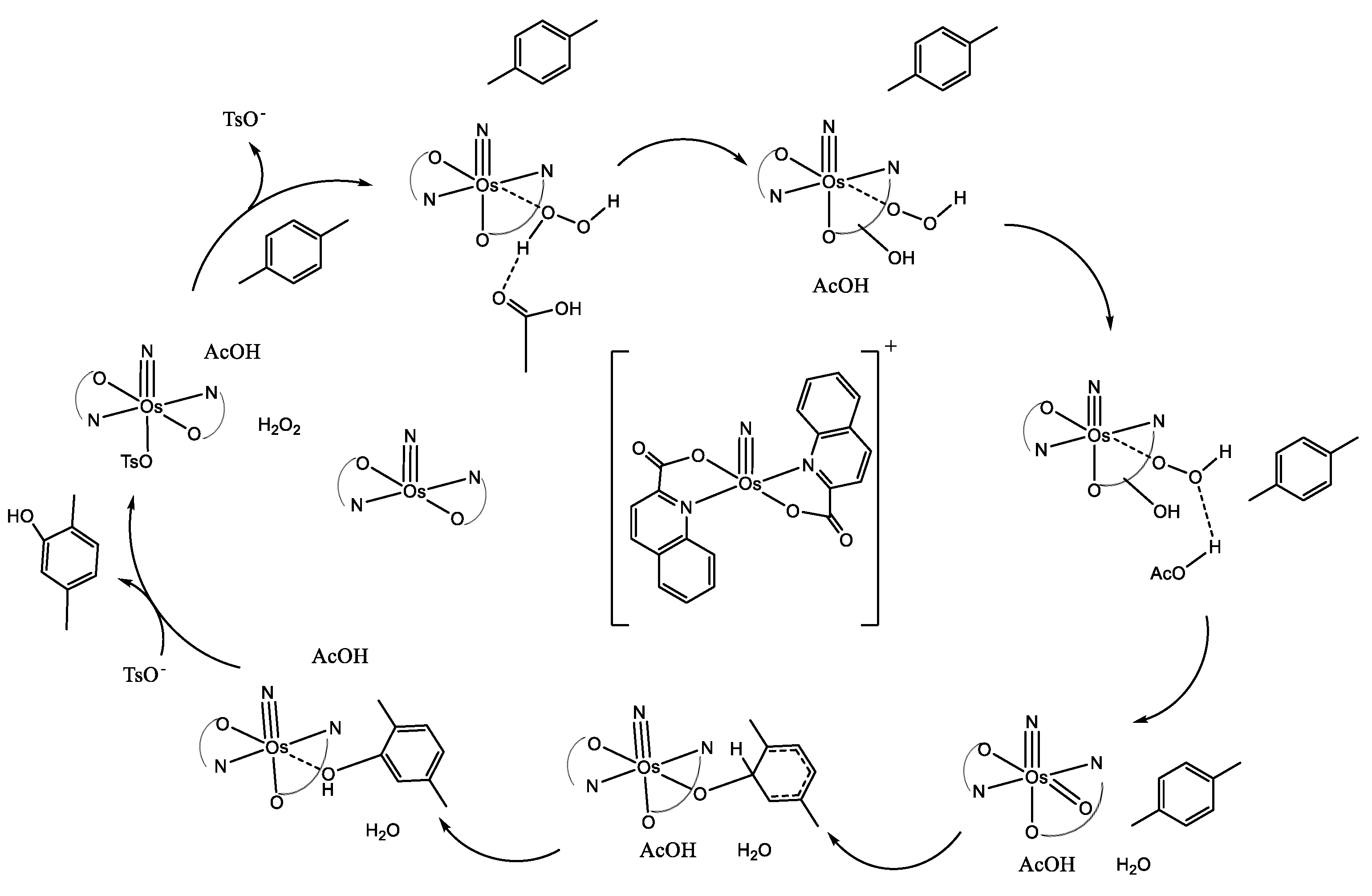
2.1. Homogeneous Catalysts
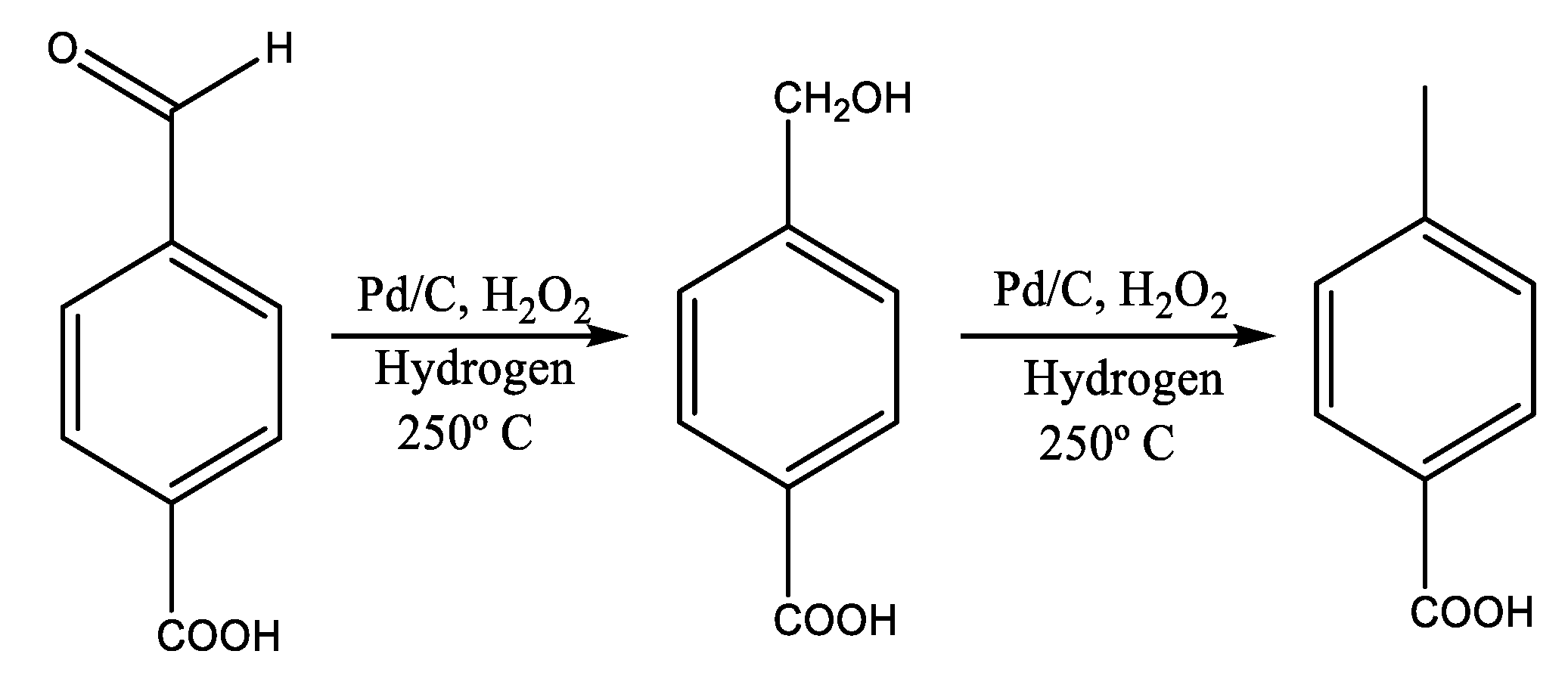
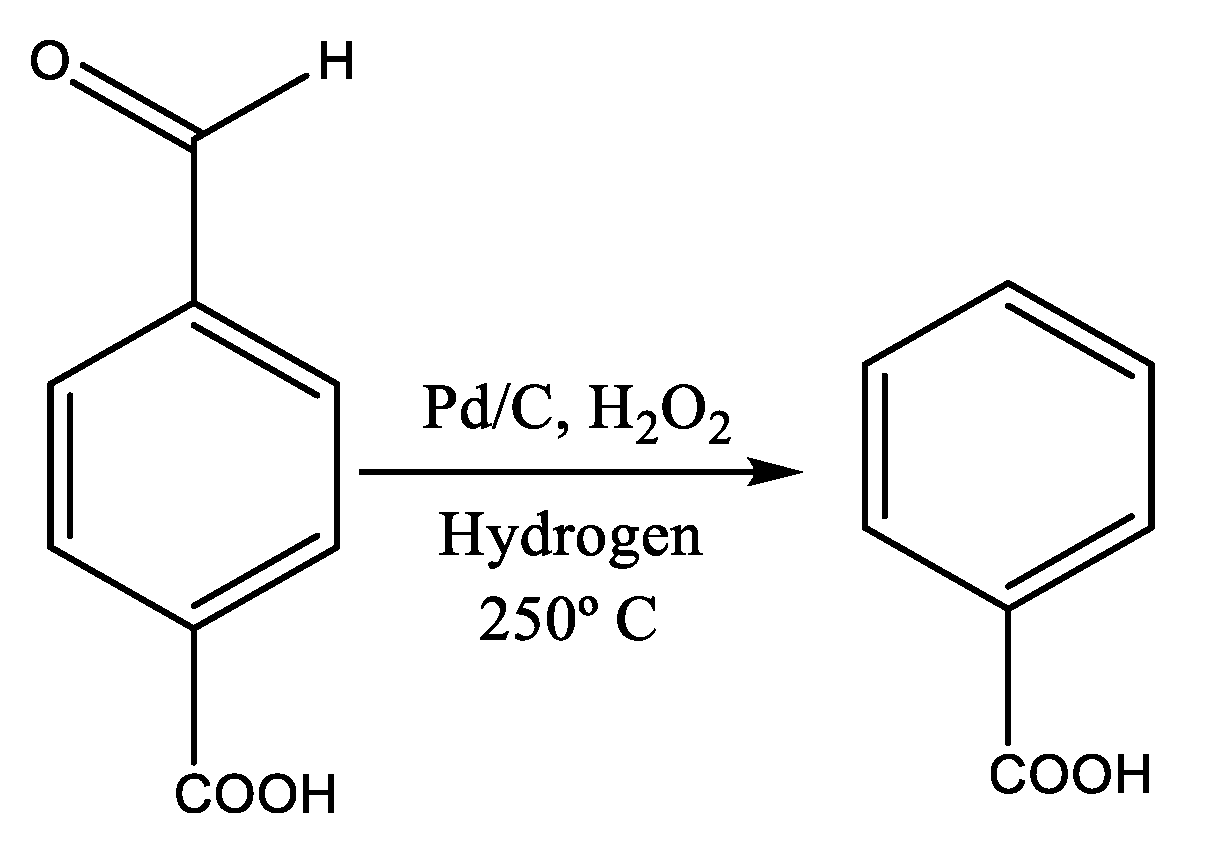
2.2. Heterogeneous Catalysts
2.3. Biocatalysis
3. Computational Studies and Simulations
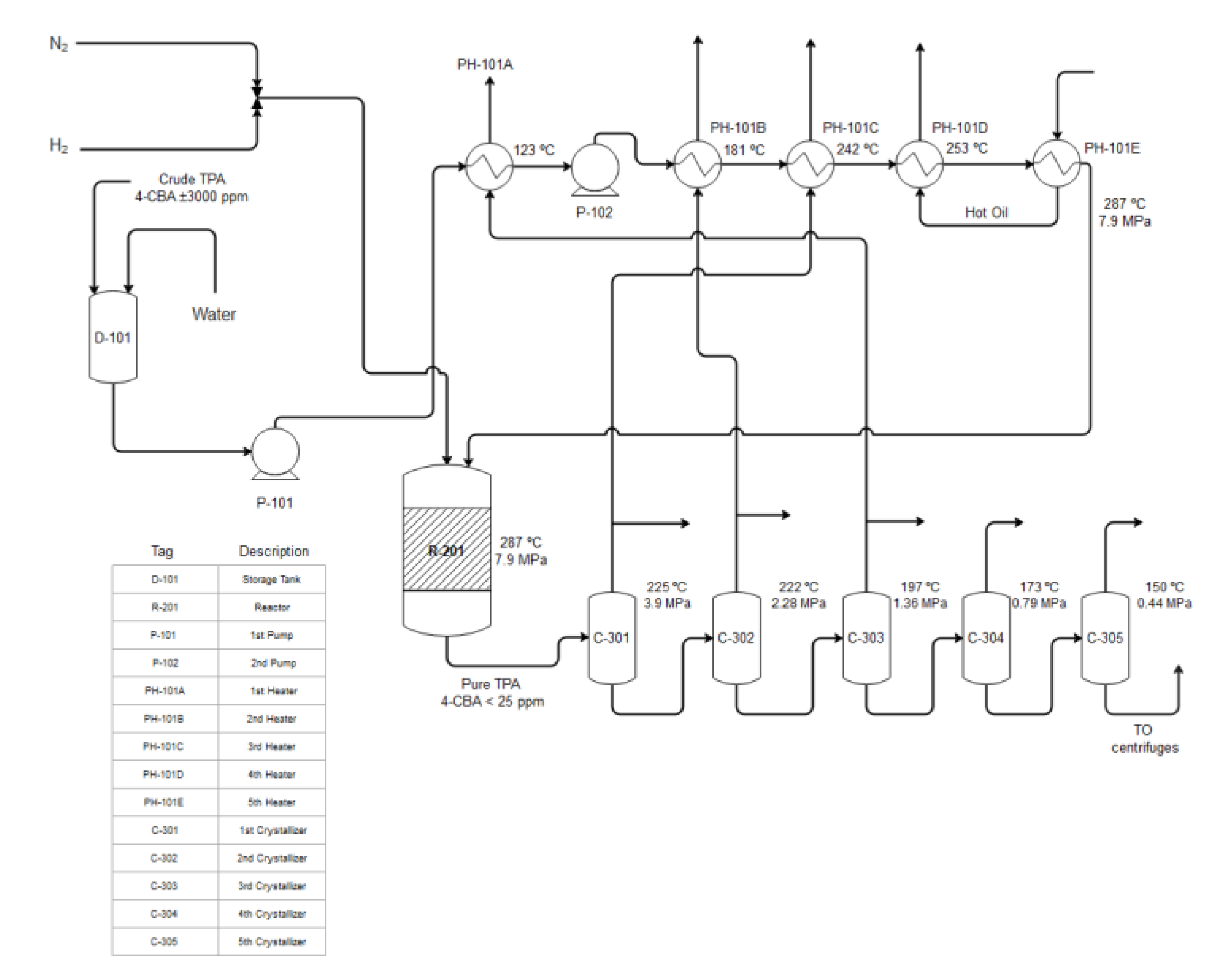
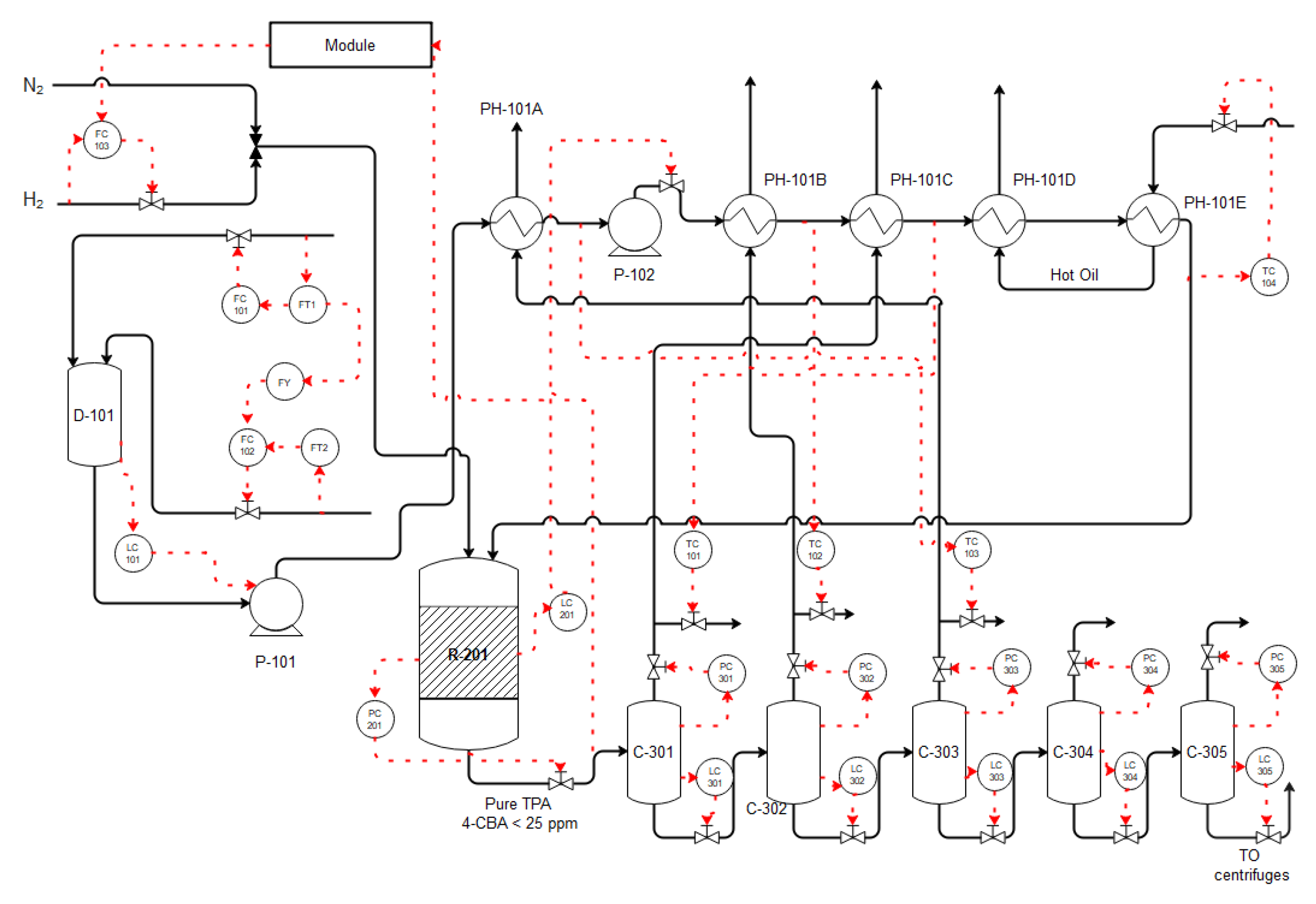
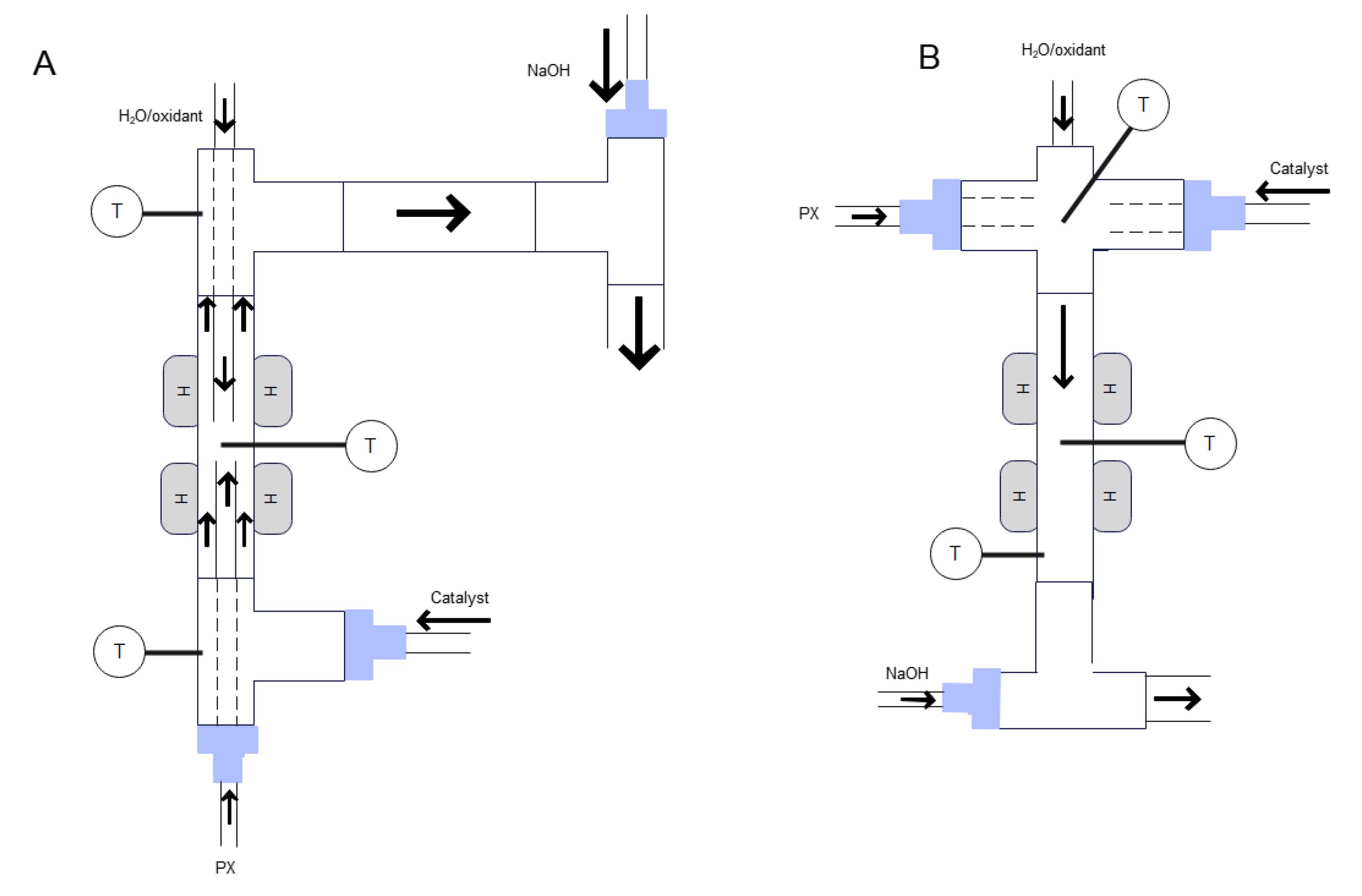
4. Reactor and Purification Step
5. Challenges and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Fadzil, N.A.M.; Rahim, M.H.A.; Maniam, G.P. A brief review of para-xylene oxidation to terephthalic acid as a model of primary C–H bond activation. Chin. J. Catal. 2014, 35, 1641–1652. [Google Scholar] [CrossRef]
- PTA market value worldwide 2015-2029 | Statista. Available online: https://www.statista.com/statistics/1244421/global-market-value-purified-terephthalic-acid/ (accessed on 14 December 2022).
- Global Purified Terephthalic Acid (PTA) Market to Grow to. Available online: https://www.globenewswire.com/en/news-release/2022/03/23/2408781/0/en/Global-Purified-Terephthalic-Acid-PTA-Market-to-Grow-to-USD-78-Billion-Growing-at-a-CAGR-of-5-6-over-the-Analysis-Period-of-2022-2028-BlueWeave-Consulting.html (accessed on 14 December 2022).
- Purified Terephthalic Acid Market Size, Share, Trends & Forecast. Available online: https://www.verifiedmarketresearch.com/product/purified-terephthalic-acid-market/ (accessed on 14 December 2022).
- PTA global market volume 2015–2029 | Statista. Available online: https://www.statista.com/statistics/1245249/purified-terephthalic-acid-market-volume-worldwide/ (accessed on 14 December 2022).
- Ciamician, G.; Silber, P. Chemische Lichtwirkungen. XXII. Autooxydationen. I. Ber. Der Dtsch. Chem. Ges. 1912, 45, 38–43. [Google Scholar] [CrossRef] [Green Version]
- Stephens, H.N. Oxidations in the benzene series by gaseous oxygen II. Alkyl benzenes with two or more carbon atoms in the side chain. J. Am. Chem. Soc. 1926, 48, 2920–2922. [Google Scholar] [CrossRef]
- Raghavendrachar, P.; Ramachandran, S. Liquid-Phase Catalytic Oxidation of p-Xylene. Ind. Eng. Chem. Res. 1992, 31, 453–462. [Google Scholar] [CrossRef]
- Sheehan, R.J. Terephthalic Acid, Dimethyl Terephthalate, and Isophthalic Acid. Ullmann’s Encycl. Ind. Chem. 2011, 26, 193. [Google Scholar] [CrossRef]
- Saffer, A.; Barker, R.S. Preparation of Aromatic Polycarboxylic Acids. U.S. Patent 2833816, 6 May 1958. [Google Scholar]
- Saffer, A.; Barker, R.S. Process for the production of aromatic polycarboxylic acids. U.S. Patent 3776949, 31 March 1959. [Google Scholar]
- Saffer, A.; Barker, R.S. Oxidation Chemical Process. U.S. Patent 3089906, 14 May 1963. [Google Scholar]
- Scheirs, J.; Long, T.E. Modern Polyesters: Chemistry and Technology of Polyesters and Copolyesters; John Wiley & Sons: Hoboken, NJ, USA, 2003; p. 750. [Google Scholar]
- Tomás, R.A.F.; Bordado, J.C.M.; Gomes, J.F.P. p -Xylene Oxidation to Terephthalic Acid: A Literature Review Oriented toward Process Optimization and Development. Chem. Rev. 2013, 113, 7421–7469. [Google Scholar] [CrossRef]
- Volanti, M.; Cespi, D.; Passarini, F.; Neri, E.; Cavani, F.; Mizsey, P.; Fozer, D. Terephthalic acid from renewable sources: Early-stage sustainability analysis of a bio-PET precursor. Green Chem. 2019, 21, 885–896. [Google Scholar] [CrossRef]
- Wan, Y.; Lee, J.M. Toward Value-Added Dicarboxylic Acids from Biomass Derivatives via Thermocatalytic Conversion. ACS Catal. 2021, 11, 2524–2560. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, J.; Dong, P.; Li, G.; Fan, X.; Yang, Y. Novel Short Process for p-Xylene Production Based on the Selectivity Intensification of Toluene Methylation with Methanol. ACS Omega 2022, 7, 1211–1222. [Google Scholar] [CrossRef]
- Chakinala, N.; Chakinala, A.G. Process Design Strategies to Produce p-Xylene via Toluene Methylation: A Review. Ind. Eng. Chem. Res. 2021, 60, 5331–5351. [Google Scholar] [CrossRef]
- Karakhanov, E.A.; Maksimov, A.L.; Zolotukhina, A.V.; Vinokurov, V.A. Oxidation of p-Xylene. Russ. J. Appl. Chem. 2018, 91, 707–727. [Google Scholar] [CrossRef]
- Plekhov, A.L.; Kushch, O.V.; Opeida, I.O.; Kompanets, M.A. Catalytic oxidation of p-xylene with molecular oxygen in the presence of N-hydroxyphthalimide. Russ. J. Appl. Chem. 2014, 87, 982–985. [Google Scholar] [CrossRef]
- Wei, X.Y.; Wang, X.M.; Li, N.; Qin, S.Y. Synthesis, O2-binding ability and catalytic oxidation performance of cobalt(II) complexes with dihydroxamic acid functionalized N-pivot lariat ethers. Chin. Chem. Lett. 2015, 26, 100–102. [Google Scholar] [CrossRef]
- Wang, F.; Tong, Z. Solely Biomass-Derived Polyethylene Terephthalate (PET): Conversion of Bio-based Isoprene and Acrolein to p-Xylene and Terephthalic Acid. ChemistrySelect 2016, 1, 5538–5541. [Google Scholar] [CrossRef]
- Mendes, M.; Ribeiro, A.P.C.; Alegria, E.C.B.A.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Liquid phase oxidation of xylenes catalyzed by the tripodal C-scorpionate iron(II) complex [FeCl2{κ3-HC(pz)3}]. Polyhedron 2017, 125, 151–155. [Google Scholar] [CrossRef]
- Silva, G.C.; Carvalho, N.M.F.; Horn, A.; Lachter, E.R.; Antunes, O.A.C. Oxidation of aromatic compounds by hydrogen peroxide catalyzed by mononuclear iron(III) complexes. J. Mol. Catal. A Chem. 2017, 426, 564–571. [Google Scholar] [CrossRef]
- Kwong, H.K.; Lo, P.K.; Yiu, S.M.; Hirao, H.; Lau, K.C.; Lau, T.C. Highly Selective and Efficient Ring Hydroxylation of Alkylbenzenes with Hydrogen Peroxide and an Osmium(VI) Nitrido Catalyst. Angew. Chem. Int. Ed. 2017, 56, 12260–12263. [Google Scholar] [CrossRef]
- Lindhorst, A.C.; Schütz, J.; Netscher, T.; Bonrath, W.; Kühn, F.E. Catalytic oxidation of aromatic hydrocarbons by a molecular iron–NHC complex. Catal. Sci. Technol. 2017, 7, 1902–1911. [Google Scholar] [CrossRef]
- Goulas, K.A.; Shiramizu, M.; Lattner, J.R.; Saha, B.; Vlachos, D.G. Oxidation of aromatic oxygenates for the production of terephthalic acid. Appl. Catal. A Gen. 2018, 552, 98–104. [Google Scholar] [CrossRef]
- Pan, H.; Li, S.; Shu, M.; Ye, Y.; Cui, Q.; Zhao, Z. P-Xylene catalytic oxidation to terephthalic acid by ozone. ScienceAsia 2018, 44, 212–217. [Google Scholar] [CrossRef]
- Sutradhar, M.; Roy Barman, T.; Alegria, E.C.B.A.; Guedes Da Silva, M.F.C.; Liu, C.M.; Kou, H.Z.; Pombeiro, A.J.L. Cu(II) complexes of N-rich aroylhydrazone: Magnetism and catalytic activity towards microwave-assisted oxidation of xylenes. Dalt. Trans. 2019, 48, 12839–12849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; He, H.; Wang, H.; Zhang, Z.; Chen, C. Efficient catalytic oxidation of methyl aromatic hydrocarbon with N-alkyl pyridinium salts. RSC Adv. 2019, 9, 38891–38896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, K.C.; Sagadevan, A.; Kundu, P. The sustainable room temperature conversion of p-xylene to terephthalic acid using ozone and UV irradiation. Green Chem. 2019, 21, 6082–6088. [Google Scholar] [CrossRef]
- Jiang, D.; Zhang, Q.; Yang, L.; Deng, Y.; Yang, B.; Liu, Y.; Zhang, C.; Fu, Z. Regulating effects of anthraquinone substituents and additives in photo-catalytic oxygenation of p-xylene by molecular oxygen under visible light irradiation. Renew. Energy 2021, 174, 928–938. [Google Scholar] [CrossRef]
- Valange, S.; Védrine, J.C. General and Prospective Views on Oxidation Reactions in Heterogeneous Catalysis. Catalysts 2018, 8, 483. [Google Scholar] [CrossRef] [Green Version]
- Qin, Z.-Z.; Su, T.-M.; Jiang, Y.-X.; Ji, H.-B.; Qin, W. guo Preparation of W-modified FeMo catalyst and its applications in the selective oxidization of p-xylene to terephthalaldehyde. Chem. Eng. J. 2014, 242, 414–421. [Google Scholar] [CrossRef]
- Kim, D.S.; Shin, Y.H.; Lee, Y.W. Synthesis of terephthalic acid by catalytic partial oxidation of p-xylene in supercritical carbon dioxide. Chem. Eng. Commun. 2014, 202, 78–84. [Google Scholar] [CrossRef]
- Mueller, R.; Li, Y.X.; Hampson, A.; Zhong, S.; Harris, C.; Marrs, C.; Rachwal, S.; Ulas, J.; Nielsson, L.; Rogers, G. Benzoxazinones as potent positive allosteric AMPA receptor modulators: Part I. Bioorg. Med. Chem. Lett. 2011, 21, 3923–3926. [Google Scholar] [CrossRef] [PubMed]
- Hehir, S.; Plourde, N.M.; Gu, L.; Poree, D.E.; Welsh, W.J.; Moghe, P.V.; Uhrich, K.E. Carbohydrate composition of amphiphilic macromolecules influences physicochemical properties and binding to atherogenic scavenger receptor A. Acta Biomater. 2012, 8, 3956–3962. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wang, L.; Zhu, Z.; Hu, Z.; Zhao, Q.; Chen, J. All Organic Sodium-Ion Batteries with Na4C8H2O6. Angew. Chem. 2014, 126, 6002–6006. [Google Scholar] [CrossRef]
- Renault, S.; Gottis, S.; Barrès, A.L.; Courty, M.; Chauvet, O.; Dolhem, F.; Poizot, P. A green Li–organic battery working as a fuel cell in case of emergency. Energy Environ. Sci. 2013, 6, 2124–2133. [Google Scholar] [CrossRef]
- Dietzel, P.D.C.; Morita, Y.; Blom, R.; Fjellvåg, H. An in situ high-temperature single-crystal investigation of a dehydrated metal-organic framework compound and field-induced magnetization of one-dimensional metal-oxygen chains. Angew. Chem. Int. Ed. Engl. 2005, 44, 6354–6358. [Google Scholar] [CrossRef] [PubMed]
- Rosi, N.L.; Kim, J.; Eddaoudi, M.; Chen, B.; O’Keeffe, M.; Yaghi, O.M. Rod packings and metal-organic frameworks constructed from rod-shaped secondary building units. J. Am. Chem. Soc. 2005, 127, 1504–1518. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Duan, D.; Wu, M.; Li, J.; Yan, Z.; Wang, W.; Zi, G.; Wang, J. One-step synthesis of 2,5-dihydroxyterephthalic acid by the oxidation of p-xylene over M-MCM-41 (M = Fe, Fe/Cu, Cu) catalysts. Chem. Eng. J. 2016, 306, 777–783. [Google Scholar] [CrossRef]
- Wang, J.; Martins, L.M.D.R.S.; Ribeiro, A.P.C.; Carabineiro, S.A.C.; Figueiredo, J.L.; Pombeiro, A.J.L. Supported C-Scorpionate Vanadium(IV) Complexes as Reusable Catalysts for Xylene Oxidation. Chem.—Asian J. 2017, 12, 1915–1919. [Google Scholar] [CrossRef]
- Samanta, S.; Srivastava, R. Thermal catalysis vs. photocatalysis: A case study with FeVO4/g-C3N4 nanocomposites for the efficient activation of aromatic and benzylic CH bonds to oxygenated products. Appl. Catal. B Environ. 2017, 218, 621–636. [Google Scholar] [CrossRef]
- Nicolae, S.; Neaţu, F.; Florea, M. Selective catalytic oxidation reaction of p-xylene on manganese–iron mixed oxide materials. Comptes Rendus Chim. 2018, 21, 354–361. [Google Scholar] [CrossRef]
- Li, Y.; Wu, M.; Chen, D.; Jiang, L.; He, J.; Luo, Z.; Wang, W.; Wang, J. One-step highly selective oxidation of p-xylene to 4-hydroxymethylbenzoic acid over Cu-MOF catalysts under mild conditions. Mol. Catal. 2019, 477, 110542. [Google Scholar] [CrossRef]
- Karakhanov, E.; Maximov, A.; Zolotukhina, A.; Vinokurov, V.; Ivanov, E.; Glotov, A. Manganese and Cobalt Doped Hierarchical Mesoporous Halloysite-Based Catalysts for Selective Oxidation of p-Xylene to Terephthalic Acid. Catalysts 2020, 10, 7. [Google Scholar] [CrossRef] [Green Version]
- Partenheimer, W. The effect of zirconium in metal/bromide catalysts during the autoxidation of p-xylene: Part I. Activation and changes in benzaldehyde intermediate formation. J. Mol. Catal. A Chem. 2003, 206, 105–119. [Google Scholar] [CrossRef]
- Partenheimer, W. The effect of zirconium in metal/bromide catalysts on the autoxidation of p-xylene: Part II. Alternative metals to zirconium and the effect of zirconium on manganese(IV) dioxide formation and precipitation with pyromellitic acid. J. Mol. Catal. A Chem. 2003, 206, 131–144. [Google Scholar] [CrossRef]
- Shimizu, A.; Tanaka, K.; Ogawa, H.; Matsuoka, Y.; Fujimori, M.; Nagamori, Y.; Hamachi, H.; Kimura, K. An Industrial Process for Adipic Acid Production by the Liquid-Phase Oxidation of Cyclohexanone with Molecular Oxygen. Bull. Chem. Soc. Jpn. 2003, 76, 1993–2001. [Google Scholar] [CrossRef]
- Cavani, F.; Ferroni, L.; Frattini, A.; Lucarelli, C.; Mazzini, A.; Raabova, K.; Alini, S.; Accorinti, P.; Babini, P. Evidence for the presence of alternative mechanisms in the oxidation of cyclohexanone to adipic acid with oxygen, catalysed by Keggin polyoxometalates. Appl. Catal. A Gen. 2011, 391, 118–124. [Google Scholar] [CrossRef]
- Trandafir, M.M.; Neaţu, S.; Bocîrnea, A.; Counsell, J.; Cavani, F.; Florea, M.; Neaţu, F. The Role of Acidity in Terephthalic Acid Synthesis from Renewable Carbon Source. ChemCatChem 2020, 12, 6248–6258. [Google Scholar] [CrossRef]
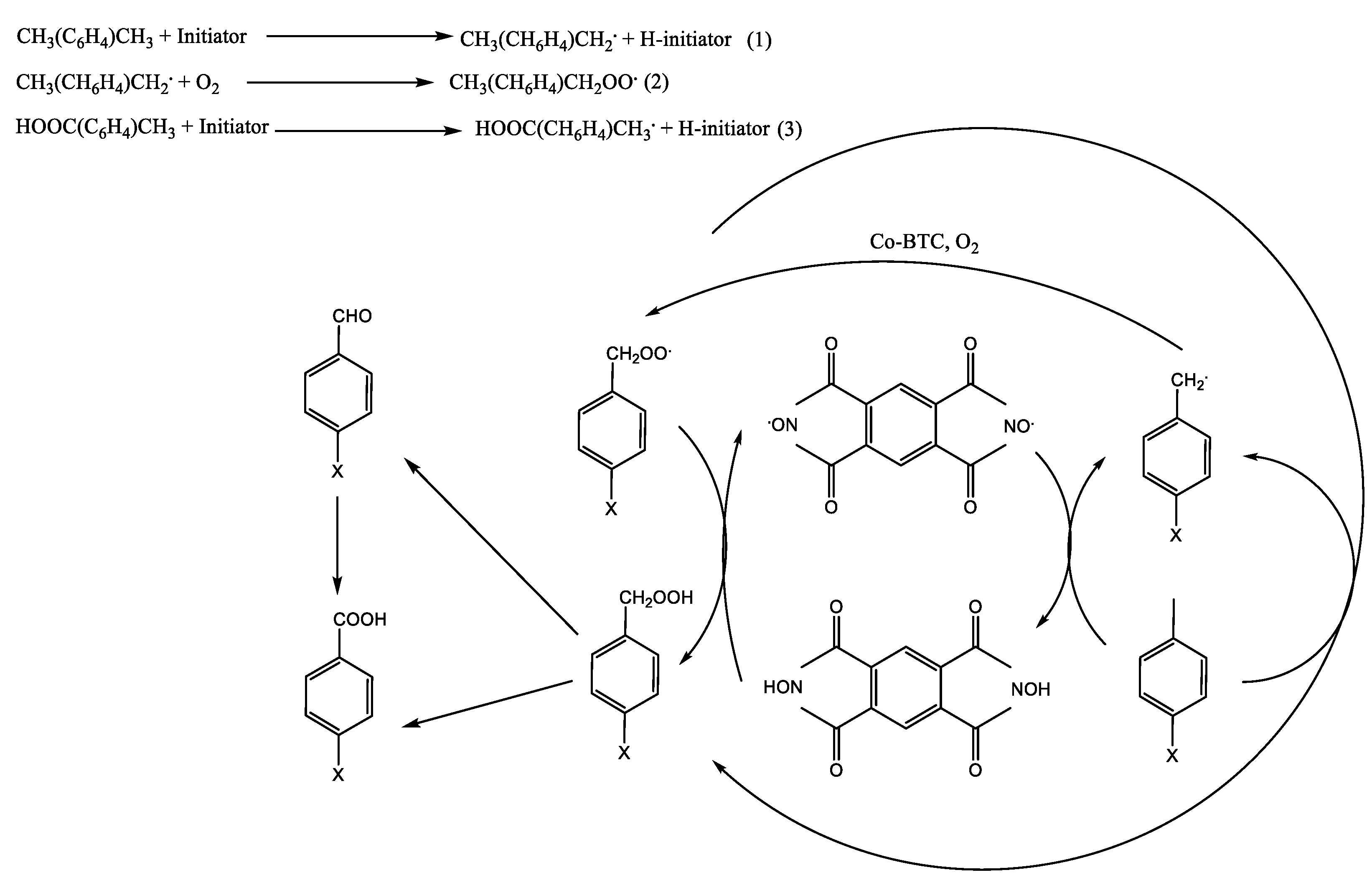
- Xu, L.; Chen, D.; Jiang, H.; Yuan, X. Efficient oxidation of p-xylene to terephthalic acid by using N,N-dihydroxypyromellitimide in conjunction with Co-benzenetricarboxylate. Appl. Catal. A Gen. 2020, 599, 117569. [Google Scholar] [CrossRef]
- Wang, Q.N.; Sun, X.; Feng, Z.; Feng, Z.; Zhang, P.; Zhang, Y.; Li, C. V-O-Ag Linkages in VAgO xMixed Oxides for the Selective Oxidation of p-Xylene to p-Methyl Benzaldehyde. ACS Catal. 2022, 12, 3323–3332. [Google Scholar] [CrossRef]
- Sarmah, K.; Mukhopadhyay, S.; Maji, T.K.; Pratihar, S. Switchable Bifunctional Bistate Reusable ZnO-Cu for Selective Oxidation and Reduction Reaction. ACS Catal. 2019, 9, 732–745. [Google Scholar] [CrossRef]
- Dutta, M.; Bania, K.K.; Pratihar, S. A Remote ‘Imidazole’-Based Ruthenium(II) Para-Cymene Pre-catalyst for the Selective Oxidation Reaction of Alkyl Arenes and Alcohols. Chem.—Asian J. 2020, 15, 926–932. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Du, J.; Zhang, C.; Liu, K.; Yu, F.; Yuan, Y.; Duan, B.; Liu, R. Selective Oxidation of Alkylarenes to the Aromatic Ketones or Benzaldehydes with Water. Org. Lett. 2022, 24, 1152–1157. [Google Scholar] [CrossRef]
- Danikiewicz, W.; Zimnicka, M. Negative ion gas-phase chemistry of arenes. Mass Spectrom. Rev. 2016, 35, 123–146. [Google Scholar] [CrossRef]
- Mohammadian, R.; Karimi Alavijeh, M.; Kamyar, N.; Amini, M.M.; Shaabani, A. Metal–organic frameworks as a new platform for molecular oxygen and aerobic oxidation of organic substrates: Recent advances. Polyhedron 2018, 156, 174–187. [Google Scholar] [CrossRef]
- Urgoitia, G.; Martin, R.S.; Herrero, M.T.; Domínguez, E. Recent Advances in Homogeneous Metal-Catalyzed Aerobic C–H Oxidation of Benzylic Compounds. Catalysts 2018, 8, 640. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Gunnoe, T.B. Advances in Rhodium-Catalyzed Oxidative Arene Alkenylation. Acc. Chem. Res. 2020, 53, 920–936. [Google Scholar] [CrossRef]
- George, K.; Kannadasan, S. Advancements in the Functionalization of Aryl C-sp2 and Alkyl C-sp3 of Toluene Derivatives via C-H Activation. Curr. Org. Chem. 2021, 25, 1783–1822. [Google Scholar] [CrossRef]
- Dong, J.J.; Fernández-Fueyo, E.; Hollmann, F.; Paul, C.E.; Pesic, M.; Schmidt, S.; Wang, Y.; Younes, S.; Zhang, W. Biocatalytic Oxidation Reactions: A Chemist’s Perspective. Angew. Chem. Int. Ed. 2018, 57, 9238–9261. [Google Scholar] [CrossRef] [PubMed]
- Hanefeld, U.; Hollmann, F.; Paul, C.E. Biocatalysis making waves in organic chemistry. Chem. Soc. Rev. 2022, 51, 594–627. [Google Scholar] [CrossRef]
- Luo, Z.W.; Lee, S.Y. Biotransformation of p-xylene into terephthalic acid by engineered Escherichia coli. Nat. Commun. 2017, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhong, W.; Liu, Y.; Luo, N.; Qian, F. Dynamic modeling and control of industrial crude terephthalic acid hydropurification process. Korean J. Chem. Eng. 2015, 32, 597–608. [Google Scholar] [CrossRef]
- Azarpour, A.; Zahedi, G. Performance analysis of crude terephthalic acid hydropurification in an industrial trickle-bed reactor experiencing catalyst deactivation. Chem. Eng. J. 2012, 209, 180–193. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, W.; Wang, X.; Luo, N.; Qian, F. Control structure design of an industrial crude terephthalic acid hydropurification process with catalyst deactivation. Comput. Chem. Eng. 2016, 88, 1–12. [Google Scholar] [CrossRef]
- Shang, J.; Sun, W.; Zhao, L.; Yuan, W.-K. Liquid phase oxidation of alkyl aromatics at low oxygen partial pressures. Chem. Eng. J. 2015, 278, 533–540. [Google Scholar] [CrossRef]
- Shang, J.; Sun, W.; Zhao, L.; Yuan, W.-K. Modeling of CO2-assisted liquid phase oxidation of para-xylene catalyzed by transition metals/bromide. Chem. Eng. Sci. 2015, 127, 52–59. [Google Scholar] [CrossRef]
- Pellegrini, R.; Agostini, G.; Groppo, E.; Piovano, A.; Leofanti, G.; Lamberti, C. 0.5wt.% Pd/C catalyst for purification of terephthalic acid: Irreversible deactivation in industrial plants. J. Catal. 2011, 280, 150–160. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, J.; Sui, Z.; Zhou, Z.; Yuan, W. Mathematical Simulation of Hydrorefining Reactor for Terephthalic Acid. Chem. React. Eng. Technol. 2008, 24, 54–60. [Google Scholar]
- Kuznetsova, N.I.; Kuznetsova, L.I.; Yakovina, O.A.; Zudin, V.N.; Bal’zhinimaev, B.S.; Bhattacharyya, A.; Walenga, J.T. Use of NH4OAc and BMIM Br in Staged Oxidation of p-Xylene and Oxidative Treatment of CTA for Obtaining TPA with a Low Content of 4-CBA. Ind. Eng. Chem. Res. 2020, 59, 1038–1044. [Google Scholar] [CrossRef]
- Pérez, E.; Thomas, M.L.; Housley, D.; Hamley, P.A.; Fraga-Dubreuil, J.; Li, J.; Lester, E.; Poliakoff, M. Selective aerobic oxidation of para-xylene in sub- and supercritical water. Part 3: Effects of geometry and mixing in laboratory scale continuous reactors. RSC Adv. 2016, 6, 11289–11294. [Google Scholar] [CrossRef]
- Li, M.; Ruddy, T.; Fahey, D.; Busch, D.H.; Subramaniam, B. Terephthalic Acid Production via Greener Spray Process: Comparative Economic and Environmental Impact Assessments with Mid-Century Process. ACS Sustain. Chem. Eng. 2014, 2, 823–835. [Google Scholar] [CrossRef]

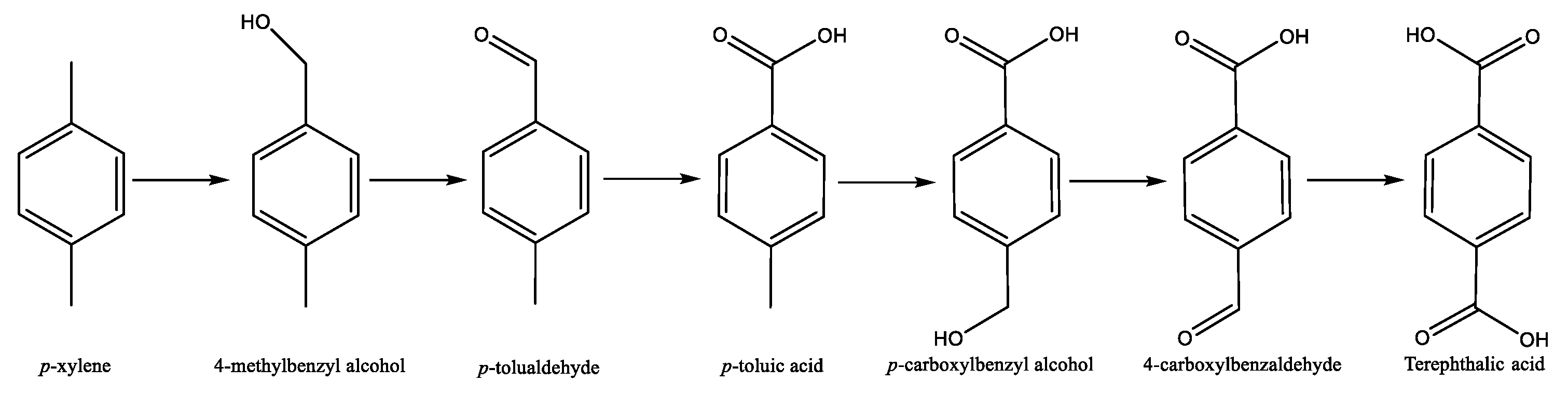

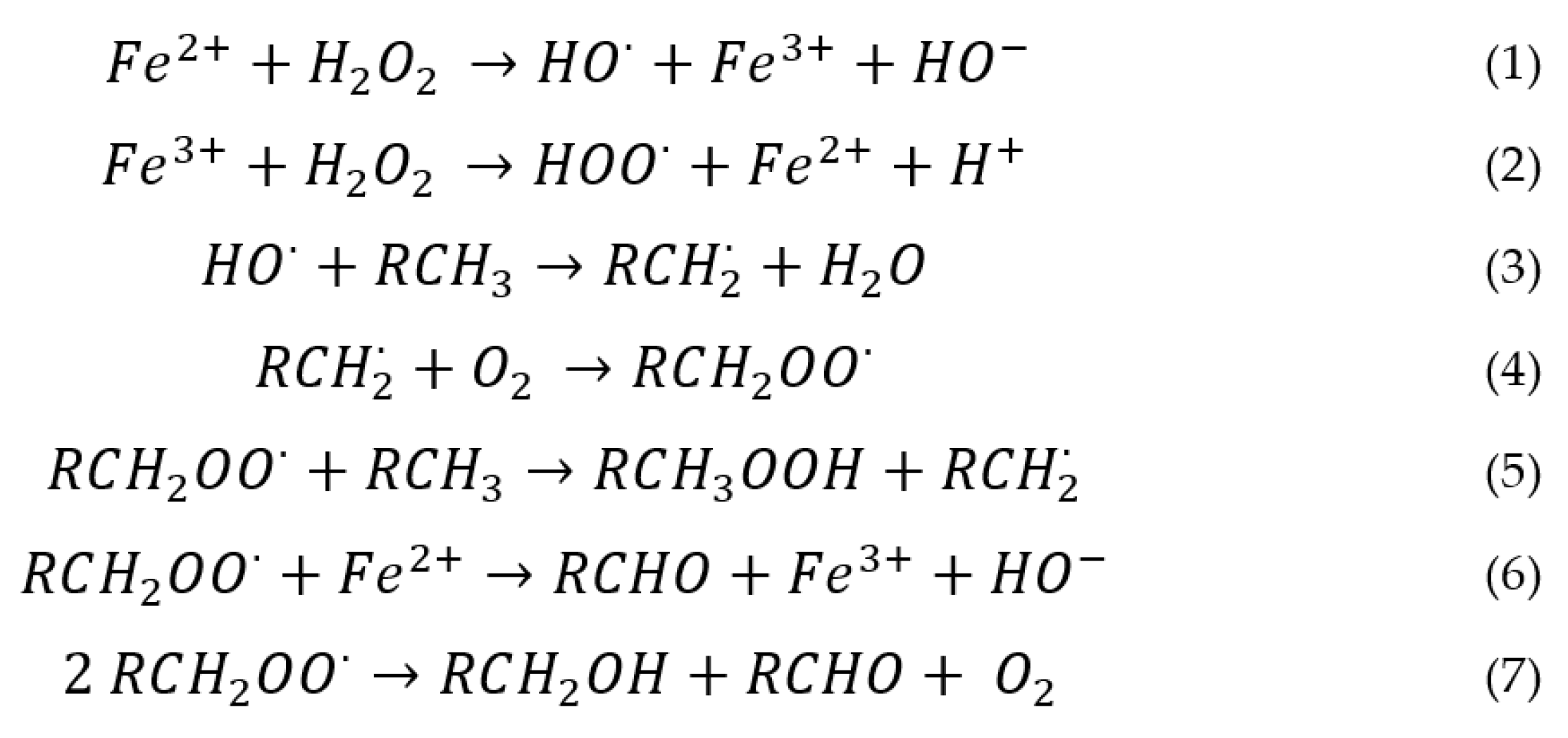

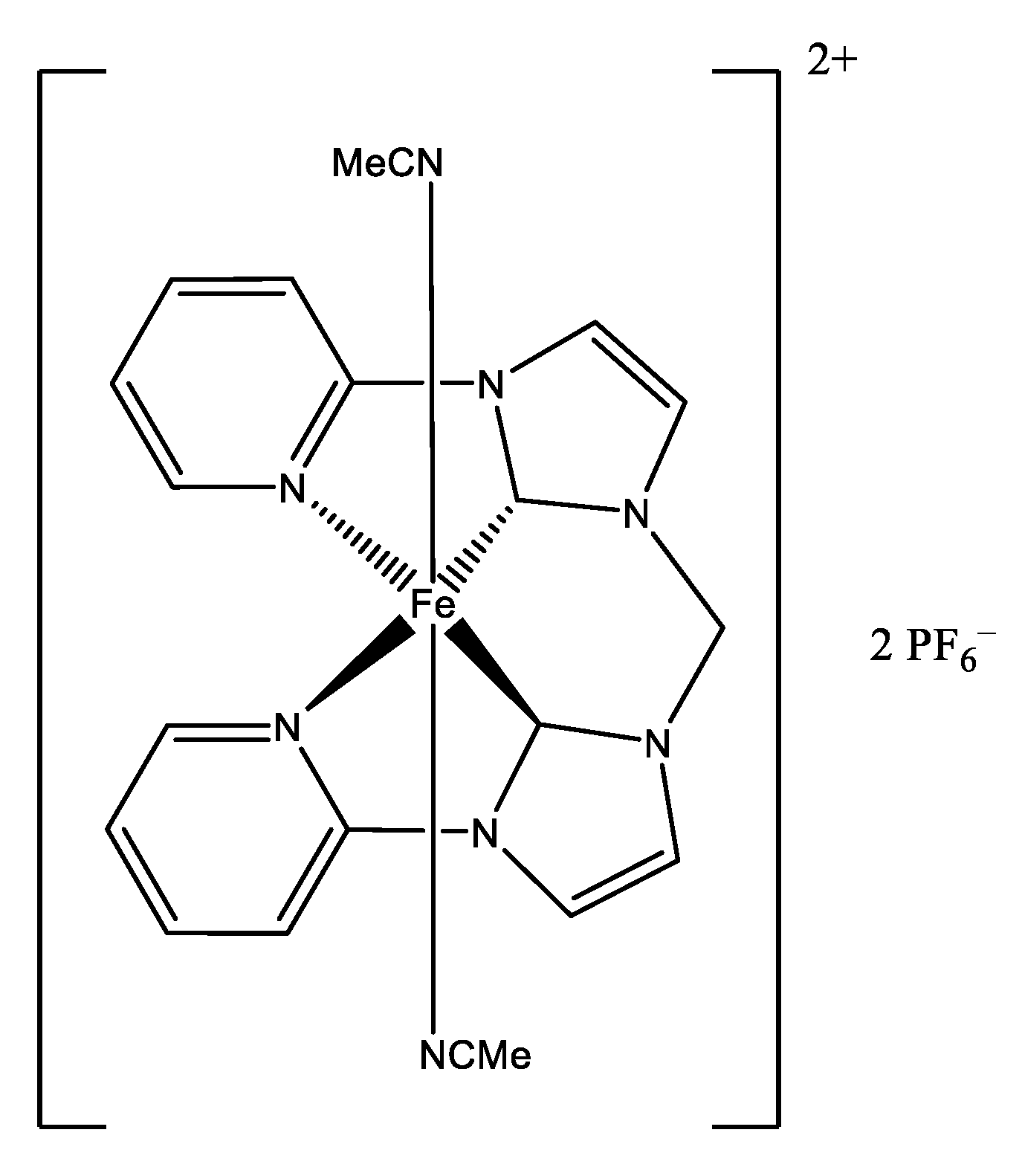
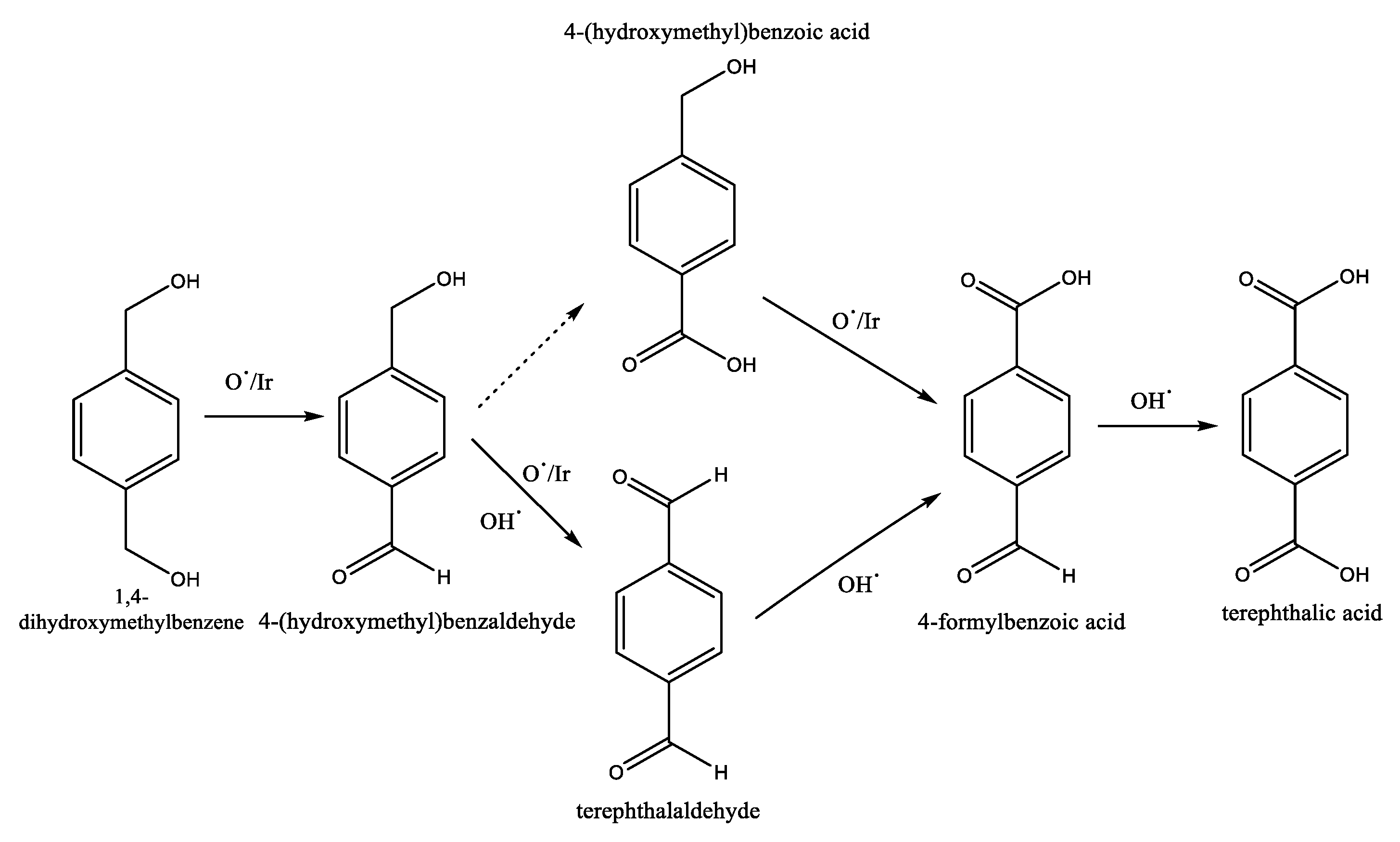


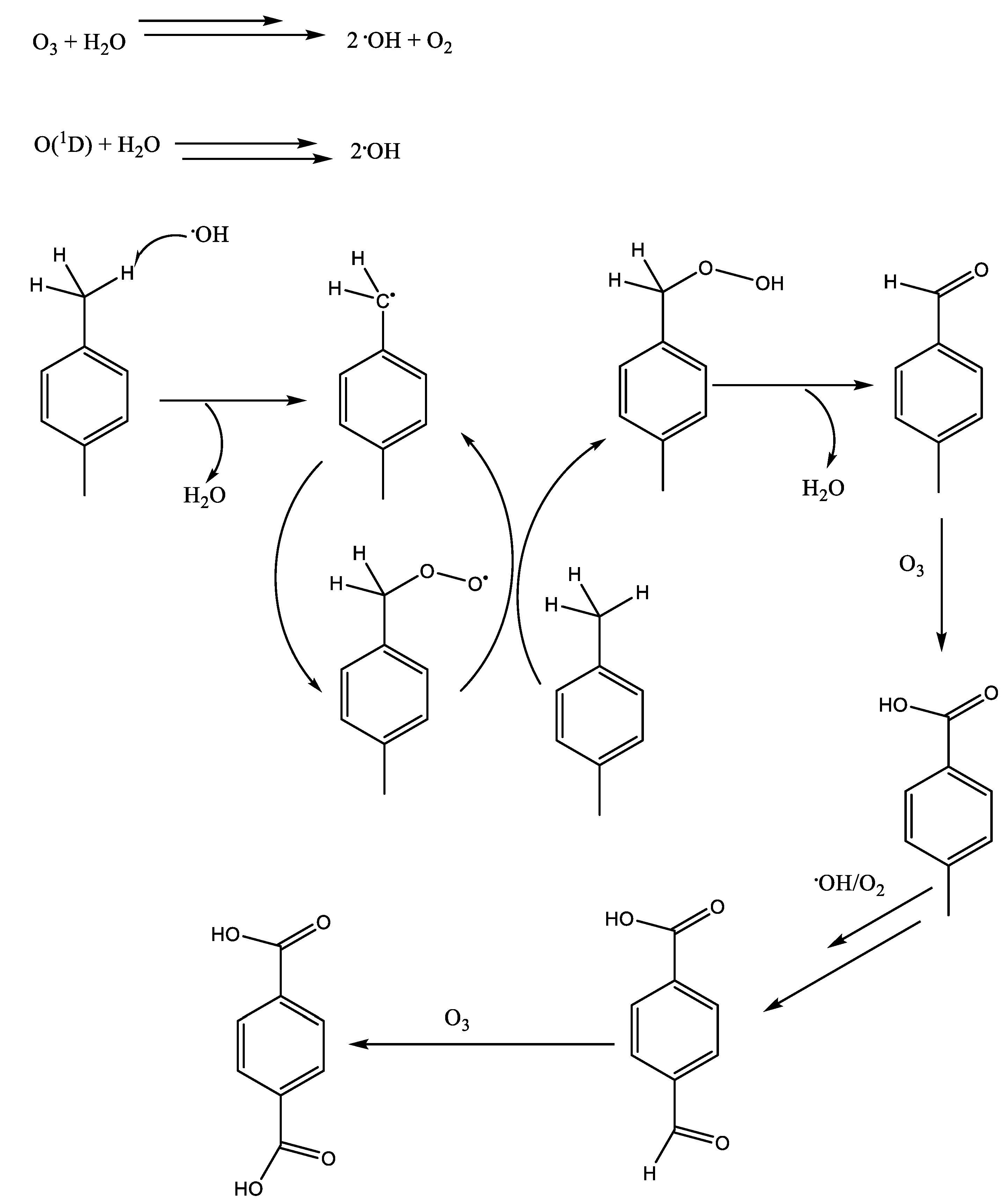
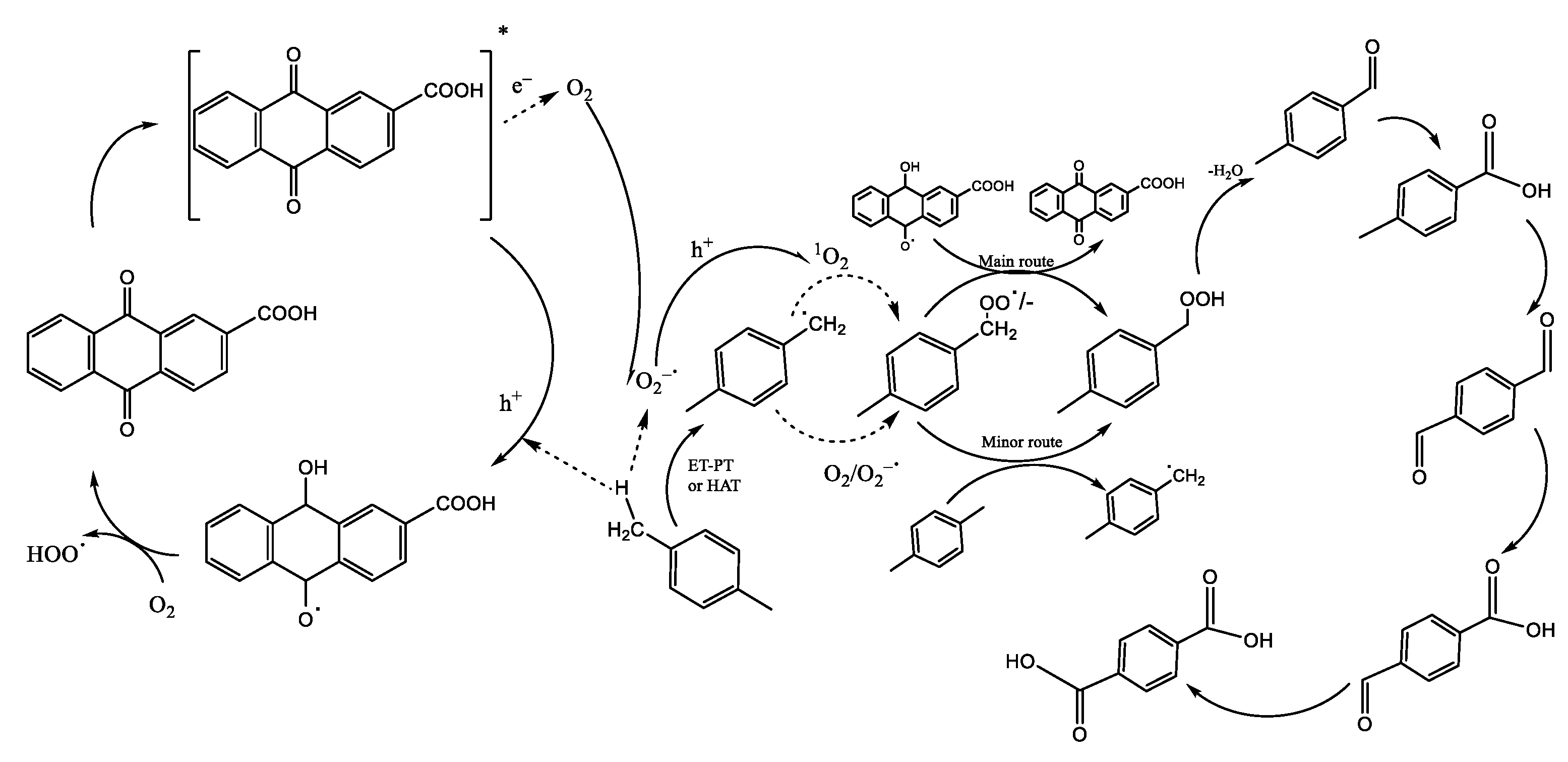

| Authors | Reaction Conditions | Substrate | Main Product | Yield % | Ref |
|---|---|---|---|---|---|
| Wei et al. | 110 °C, Co(II) aza-crowned dihydroxamic acid complexes, diglyme, O2, 5 h | 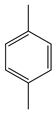 |  | 38 (a) | [21] |
| Wang and Tong | Reflux, acetic acid, CoAc + MnAc + NHPI, O2, 14 h | 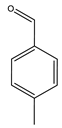 |  | 91 (b) | [22] |
| Mendes et al. | 35 °C, NCMe, H2O2, nitric acid (10:1 additive: catalyst), 5 min | 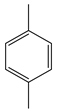 |  | 22 (c) | [23] |
| Goulas et al. | 100 °C, O2, Ir/C, 20 h | 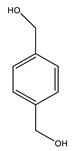 | 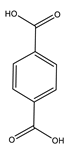 | 76 (c) | [27] |
| Pan et al. | 80 °C, CoAc, acetic acid, O3, 6 h | 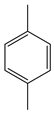 | 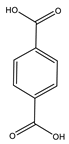 | 64 (a) | [28] |
| Hwang et al. | r.t., NCMe + H2O, O2 + O3, | 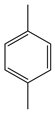 |  | 94 (a) | [31] |
| Authors | Reaction Conditions | Substrate | Main Product | Yield % | Ref |
|---|---|---|---|---|---|
| Wang et al. | 80 °C, vanadium(IV) C-scorpionate@CNT, 5 h MW irradiation, solvent-free, TBHP 70%, nitric acid (10:1 additive: catalyst) | 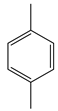 | 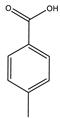 | 43 (a) | [43] |
| Samanta and Srivastava | 25 °C, FeVO4:g-C3N4, 4 h, H2O2 30%, 250 W high-pressure visible lamp, NCMe | 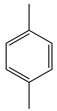 |  | 34 (a) | [44] |
| Nicolae et al. | 100 °C, Mn/Fe/O_CIT, TBHP 70%, 24 h, NCMe | 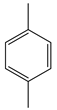 |  | 93 (b) | [45] |
| Ying Lo et al. | 30 °C, Cu-MOF, H2O2 30%, NCMe, 5 h | 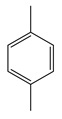 |  | 85 (b) | [46] |
| Xu et al. | 150 °C, NCMe, O2, Co-BTC + NHPI, 12 h | 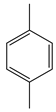 | 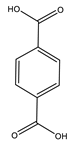 | 96 (b) | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapa, H.M.; Martins, L.M.D.R.S. p-Xylene Oxidation to Terephthalic Acid: New Trends. Molecules 2023, 28, 1922. https://doi.org/10.3390/molecules28041922
Lapa HM, Martins LMDRS. p-Xylene Oxidation to Terephthalic Acid: New Trends. Molecules. 2023; 28(4):1922. https://doi.org/10.3390/molecules28041922
Chicago/Turabian StyleLapa, Hugo M., and Luísa M. D. R. S. Martins. 2023. "p-Xylene Oxidation to Terephthalic Acid: New Trends" Molecules 28, no. 4: 1922. https://doi.org/10.3390/molecules28041922
APA StyleLapa, H. M., & Martins, L. M. D. R. S. (2023). p-Xylene Oxidation to Terephthalic Acid: New Trends. Molecules, 28(4), 1922. https://doi.org/10.3390/molecules28041922







