Optimization of Microalgal Biomass Production in Vertical Tubular Photobioreactors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgae Strains
2.2. Preincubation Conditions
2.3. Experimental Setup
2.4. Analytical Methods
2.5. Statistical Analysis
3. Results and Discussion
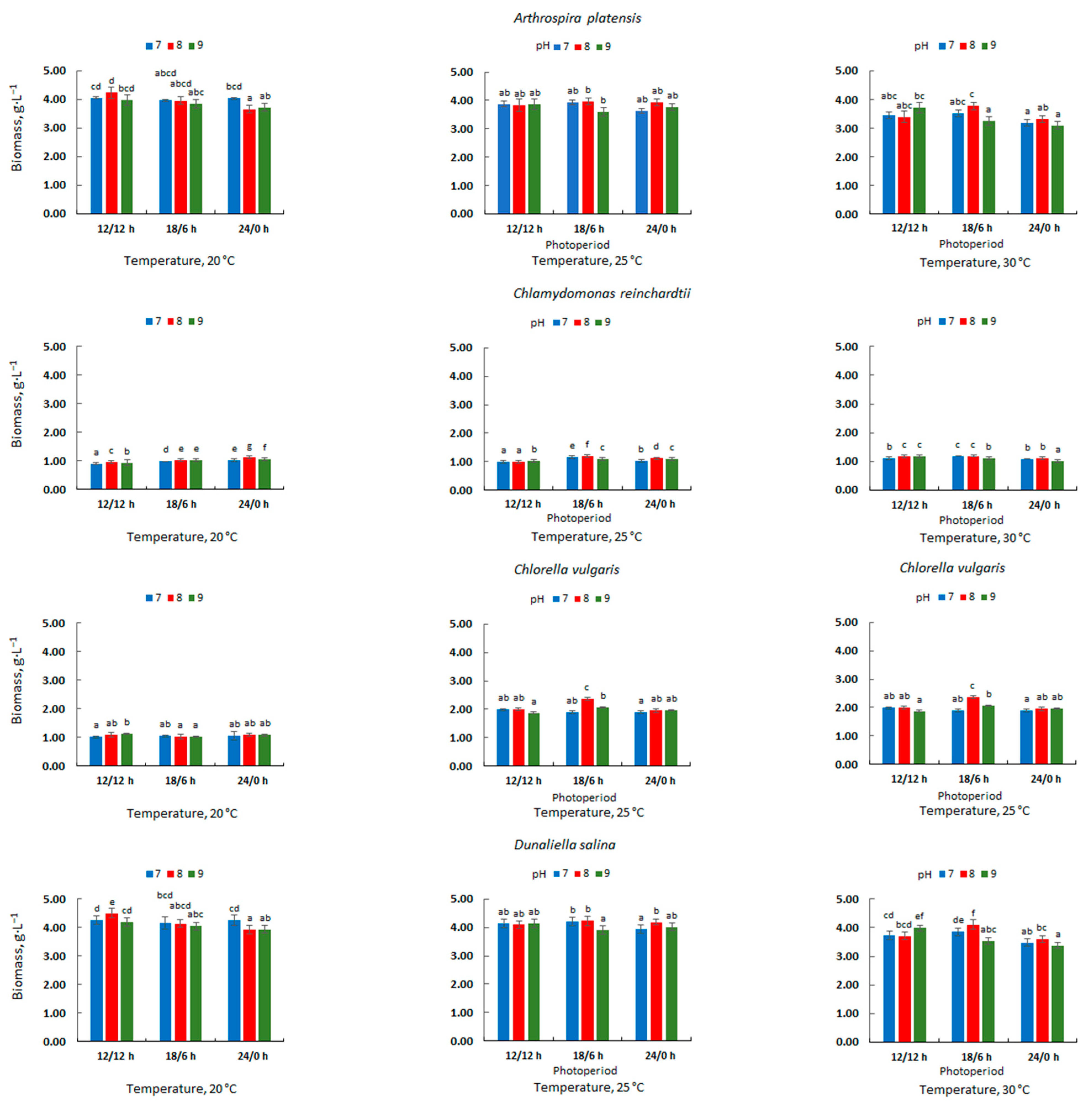
3.1. Microalgal Biomass Concentration and Productivity
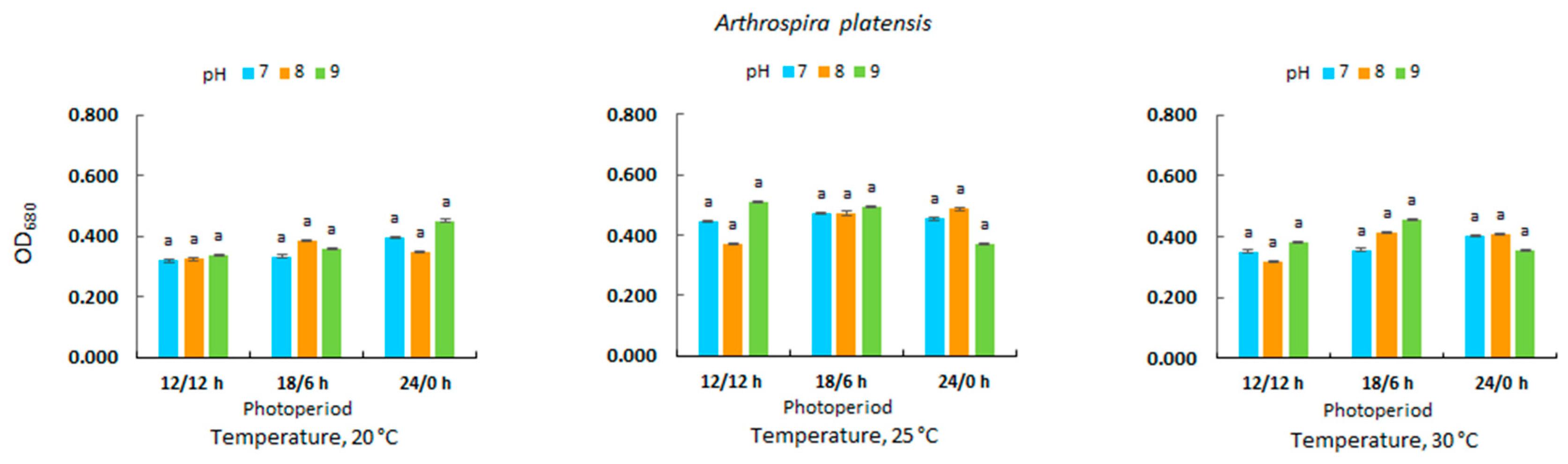
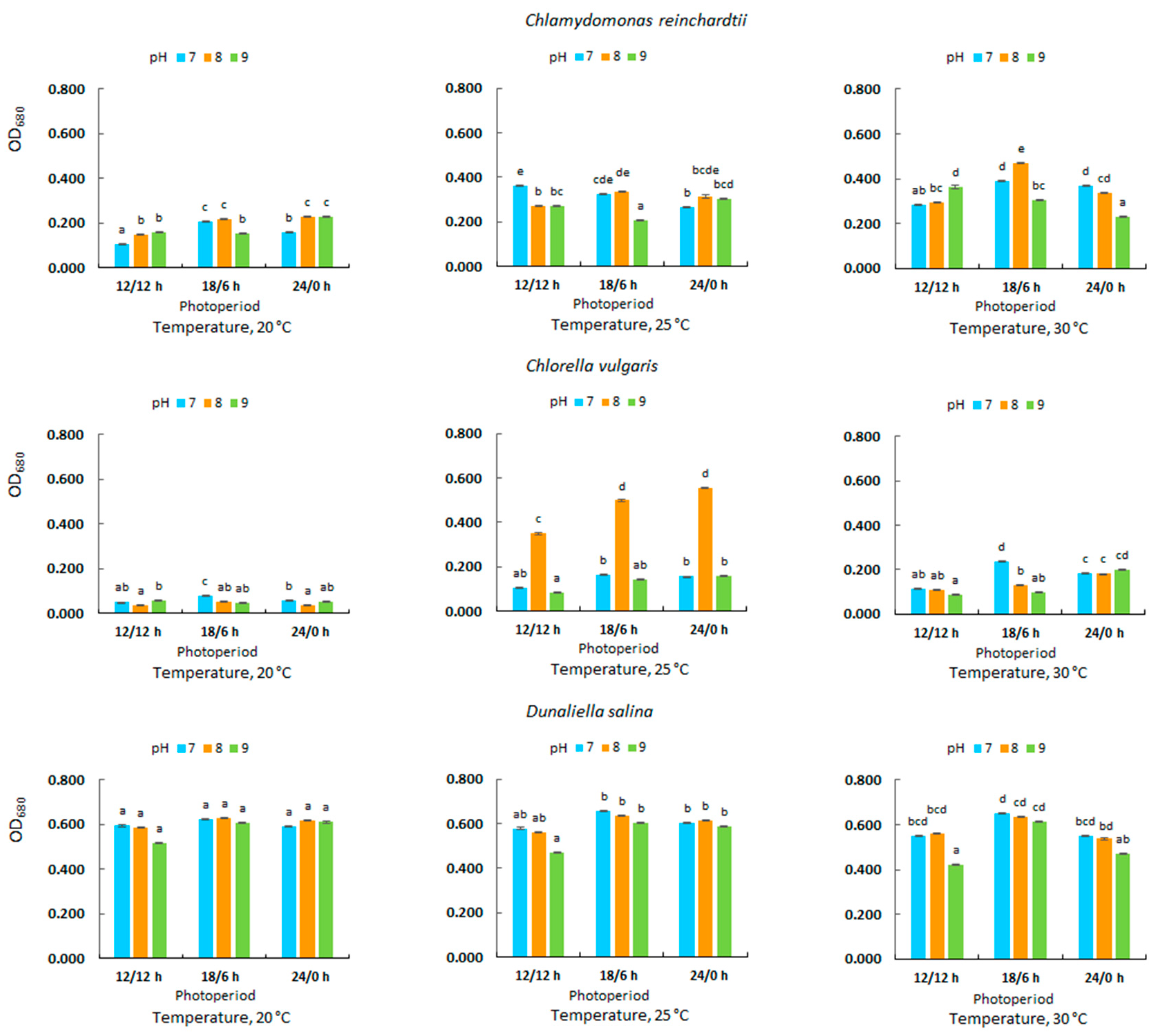
3.2. Optical Density in Microalgal Culture
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luo, Z.; Zhou, J. Thermal Conversion of Biomass. In Handbook of Climate Change Mitigation; Chen, W.Y., Seiner, J., Suzuki, T., Lackner, M., Eds.; Springer: New York, NY, USA, 2012. [Google Scholar] [CrossRef]
- Driver, T.; Bajhaiya, A.; Pittman, J.K. Potential of Bioenergy Production from Microalgae. Curr. Sustain. Renew. Energy Rep. 2014, 1, 94–103. [Google Scholar] [CrossRef] [Green Version]
- Baležentis, T.; Streimikiene, D.; Zhang, T.; Liobikiene, G. The role of bioenergy in greenhouse gas emission reduction in EU countries: An Environmental Kuznets Curve modelling. Resour. Conserv. Recycl. 2019, 142, 225–231. [Google Scholar] [CrossRef]
- Garba, A. Biomass Conversion Technologies for Bioenergy Generation: An Introduction. In Biotechnological Applications of Biomass; Basso, T.P., Basso, T.O., Basso, L.C., Eds.; IntechOpen: London, UK, 2020; Available online: https://www.intechopen.com/chapters/73832 (accessed on 18 February 2023). [CrossRef]
- Hoang, D.L.; Davis, C.; Moll, H.C.; Nonhebel, S. Can Multiple Uses of Biomass Limit the Feedstock Availability for Future Biogas Production? An Overview of Biogas Feedstocks and Their Alternative Uses. Energies 2020, 13, 2747. [Google Scholar] [CrossRef]
- Nethravathy, M.U.; Mehar, J.G.; Mudliar, S.N.; Shekh, A.Y. Recent Advances in Microalgal Bioactives for Food, Feed, and Healthcare Products: Commercial Potential, Market Space, and Sustainability. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1882–1897. [Google Scholar] [CrossRef] [Green Version]
- Lucakova, S.; Branyikova, I.; Hayes, M. Microalgal Proteins and Bioactives for Food, Feed, and Other Applications. Appl. Sci. 2022, 12, 4402. [Google Scholar] [CrossRef]
- Vyas, S.; Patel, A.; Risse, E.N.; Krikigianni, E.; Rova, U.; Christakopoulos, P.; Matsakas, L. Biosynthesis of microalgal lipids, proteins, lutein, and carbohydrates using fish farming wastewater and forest biomass under photoautotrophic and heterotrophic cultivation. Bioresour. Technol. 2022, 359, 127494. [Google Scholar] [CrossRef]
- Sharma, P.K.; Saharia, M.; Srivstava, R.; Kumar, S.; Sahoo, L. Tailoring Microalgae for Efficient Biofuel Production. Front. Mar. Sci. 2018, 5, 382. [Google Scholar] [CrossRef]
- Tiwari, A.; Kiran, T. Biofuels from Microalgae. In Advances in Biofuels and Bioenergy; Nageswara-Rao, M., Soneji, J.R., Eds.; IntechOpen: London, UK, 2018; Available online: https://www.intechopen.com/chapters/59007 (accessed on 18 February 2023). [CrossRef] [Green Version]
- Zewdie, D.T.; Ali, A.Y. Cultivation of microalgae for biofuel production: Coupling with sugarcane-processing factories. Energ. Sustain. Soc. 2020, 10, 27. [Google Scholar] [CrossRef]
- Hawrot-Paw, M.; Ratomski, P.; Koniuszy, A.; Golimowski, W.; Teleszko, M.; Grygier, A. Fatty Acid Profile of Microalgal Oils as a Criterion for Selection of the Best Feedstock for Biodiesel Production. Energies 2021, 14, 7334. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.; Xu, G.; Li, F.; Li, X. A review on biodiesel production from microalgae: Influencing parameters and recent advanced technologies. Front. Microbiol. 2022, 13, 970028. [Google Scholar] [CrossRef]
- Limongi, A.R.; Viviano, E.; De Luca, M.; Radice, R.P.; Bianco, G.; Martelli, G. Biohydrogen from Microalgae: Production and Applications. Appl. Sci. 2021, 11, 1616. [Google Scholar] [CrossRef]
- Li, S.; Li, F.; Zhu, X.; Liao, Q.; Chang, J.-S.; Ho, S.-H. Biohydrogen production from microalgae for environmental sustainability. Chemosphere 2022, 291, 132717. [Google Scholar] [CrossRef] [PubMed]
- Shahi, T.; Beheshti, B.; Zenouzi, A.; Almasi, M. Bio-oil production from residual biomass of microalgae after lipid extraction: The case of Dunaliella sp. Biocatal. Agric. Biotechnol. 2020, 23, 101494. [Google Scholar] [CrossRef]
- Lakatos, G.E.; Ranglová, K.; Manoel, J.C.; Grivalský, T.; Kopecký, J.; Masojídek, J. Bioethanol production from microalgae polysaccharides. Folia Microbiol. 2019, 64, 627–644. [Google Scholar] [CrossRef]
- Ranga Rao, A.; Sarada, R.; Ravishankar, G.A. Influence of CO2 on growth and hydrocarbon production in Botryococcus braunii. J. Microbiol. Biotechnol. 2007, 17, 414–419. [Google Scholar]
- Milano, J.; Ong, H.C.; Masjuki, H.H.; Chong, W.T.; Lam, M.K.; Loh, P.K.; Vellayan, V. Microalgae biofuels as an alternative to fossil fuel for power generation. Renew. Sustain. Energy Rev. 2016, 58, 180–197. [Google Scholar] [CrossRef]
- Torres, A.; Padrino, S.; Brito, A.; Díaz, L. Biogas production from anaerobic digestion of solid microalgae residues generated on different processes of microalgae-to-biofuel production. Biomass Conv. Bioref. 2021. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Bezerra, P.Q.M.; Moreira, J.B.; Molina, A.N.; de Morais, M.G. Biogas from Microalgae: Production Approaches and Strategies for Process Optimization. In Role of Microbes in Industrial Products and Processes; Kumar, S., Kumar, N., Shahid-ul-Islam, Eds.; Scrivener Publishing: Beverly, MA, USA, 2022. [Google Scholar] [CrossRef]
- Wu, N.; Moreira, C.M.; Zhang, Y.; Doan, N.; Yang, S.; Phlips, E.J.; Svoronos, S.A.; Pullammanappallil, P.C. Techno-Economic Analysis of Biogas Production from Microalgae through Anaerobic Digestion. In Anaerobic Digestion; Rajesh, B.J., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Mussgnug, J.H.; Klassen, V.; Schlüter, A.; Kruse, O. Microalgae as substrates for fermentative biogas production in a combined biorefinery concept. J. Biotechnol. 2010, 150, 51–56. [Google Scholar] [CrossRef]
- Ward, A.J.; Lewis, D.M.; Green, F.B. Anaerobic digestion of algae biomass: A review. Algal Res. 2014, 5, 204–214. [Google Scholar] [CrossRef]
- Klassen, V.; Blifernez-Klassen, O.; Wibberg, D.; Winkler, A.; Kalinowski, J.; Posten, C.; Kruse, O. Highly efficient methane generation from untreated microalgae biomass. Biotechnol. Biofuels 2017, 10, 186. [Google Scholar] [CrossRef] [Green Version]
- Veerabadhran, M.; Gnanasekaran, D.; Wei, J.; Yang, F. Anaerobic digestion of microalgal biomass for bioenergy production, removal of nutrients and microcystin: Current status. J. Appl. Microbiol. 2021, 131, 1639–1651. [Google Scholar] [CrossRef] [PubMed]
- Baltrėnas, P.; Misevičius, A. Biogas production experimental research using algae. J. Environ. Health Sci. Eng. 2015, 13, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueroa-Torres, G.M.; Pittman, J.K.; Theodoropoulos, C. Optimisation of microalgal cultivation via nutrient-enhanced strategies: The biorefinery paradigm. Biotechnol. Biofuels 2021, 14, 64. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, M.; Vecchi, V.; Barera, S.; Dall’Osto, L. Biomass from microalgae: The potential of domestication towards sustainable biofactories. Microb. Cell Fact. 2018, 17, 173. [Google Scholar] [CrossRef] [Green Version]
- Skorupskaite, V.; Makareviciene, V.; Levisauskas, D. Optimization of mixotrophic cultivation of microalgae Chlorella sp. for biofuel production using response surface methodology. Algal Res. 2015, 7, 45–50. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Freitas, B.C.B.; Santos, T.D.; Mitchell, B.G.; Morais, M.G. Chapter 9—Open pond systems for microalgal culture. In Biomass, Biofuels, Biochemicals, Biofuels from Algae, 2nd ed.; Pandey, A., Chang, J.-S., Soccol, C.R., Lee, D.-J., Chisti, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 199–223. [Google Scholar] [CrossRef]
- Al-Dailami, A.; Koji, I.; Ahmad, I.; Goto, M. Potential of Photobioreactors (PBRs) in Cultivation of Microalgae. J. Adv. Res. App. Sc. Eng. Tech. 2022, 27, 32–44. [Google Scholar] [CrossRef]
- Ozkurt, I. Qualifying of safflower and algae for energy. Energy Educ. Sci. Technol. Part A 2009, 23, 145–151. [Google Scholar]
- Izadpanah, M.; Gheshlaghi, R.; Mahdavi, M.A.; Elkamel, A. Effect of light spectrum on isolation of microalgae from urban wastewater and growth characteristics of subsequent cultivation of the isolated species. Algal Res. 2018, 29, 154–158. [Google Scholar] [CrossRef]
- Maltsev, Y.; Maltseva, K.; Kulikovskiy, M.; Maltseva, S. Influence of Light Conditions on Microalgae Growth and Content of Lipids, Carotenoids, and Fatty Acid Composition. Biology 2021, 10, 1060. [Google Scholar] [CrossRef]
- Baer, S.; Heining, M.; Schwerna, P.; Buchholz, R.; Hübner, H. Optimization of spectral light quality for growth and product formation in different microalgae using a continuous photobioreactor. Algal Res. 2016, 14, 109–115. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Grobbelaar, J.U. Algal nutrition. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Richmond, A., Ed.; Blackwell: Oxford, UK, 2004; pp. 97–115. [Google Scholar]
- Carvalho, A.P.; Silva, S.O.; Baptista, J.M.; Malcata, F.X. Light requirements in microalgal photobioreactors: An overview of biophotonic aspects. Appl. Microbiol. Biotechnol. 2011, 89, 1275–1288. [Google Scholar] [CrossRef]
- Krzemińska, I.; Pawlik-Skowrońska, B.; Trzcińska, M.; Tys, J. Influence of photoperiods on the growth rate and biomass productivity of green microalgae. Bioprocess Biosyst. Eng. 2014, 37, 735–741. [Google Scholar] [CrossRef] [Green Version]
- Blanken, W.; Cuaresma, M.; Wijffels, R.H.; Janssen, M. Cultivation of microalgae on artificial light comes at a cost. Algal Res. 2013, 2, 333–340. [Google Scholar] [CrossRef]
- Radzun, K.A.; Wolf, J.; Jakob, G.; Zhang, E.; Stephens, E.; Ross, I.; Hankamer, B. Automated nutrient screening system enables high-throughput optimisation of microalgae production conditions. Biotechnol. Biofuels 2015, 8, 65. [Google Scholar] [CrossRef] [Green Version]
- Gao, K.; Xue, C.; Yang, M.; Li, L.; Qian, P.; Gao, Z.; Gao, Z.; Deng, X. Optimization of light intensity and photoperiod for growing Chlorella sorokiniana on cooking cocoon wastewater in a bubble-column bioreactor. Algal Res. 2022, 62, 102612. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of temperature and light on the growth of algae species: A review. Renew. Sust. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Bechet, Q.; Laviale, M.; Arsapin, N.; Bonnefond, H.; Bernard, O. Modeling the impact of high temperatures on microalgal viability and photosynthetic activity. Biotechnol. Biofuels 2017, 10, 136. [Google Scholar] [CrossRef] [Green Version]
- Morales, M.; Sánchez, L.; Revah, S. The impact of environmental factors on carbon dioxide fixation by microalgae. FEMS Microbiol. Lett. 2018, 365, fnx262. [Google Scholar] [CrossRef] [Green Version]
- Bleeke, F.; Rwehumbiza, V.M.; Winckelmann, D.; Klöck, G. Isolation and Characterization of New Temperature Tolerant Microalgal Strains for Biomass Production. Energies 2014, 7, 7847–7856. [Google Scholar] [CrossRef] [Green Version]
- Serra-Maia, R.; Bernard, O.; Gonçalves, A.; Bensalem, S.; Lopes, F. Influence of temperature on Chlorella vulgaris growth and mortality rates in a photobioreactor. Algal Res. 2016, 18, 352–359. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T. Potential of using organic fertilizer to cultivate Chlorella vulgaris for biodiesel production. Appl. Energy 2012, 94, 303–308. [Google Scholar] [CrossRef]
- Chowdury, K.; Nahar, N.; Deb, U. The Growth Factors Involved in Microalgae Cultivation for Biofuel Production: A Review. Comput. Water Energy Environ. Eng. 2020, 9, 185–215. [Google Scholar] [CrossRef]
- Gao, K. Approaches and involved principles to control pH/pCO2 stability in algal cultures. J. Appl. Phycol. 2021, 33, 3497–3505. [Google Scholar] [CrossRef]
- Shekh, A.; Sharma, A.; Schenk, P.M.; Kumar, G.; Mudliar, S. Microalgae cultivation: Photobioreactors, CO2 utilization, and value-added products of industrial importance. J. Chem. Technol. Biotechnol. 2022, 97, 1064–1085. [Google Scholar] [CrossRef]
- Ismaiel, M.M.S.; El-Ayouty, Y.M.; Piercey-Normore, M. Role of pH on antioxidants production by Spirulina (Arthrospira) platensis. Braz. J. Microbiol. 2016, 47, 298–304. [Google Scholar] [CrossRef] [Green Version]
- Sąsiadek, M.; Hawrot-Paw, M. Microalgae biomass production in vertical tubular photobioreactors. In Improvement of Technologies Agricultural Production, Including Renewable Energy, Taking into Account the Requirements of Sustainable Development; Romaniuk, W., Ed.; ITP: Warszawa-Falenty, Poland, 2021. (In Polish) [Google Scholar]
- Guillard, R.R.L.; Ryther, J.J. Studies of marine planktonic diatoms, I. Cyclotella nana Hustedt and Detonula confervacea (cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Zhou, T.; Cao, L.; Cai, Y.; Wang, Y.; Cui, X.; Yan, H.; Ruan, R.; Zhang, Q. Effects of Culture Conditions on the Performance of Arthrospira platensis and Its Production of Exopolysaccharides. Foods 2022, 11, 2020. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Chirasuwan, N.; Srinorasing, T.; Attasat, S.; Nopharatana, A.; Bunnag, B. Enhanced biomass and phycocyanin production of Arthrospira (Spirulina) platensis by a cultivation management strategy: Light intensity and cell concentration. Bioresour. Technol. 2022, 343, 126077. [Google Scholar] [CrossRef]
- Madkour, F.F.; Kamil, A.E.-W.; Nasr, H.S. Production and nutritive value of Spirulina platensis in reduced cost media. Egypt. J. Aquat. Res. 2012, 38, 51–57. [Google Scholar] [CrossRef] [Green Version]
- De Castro, G.F.P.S.; Rizzo, R.F.; Passos, T.S.; dos Santos, B.N.C.; Dias, D.S.; Domingues, J.R.; Araújo, K.G.L. Biomass production by Arthrospira platensis under different culture conditions. Food Science and Technology Biomass production by Arthrospira platensis under different culture conditions. Food Sci. Technol. 2015, 35, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Vargas, S.R.; Zaiat, M.; Calijuri, M.d. Influence of Sulfur and Light Intensity in Nutrient Removal, and Hydrogen and Ethanol Production by Improved Biomass of Chlamydomonas reinhardtii in Batch Anaerobic Photobioreactors. Bioenerg. Res. 2022, 15, 218–229. [Google Scholar] [CrossRef]
- Abd El Baky, H.; El Baroty, G.S. Optimization cultivation of Chlamydomonas reinhardtii in a tubular photobioreactor (2000 Liter) for biomass and green bioenergy (biodiesel) production. Not. Bot. Horti Agrobot. 2020, 48, 1439–1457. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of CO2 concentration on algal growth: A review. Renew. Sust. Energ. Rev. 2014, 38, 172–179. [Google Scholar] [CrossRef]
- Filali, R.; Tian, H.; Micheils, E.; Taidi, B. Evaluation of the Growth Performance of Microalgae Based on Fine pH Changes. Austin J. Biotechnol. Bioeng. 2021, 8, 1109. [Google Scholar]
- Ratomski, P.; Hawrot-Paw, M. Production of Chlorella vulgaris Biomass in Tubular Photobioreactors during Different Culture Conditions. Appl. Sci. 2021, 11, 3106. [Google Scholar] [CrossRef]
- Xu, Y.; Ibrahim, I.M.; Harvey, P.J. The influence of photoperiod and light intensity on the growth and photosynthesis of Dunaliella salina (chlorophyta) CCAP 19/30. Plant Physiol. Biochem. 2016, 106, 305–315. [Google Scholar] [CrossRef] [Green Version]
- Ras, M.; Steyer, J.P.; Bernard, O. Temperature effect on microalgae: A crucial factor for outdoor production. Rev. Environ. Sci. Biotechnol. 2013, 12, 153–164. [Google Scholar] [CrossRef] [Green Version]
- Gastelum-Franco, J.; Esparza-Leal, H.; García-Ulloa, M.; López-Álvarez, E.; Muy-Rangel, M.; Pérez-Rubio, V.; Ulloa-Mercado, R.; Montiel-Montoya, J. Preliminary evaluation of the green microalga Dunaliella salina as a potential feedstock for biodiesel: Effect of molasses on growth and lipid profile. Lat. Am. J. Aquat. Res. 2021, 49, 763–772. [Google Scholar] [CrossRef]
- Lv, H.; Wang, Q.E.; Wang, S.; Qi, B.; He, J.; Jia, S. Enhancing biomass production of Dunaliella salina via optimized combinational application of phytohormones. Aquaculture 2019, 503, 146–155. [Google Scholar] [CrossRef]
- Morowvat, M.H.; Ghasemi, Y. Culture medium optimization for enhanced β-carotene and biomass production by Dunaliella salina in mixotrophic culture. Biocatal. Agric. Biotechnol. 2016, 7, 217–223. [Google Scholar] [CrossRef]
- Ahmed, R.A.; He, M.; Aftab, R.A.; Zheng, S.; Nagi, M.; Bakri, R.; Wang, C. Bioenergy application of Dunaliella salina SA 134 grown at various salinity levels for lipid production. Sci. Rep. 2017, 7, 8118. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.; Park, J.M.; Gim, G.H.; Jeong, S.H.; Kang, C.M.; Kim, D.J.; Kim, S.W. Optimization of culture conditions and comparison of biomass productivity of three green algae. Bioprocess Biosyst. Eng. 2012, 35, 19–27. [Google Scholar] [CrossRef]
- Saxena, R.; Rodríguez-Jasso, R.M.; Chávez-Gonzalez, M.L.; Aguilar, C.N.; Quijano, G.; Ruiz, H.A. Strategy Development for Microalgae Spirulina platensis Biomass Cultivation in a Bubble Photobioreactor to Promote High Carbohydrate Content. Fermentation 2022, 8, 374. [Google Scholar] [CrossRef]
- Hussin, A.A.; To, S.W.; Sani, M.H.; Amin, M.F.M.; Kamaroddin, M.F. Optimisation and growth kinetic analysis of Microalgae, Arthrospira platensis in 2-L Photobioreactors. In Proceedings of the 3rd International Conference on Tropical Resources and Sustainable Sciences, Kelantan, Malezja, 14–15 July 2021; Volume 842, p. 012036. [Google Scholar] [CrossRef]
- Bao, Y.; Wen, S.; Cong, W.; Wu, X.; Ning, Z. An Optical-Density-Based Feedback Feeding Method for Ammonium Concentration Control in Spirulina platensis Cultivation. J. Microbiol. Biotechnol. 2012, 22, 967–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Slavens, S.; Crunkleton, D.W.; Johannes, T.W. Interactive effect of light quality and temperature on Chlamydomonas reinhardtii growth kinetics and lipid synthesis. Algal Res. 2021, 53, 102127. [Google Scholar] [CrossRef]
- Zhu, Z.; Cao, H.; Li, X.; Rong, J.; Cao, X.; Tian, J. A Carbon Fixation Enhanced Chlamydomonas reinhardtii Strain for Achieving the Double-Win Between Growth and Biofuel Production Under Non-stressed Conditions. Front. Bioeng. Biotechnol. 2021, 8, 603513. [Google Scholar] [CrossRef]
- Ammar, S.H. Cultivation of Microalgae Chlorella vulgaris in Airlift photobioreactor for Biomass Production using commercial NPK Nutrients. Al-Khawarizmi Eng. J. 2016, 12, 90–99. [Google Scholar]
- Deniz, I. Determination of growth conditions for Chlorella vulgaris. Mar. Sci. Tech. Bull. 2020, 9, 114–117. [Google Scholar] [CrossRef]
- Akter, R.; Hossain, S.M.M.; Zhe, W.; Kermanee, P.; Juntawong, N. Enhanced lipid production in Dunaliella salina grown under high light intensity by shifting the culture from high to low nitrogen concentration. Adv. Environ. Biol. 2016, 10, 18–29. [Google Scholar]
- Demirel, Z. Monitoring of growth and biochemical composition of Dunaliella salina and Dunaliella polymorpha in different photobioreactors. Aquat. Res. 2022, 5, 136–145. [Google Scholar] [CrossRef]
- Saha, S.K.; Kazipet, N.; Murray, P. The Carotenogenic Dunaliella salina CCAP 19/20 Produces Enhanced Levels of Carotenoid under Specific Nutrients Limitation. Biomed Res. Int. 2018, 2018, 7532897. [Google Scholar] [CrossRef] [Green Version]
- Guidi, F.; Gojkovic, Z.; Venuleo, M.; Assunçao, P.A.C.J.; Portillo, E. Long-Term Cultivation of a Native Arthrospira platensis (Spirulina) Strain in Pozo Izquierdo (Gran Canaria, Spain): Technical Evidence for a Viable Production of Food-Grade Biomass. Processes 2021, 9, 1333. [Google Scholar] [CrossRef]
- Banerjee, S.; Ray, A.; Das, D. Optimization of Chlamydomonas reinhardtii cultivation with simultaneous CO2 sequestration and biofuels production in a biorefinery framework. Sci. Total Environ. 2021, 762, 143080. [Google Scholar] [CrossRef]
- Kong, Q.X.; Li, L.; Martinez, B.; Chen, P.; Ruan, R. Culture of Microalgae Chlamydomonas reinhardtii in Wastewater for Biomass Feedstock Production. Appl. Biochem. Biotechnol. 2010, 160, 9. [Google Scholar] [CrossRef] [PubMed]
- Anyanwu, R.C.; Rodriguez, C.; Durrant, A.; Olabi, A.G. Evaluation of Growth Rate and Biomass Productivity of Scenedesmus quadricauda and Chlorella vulgaris under Different LED Wavelengths and Photoperiods. Sustainability 2022, 14, 6108. [Google Scholar] [CrossRef]
- Amini Khoeyi, Z.; Seyfabadi, J.; Ramezanpour, Z. Effect of light intensity and photoperiod on biomass and fatty acid composition of the microalgae, Chlorella vulgaris. Aquacult. Int. 2012, 20, 41–49. [Google Scholar] [CrossRef]
- Abarna, K.M.; Velu, R.; Padmavathy, P.; Jawahar, P.; Uma, A.; Kalidas, C.; Shukla, S.P. Effect of seaweed-based liquid extracts on biomass production and lipid accumulation in Nannochloropsis oculata and Dunaliella salina. Turk. J. Bot. 2022, 46, 4. [Google Scholar] [CrossRef]
- Borovkov, A.B.; Gudvilovich, I.N.; Avsiyan, A.L. Scale-up of Dunaliella salina cultivation: From strain selection to open ponds. J. Appl. Phycol. 2020, 32, 1545–1558. [Google Scholar] [CrossRef]
- Barten, R.; Djohan, Y.; Evers, W.; Wijffels, R.; Barbosa, M. Towards industrial production of microalgae without temperature control: The effect of diel temperature fluctuations on microalgal physiology. J. Biotechnol. 2021, 336, 56–63. [Google Scholar] [CrossRef]
- Maroneze, M.M.; Siqueira, S.F.; Vendruscolo, R.G.; Wagner, R.; de Menezes, C.R.; Zepka, L.Q.; Jacob-Lopes, E. The role of photoperiods on photobioreactors—A potential strategy to reduce costs. Bioresour. Technol. 2016, 219, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Atta, M.; Idris, A.; Bukhari, A.; Wahidin, S. Intensity of blue LED light: A potential stimulus for biomass and lipid content in fresh water microalgae Chlorella vulgaris. Bioresour. Technol. 2013, 148, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Fujihara, Y.; Vavricka, C.J.; Chang, J.-S.; Hasunuma, T.; Kondo, A. Light/dark cycling causes delayed lipid accumulation and increased photoperiod-based biomass yield by altering metabolic flux in oleaginous Chlamydomonas sp. Biotechnol. Biofuels 2019, 12, 39. [Google Scholar] [CrossRef]
- Moazami-Goudarzi, M.; Colman, B. Changes in carbon uptake mechanisms in two green marine algae by reduced seawater pH. J. Exp. Mar. Biol. Ecol. 2012, 413, 94–99. [Google Scholar] [CrossRef]
- Elalami, D.; Oukarroum, A.; Barakat, A. Anaerobic digestion and agronomic applications of microalgae for its sustainable valorization. RSC Adv. 2021, 11, 26444–26462. [Google Scholar] [CrossRef]
- Schagerl, M.; Siedler, R.; Konopáčová, E.; Ali, S.S. Estimating Biomass and Vitality of Microalgae for Monitoring Cultures: A Roadmap for Reliable Measurements. Cells 2022, 11, 2455. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.N.; Zachleder, V.; Vítová, M.; Barbosa, M.J.; Bišová, K. Starch Production in Chlamydomonas reinhardtii through Supraoptimal Temperature in a Pilot-Scale Photobioreactor. Cells 2021, 10, 1084. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Singh, G.P.; Sharma, V.K. Effects of Culture Conditions on Growth and Biochemical Profile of Chlorella Vulgaris. J. Plant Pathol. Microb. 2012, 3, 1000131. [Google Scholar] [CrossRef] [Green Version]
- Ratomski, P.; Hawrot-Paw, M. Influence of Nutrient-Stress Conditions on Chlorella vulgaris Biomass Production and Lipid Content. Catalysts 2021, 11, 573. [Google Scholar] [CrossRef]
- Griffiths, M.J.; Garcin, C.; van Hille, R.P.; Harrison, S.T.L. Interference by pigment in the estimation of microalgal biomass concentration by optical density. J. Microbiol. Methods 2011, 85, 119–123. [Google Scholar] [CrossRef]
- Chioccioli, M.; Hankamer, B.; Ross, I.L. Flow Cytometry Pulse Width Data Enables Rapid and Sensitive Estimation of Biomass Dry Weight in the Microalgae Chlamydomonas reinhardtii and Chlorella vulgaris. PLoS ONE 2014, 9, e97269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srirangan, S.; Sauer, M.L.; Howard, B.; Dvora, M.; Dums, J.; Backman, P.; Sederoff, H. Interaction of Temperature and Photoperiod Increases Growth and Oil Content in the Marine Microalgae Dunaliella viridis. PLoS ONE 2015, 10, e0127562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Havlik, I.; Beutel, S.; Scheper, T.; Reardon, K.F. On-Line Monitoring of Biological Parameters in Microalgal Bioprocesses Using Optical Methods. Energies 2022, 15, 875. [Google Scholar] [CrossRef]

| Photobioreactor Capacity [L] | CO2 Dose | Light Intensity [µmol m−2 s−1] | Temperature [°C] | Photoperiod [h; Light/Dark] | pH |
|---|---|---|---|---|---|
| 2.5 | atmospheric concentration | 130 | 20 | 12/12 | 7 |
| 25 | 16/8 | 8 | |||
| 30 | 24/0 | 9 |
| Microalgae | Cultivation Conditions | Amount of Biomass [g·L−1] | Photobioreactor Type/Capacity [L] | References | ||
|---|---|---|---|---|---|---|
| Temperature [°C] | Photoperiod [h/h Light/Dark] | pH | ||||
| A. platensis | 20 | 12/12 | 8 | 4.24 | Tubular: 2.5 | This study |
| Not controlled | 13/11 | 9–10 | 0.93 | Bubble 10.1 | [72] | |
| Room temp. | 24/0 | 9 | 1.50 | Bubble: 2 | [73] | |
| 30 | 24/0 | 9.6 | 3.06 | Airlift: 2.5 | [74] | |
| Ch. reinchardtii | 30 | 18/6 | 7–8 | 1.19 | Tubular; 2.5 | This study |
| 24–32 | n.r 1 | n.r | 0.5–0.7 | Flask; 0.25 | [75] | |
| 32 | 24/0 | 6.2 | 1.07 | Flask; 0.5 | [60] | |
| 25 | 14/10 | n.r. | 1.74 | Flat plate; 1.5 | [76] | |
| C. vulgaris | 25 | 18/6 | 8 | 2.37 | Tubular; 2.5 | This study |
| 25 | n.r | 7 | 0.824 | Baffled; 5 | [63] | |
| 20–25 | 20/4 | 4–7 | 0.317 | Airlift; 6.6 | [77] | |
| 25 | 24/0 | 9 | 0.150–0.205 | Flask; 0.25 | [78] | |
| D. salina | 20 | 12/12 | 8 | 4.50 | Tubular; 2.5 | This study |
| 25 | n.r | n.r | 2.13 | Bubble; 0.25 | [79] | |
| 20 | 18/6 | n.r | 0.801 | Bubble; 2 | [80] | |
| 22 | 16/8 | 7.7 | 0.025 | Flask; 0.25 | [81] | |
| Temperature [°C] | Photoperiod [Light/Dark] | pH | Biomass Productivity [g·L−1·d−1] | |||
|---|---|---|---|---|---|---|
| Arthrospira platensis | Chlamydomonas reinchardtii | Chlorella vulgaris | Dunaliella salina | |||
| 7 | 0.69 ± 0.03 | 0.06 ± 0.01 | 0.10 ± 0.00 | 0.77 ± 0.03 | ||
| 12/12 | 8 | 0.72 ± 0.04 | 0.07 ± 0.00 | 0.11 ± 0.01 | 0.81 ± 0.04 | |
| 9 | 0.68 ± 0.03 | 0.06 ± 0.01 | 0.12 ± 0.03 | 0.74 ± 0.03 | ||
| 7 | 0.66 ± 0.03 | 0.08± 0.01 | 0.29 ± 0.02 | 0.72 ± 0.03 | ||
| 20 | 18/6 | 8 | 0.63 ± 0.03 | 0.08 ± 0.01 | 0.30 ± 0.02 | 0.69 ± 0.02 |
| 9 | 0.66 ± 0.03 | 0.08 ± 0.01 | 0.26 ± 0.01 | 0.71 ± 0.03 | ||
| 7 | 0.50 ± 0.03 | 0.10 ± 0.02 | 0.20 ± 0.00 | 0.59 ± 0.03 | ||
| 24/0 | 8 | 0.50 ± 0.02 | 0.11± 0.01 | 0.19 ± 0.00 | 0.60 ± 0.03 | |
| 9 | 0.53 ± 0.02 | 0.11 ± 0.01 | 0.20 ± 0.00 | 0.64 ± 0.02 | ||
| 7 | 0.67 ± 0.03 | 0.08 ± 0.01 | 0.10 ± 0.01 | 0.75 ± 0.04 | ||
| 12/12 | 8 | 0.66 ± 0.02 | 0.08 ± 0.01 | 0.10 ± 0.01 | 0.71 ± 0.03 | |
| 9 | 0.65 ± 0.03 | 0.08 ± 0.01 | 0.10 ± 0.01 | 0.72 ± 0.03 | ||
| 7 | 0.65 ± 0.02 | 0.11 ± 0.01 | 0.27 ± 0.01 | 0.71 ± 0.03 | ||
| 25 | 18/6 | 8 | 0.65 ± 0.02 | 0.12 ± 0.01 | 0.36 ± 0.01 | 0.71 ± 0.03 |
| 9 | 0.59 ± 0.02 | 0.10 ± 0.01 | 0.31 ± 0.01 | 0.65 ± 0.02 | ||
| 7 | 0.51 ± 0.02 | 0.12 ± 0.02 | 0.19 ± 0.00 | 0.61 ± 0.03 | ||
| 24/0 | 8 | 0.56 ± 0.03 | 0.12 ± 0.01 | 0.20 ± 0.00 | 0.67 ± 0.03 | |
| 9 | 0.49 ± 0.03 | 0.10 ± 0.01 | 0.20 ± 0.00 | 0.58 ± 0.04 | ||
| 7 | 0.69 ± 0.02 | 0.08 ± 0.01 | 0.10 ± 0.01 | 0.77 ± 0.03 | ||
| 12/12 | 8 | 0.62 ± 0.02 | 0.11 ± 0.01 | 0.11 ± 0.02 | 0.69 ± 0.03 | |
| 9 | 0.64 ± 0.03 | 0.09 ± 0.00 | 0.11 ± 0.00 | 0.71 ± 0.03 | ||
| 7 | 0.60 ± 0.02 | 0.08 ± 0.01 | 0.27 ± 0.01 | 0.65 ± 0.02 | ||
| 30 | 18/6 | 8 | 0.63 ± 0.03 | 0.10 ± 0.01 | 0.28 ± 0.01 | 0.68 ± 0.03 |
| 9 | 0.61 ± 0.02 | 0.10 ± 0.01 | 0.29 ± 0.01 | 0.65 ± 0.02 | ||
| 7 | 0.47 ± 0.02 | 0.10 ± 0.01 | 0.20 ± 0.01 | 0.56 ± 0.02 | ||
| 24/0 | 8 | 0.49 ± 0.03 | 0.10 ± 0.01 | 0.19 ± 0.00 | 0.59 ± 0.03 | |
| 9 | 0.47 ± 0.02 | 0.08 ± 0.01 | 0.20 ± 0.01 | 0.56 ± 0.02 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hawrot-Paw, M.; Sąsiadek, M. Optimization of Microalgal Biomass Production in Vertical Tubular Photobioreactors. Energies 2023, 16, 2429. https://doi.org/10.3390/en16052429
Hawrot-Paw M, Sąsiadek M. Optimization of Microalgal Biomass Production in Vertical Tubular Photobioreactors. Energies. 2023; 16(5):2429. https://doi.org/10.3390/en16052429
Chicago/Turabian StyleHawrot-Paw, Małgorzata, and Magdalena Sąsiadek. 2023. "Optimization of Microalgal Biomass Production in Vertical Tubular Photobioreactors" Energies 16, no. 5: 2429. https://doi.org/10.3390/en16052429





