Antimicrobial Nano-Zinc Oxide Biocomposites for Wound Healing Applications: A Review
Abstract
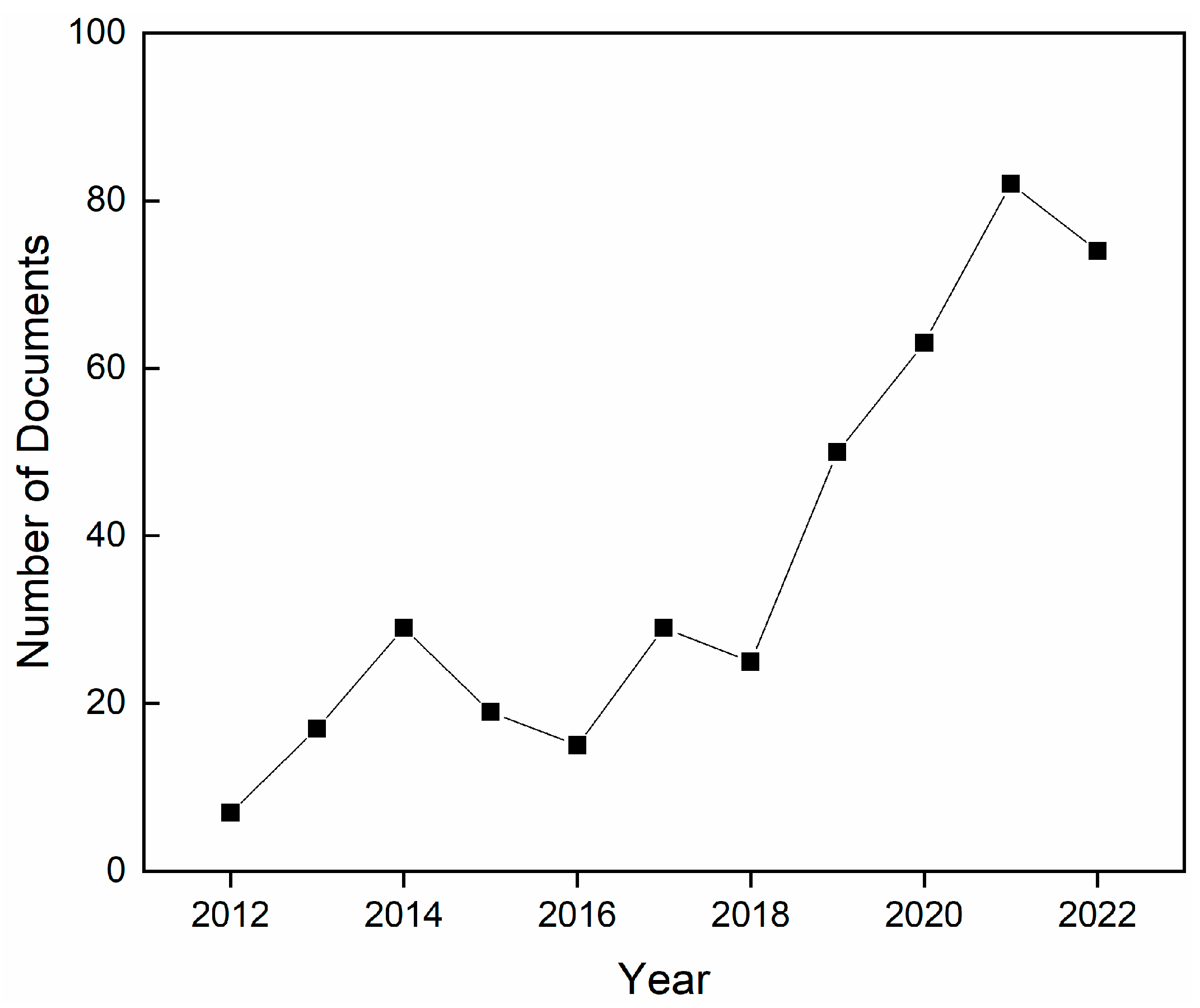
:1. Introduction
2. Biomacromolecules for Wound Dressings
2.1. Overview
2.2. Polysaccharide-Based Polymers
2.3. Protein-Based Polymers
3. Nanostructured Zinc Oxide (nZnO)
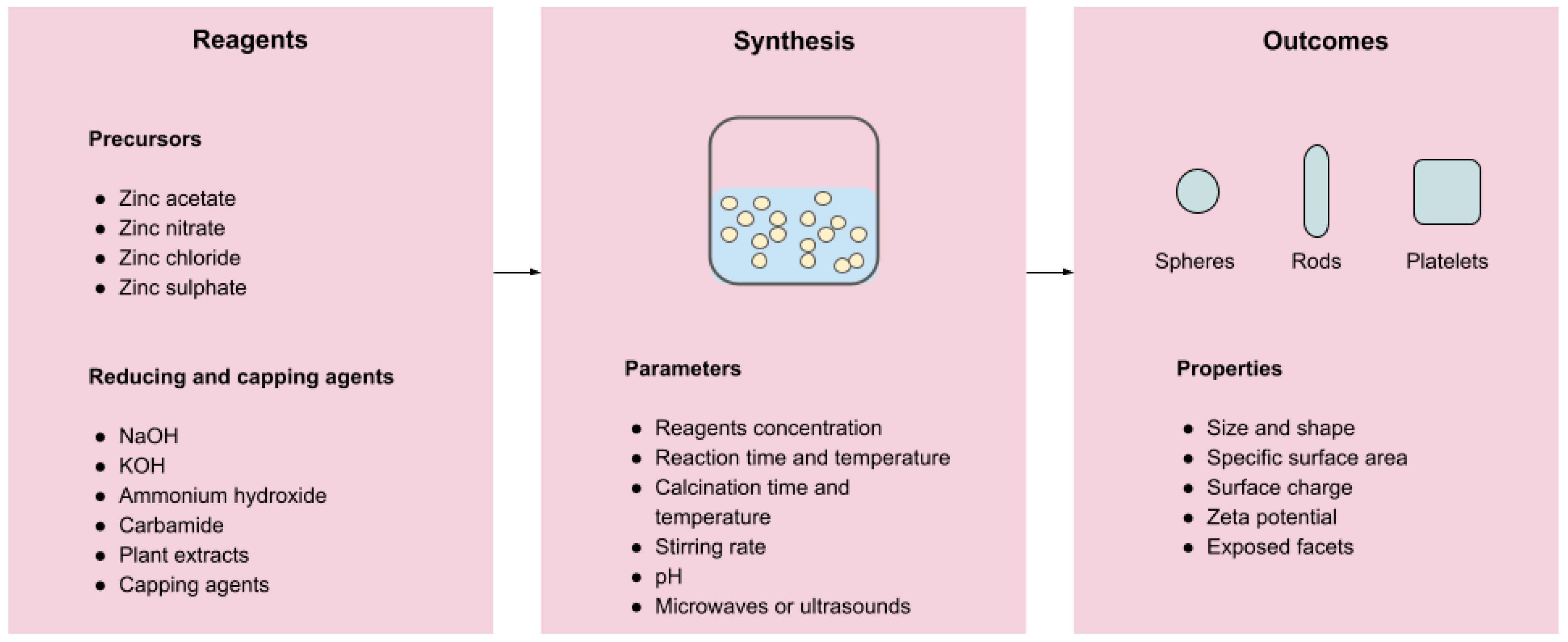
3.1. Preparation Methods
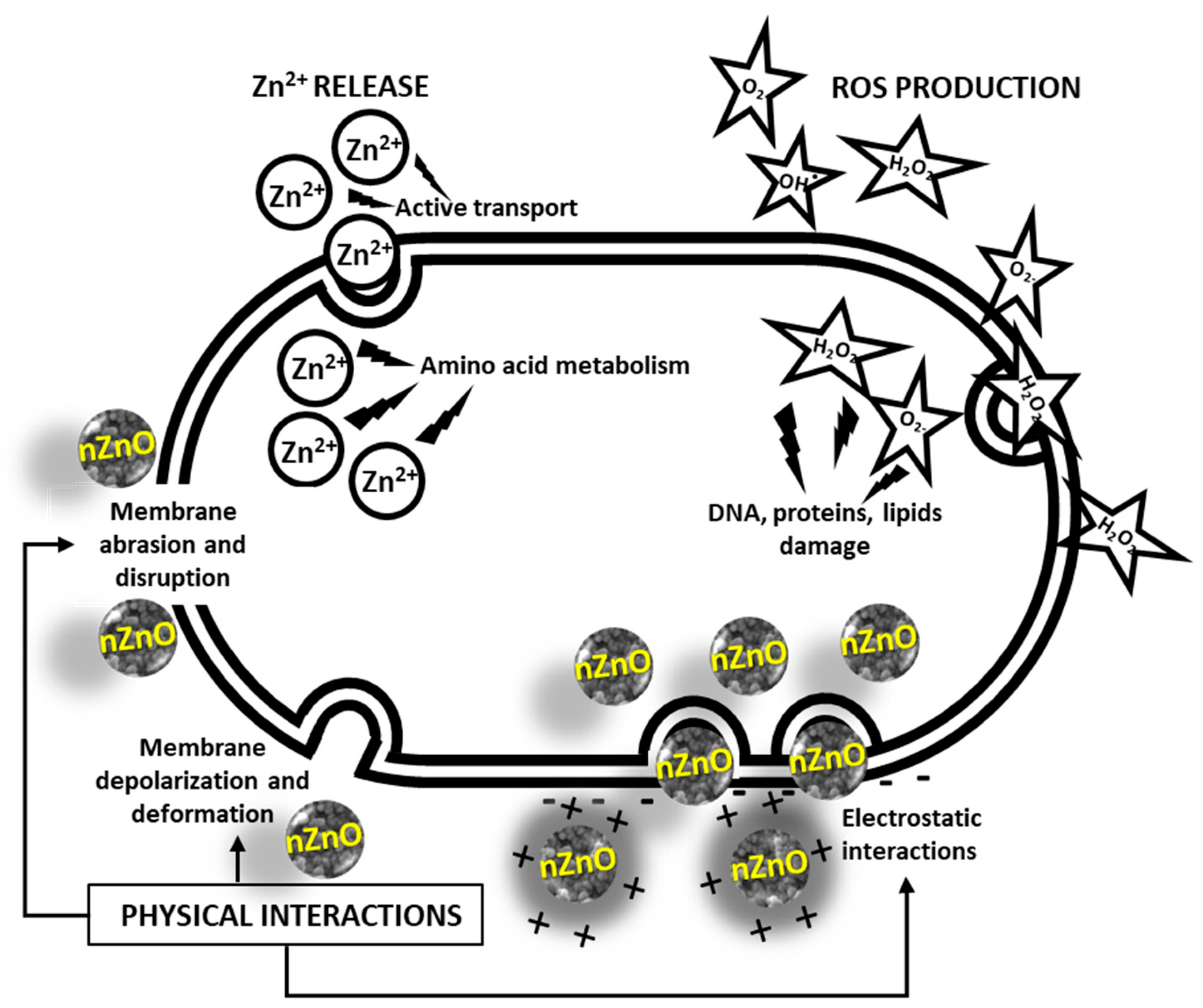
3.2. Antimicrobial Mechanisms
- Production of Reactive Oxygen Species (ROS). Zinc oxide is a direct, 3.3 eV-bandgap semiconductor. Therefore, electron–hole pairs can be created across its conduction and valence bands upon exposure to UV radiation. In the presence of water and oxygen molecules, these charge carriers subsequently trigger redox reactions at the surface of the material, leading to the production of ROS, such as hydroxyl radicals OH∙, hydrogen peroxide H2O2, and superoxide anion O2−. The high reactivity of these species is known to be responsible for the fatal oxidative damage and disruption of DNA, proteins, and lipids, ultimately leading to cell death [153,154].
- Physical interactions. ZnO NPs accumulate in the proximity of bacterial cells. The contact is promoted by electrostatic interactions. In fact, bacterial cells are negatively charged, while ZnO NPs typically possess a positive charge in aqueous suspensions. In turn, this causes membrane depolarization and deformation, as well as abrasion caused by nZnO’s sharp surface edges, ultimately leading to cell death [153]. A graphical representation of these three mechanisms is offered in Figure 3.
4. Biomacromolecule—nZnO Composites
4.1. Preparation Methods
4.2. Mechanical and Functional Properties
4.2.1. Mechanical Properties
4.2.2. Surface Roughness and Wettability
4.2.3. Porosity and Swelling
4.2.4. Gas Barrier Properties
4.2.5. Ultraviolet Light Barrier
5. Applications in Wound Healing
5.1. Controlled Drug Release
5.2. Antimicrobial Properties
| Matrix Type | nZnO (% wt) | Test Type * | Tested Microorganisms | Test Results ** | Ref. |
|---|---|---|---|---|---|
| Polyurethane/chitosan with mahua oil | 5 | Disk diffusion (10) | S. aureus | 20 | [99] |
| E. coli | 25 | ||||
| Chitosan/Poly(vinyl alcohol) | 5 | Disk diffusion | S. aureus | 14 | [100] |
| E. coli | 17 | ||||
| C. albicans | 2 | ||||
| A. niger | 2 | ||||
| Gelatin/hyaluronic acid/chitosan and asiatic acid | - | Disk diffusion (3) | S. aureus | 4.9 ± 0.6 | [83] |
| E. coli | 5.3 ± 0.2 | ||||
| Alginate | 5 | Disk diffusion (13) | MRSA | 15 ± 2 | [41] |
| Chitosan | 2 | End-point assay | B. subtilis | 3 mg/mL | [48] |
| E. coli | 6 mg/mL | ||||
| Bacterial cellulose | - | Disk diffusion (20) | E. coli | 27 ± 0 | [50] |
| P. aeruginosa | 25 ± 1 | ||||
| S. aureus | 28.6 ± 1.15 | ||||
| C. freundii | 26 ± 0 | ||||
| Chitosan–cellulose | 10–30 (% w/v) | Disk diffusion | S. aureus | 13.75 ± 1.50 | [51] |
| T. rubrum | 12.00 ± 1.82 | ||||
| PVA/chitosan/CNC | 5 | Disk diffusion | S. aureus | 6.3 | [52] |
| S. choleraesuis | 4.9 | ||||
| Bacterial cellulose | 5 | Disk diffusion (5) | S. aureus | 11.8 | [53] |
| E. coli | 5.6 | ||||
| Carboxymethylcellulose | - | Disk diffusion (10) | S. aureus | 13 | [54] |
| B. subtilis | 16 | ||||
| P. aeruginosa | 20 | ||||
| E. coli | 16 | ||||
| Gelatin | - | End-point assay | L. monocytogenes | 1 LogCFU/mL after 12 h | [56] |
| E. coli | c.a. 5.3 LogCFU/mL after 12 h | ||||
| Carboxymethylchitosan | - | End-point assay | S. aureus | 99% reduction in viability after 4 h | [58] |
| E. coli | 99% reduction in viability after 6 h | ||||
| Chitosan–carboxymethyl cellulose–oleic acid | 2 | Disk diffusion (4) | A. niger | 30.10 ± 1.50 | [59] |
| Gelatin-chitosan nanofibers | 5 | Disk diffusion (12) | E. coli | 25.06 ± 0.24 | [84] |
| S. aureus | 33.13 ± 0.67 | ||||
| P. aeruginosa | 12.95 ± 0.18 | ||||
| Gelatin/β-glucan | - | Disk diffusion (12) | S. typhimurium | 13.2 ± 1.72 | [85] |
| E. coli | 15.0 ± 1.52 | ||||
| S. aureus | 17.0 ± 2.10 | ||||
| P. aeruginosa | 14.2 ± 1.32 | ||||
| Gum acacia/poly(acrylate) | - | Disk diffusion | E. coli | 32 ± 0.7 | [61] |
| Hyaluronic acid | - | Disk diffusion (6) | E. coli | 19 | [75] |
| S. aureus | 11 | ||||
| Methacrylated hyaluronic acid/elastin-like polypeptide | 0.2 | End-point assay | MRSA | 28.3 ± 4.7 CFU | [76] |
| Keratin | 5 | Disk diffusion (5) | E. coli | 7.7 ± 1 | [79] |
| S. aureus | 13.5 ± 1.3 | ||||
| Sago-starch | 5 | Disk diffusion | S. aureus | 80 mm2 | [62] |
| Oxidised starch | - | Disk diffusion (5) | S. aureus | 100% inhibition | [64] |
| E. coli | 8–11 mm | ||||
| Carrageenan | - | End-point assay | L. monocytogenes | 9 LogCFU/mL after 12 h | [66] |
| E. coli | 0 LogCFU/mL after 12 h | ||||
| Chitosan/cellulose/acetate phthalate | 5 | Disk diffusion (10) | S. aureus | 23 ± 0.35 | [71] |
| E. coli | 24 ± 0. 47 | ||||
| Poly(lactic acid) | - | End-point assay | E. coli | 3.31 LogCFU/mL after 12 h | [204] |
| Poly(vinyl alcohol)/chitosan | 1 | Disk diffusion (20) | S. aureus | 26 | [192] |
| E. coli | 25 | ||||
| Poly(caprolactone) nanofibers | 5 | Disk diffusion (6) | E. coli | 8.76 ± 1.2 | [194] |
| S. aureus | 9.98 ± 0.6 | ||||
| Chitosan | - | End-point assay | E. coli | 2.5 ± 0.421 × 107 CFU/g | [189] |
| S. aureus | 9 ± 0.367 × 107 | ||||
| Regenerated bacterial cellulose | 2 | Disk diffusion (15) | E. coli | 41 | [191] |
| Cellulose | - | Disk diffusion | S. aureus | 10.40 ± 0.50 | [196] |
| T. rubrum | 9.20 ± 0.20 | ||||
| Starch | 5 | End-point assay | E. coli | ~65% inhibitory rate | [207] |
| S. aureus | 100% inhibitory rate | ||||
| Chitosan–cellulose | - | Disk diffusion | S. aureus | 13.75 ± 1.50 | [51] |
| T. rubrum | 12.00 ± 1.82 | ||||
| Gelatin nanofibers | 3 | Disk diffusion (10) | S. aureus | 27 ± 1 | [212] |
| Hydroxyethylcellulose | 0.2 | End-point assay | E. coli | 60.2% inhibition | [225] |
| S. aureus | 91.5% inhibition | ||||
| Poly(lactic acid)/acetylated cellulose nanocrystals | 5 | End-point assay | S. aureus | 100% growth inhibition rate | [226] |
| E. coli | 100% growth inhibition rate | ||||
| Carboxymethylcellulose | - | Disk diffusion (5) | E. coli | 20 ± 2 | [227] |
| S. aureus | 28 ± 2 | ||||
| Chitosan/poly(vinyl alcohol) nanofiber | - | Disk diffusion (15) | E. coli | 20.2 ± 1.0 | [101] |
| B. subtilis | 15.5 ± 0.8 | ||||
| S. aureus | 21.5 ± 0.5 | ||||
| P. aeruginosa | 21.8 ± 1.5 | ||||
| Cellulose/chitosan nanofibers | 5 | Disk diffusion (12) | E. coli | 25.06 ± 0.24 | [228] |
| S. aureus | 33.13 ± 0.67 | ||||
| P. aeruginosa | 12.95 ± 0.18 | ||||
| Ultrasonicated poly(lactic acid) | 1 | Disk diffusion (10) | E. coli | 21.17 ± 0.07 | [229] |
| S. aureus | 18.13 ± 0.08 | ||||
| Bacterial cellulose | - | Disk diffusion (6) | E. coli | 0 | [230] |
| B. subtilis | 4 ± 0.13 | ||||
| C. albicans | 0 |
5.3. Wound-Healing Properties
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. Prevalence and incidence of chronic wounds and related complications: A protocol for a systematic review. Syst. Rev. 2016, 5, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Medtech Europe. The Burden of Wounds on EU Healthcare Systems. Available online: https://www.medtecheurope.org/wp-content/uploads/2015/10/290902009_MTE_The-Burden-of-Wounds-on-EU-Healthcare-Systems_Brochure.pdf (accessed on 1 December 2022).
- EWMA. The Impact of Patient Health and Lifestyle Factors on Wound Healing. 2022. Available online: https://ewma.org/what-we-do/projects/lifestyle-factors (accessed on 1 December 2022).
- Eaglstein, W.H.; Kirsner, R.S.; Robson, M.C. Food and Drug Administration (FDA) drug approval end points for chronic cutaneous ulcer studies. Wound Repair Regen. 2012, 20, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Järbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2018, 27, 114–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burns. Available online: https://www.who.int/news-room/fact-sheets/detail/burns (accessed on 1 December 2022).
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy: Perspective article. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [Green Version]
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn wound infections. Clin. Microbiol. Rev. 2006, 19, 403–434. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, P.; Frew, Q.; Green, A.; Martin, R.; Dziewulski, P. Cause of death and correlation with autopsy findings in burns patients. Burns 2013, 39, 583–588. [Google Scholar] [CrossRef]
- Baker, C.C.M.; Miller, C.L.; Trunkey, D.D.M. Predicting Fatal Sepsis in Burn Patients. J. Trauma Inj. Infect. Crit. Care 1979, 19, 641–648. [Google Scholar] [CrossRef]
- Wangoye, K.; Mwesigye, J.; Tungotyo, M.; Samba, S.T. Chronic wound isolates and their minimum inhibitory concentrations against third generation cephalosporins at a tertiary hospital in Uganda. Sci. Rep. 2022, 12, 1195. [Google Scholar] [CrossRef]
- Filius, P.M.; Gyssens, I.C. Impact of Increasing Antimicrobial Resistance on Wound Management. Am. J. Clin. Dermatol. 2002, 3, 1–7. [Google Scholar] [CrossRef]
- Puca, V.; Marulli, R.Z.; Grande, R.; Vitale, I.; Niro, A.; Molinaro, G.; Prezioso, S.; Muraro, R.; Di Giovanni, P. Microbial Species Isolated from Infected Wounds and Antimicrobial Resistance Analysis: Data Emerging from a Three-Years Retrospective Study. Antibiotics 2021, 10, 1162. [Google Scholar] [CrossRef]
- Guan, H.; Dong, W.; Lu, Y.; Jiang, M.; Zhang, D.; Aobuliaximu, Y.; Dong, J.; Niu, Y.; Liu, Y.; Guan, B.; et al. Distribution and Antibiotic Resistance Patterns of Pathogenic Bacteria in Patients With Chronic Cutaneous Wounds in China. Front. Med. 2021, 8, 609584. [Google Scholar] [CrossRef]
- Boateng, J.; Catanzano, O. Advanced Therapeutic Dressings for Effective Wound Healing—A Review. J. Pharm. Sci. 2015, 104, 3653–3680. [Google Scholar] [CrossRef] [Green Version]
- Dong, R.; Guo, B. Smart wound dressings for wound healing. Nano Today 2021, 41, 101290. [Google Scholar] [CrossRef]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. BioMedicine 2015, 5, 24–28. [Google Scholar] [CrossRef]
- Broussard, K.C.; Powers, J.G. Wound Dressings: Selecting the Most Appropriate Type. Am. J. Clin. Dermatol. 2013, 14, 449–459. [Google Scholar] [CrossRef]
- Holloway, S.; Harding, K.G. Wound dressings. Surgery 2022, 40, 25–32. [Google Scholar] [CrossRef]
- Pormohammad, A.; Monych, N.; Ghosh, S.; Turner, D.; Turner, R. Nanomaterials in Wound Healing and Infection Control. Antibiotics 2021, 10, 473. [Google Scholar] [CrossRef]
- Shalaby, M.A.; Anwar, M.M.; Saeed, H. Nanomaterials for application in wound Healing: Current state-of-the-art and future perspectives. J. Polym. Res. 2022, 29, 91. [Google Scholar] [CrossRef]
- Batool, Z.; Muhammad, G.; Iqbal, M.M.; Aslam, M.S.; Raza, M.A.; Sajjad, N.; Abdullah, M.; Akhtar, N.; Syed, A.; Elgorban, A.M.; et al. Hydrogel assisted synthesis of gold nanoparticles with enhanced microbicidal and in vivo wound healing potential. Sci. Rep. 2022, 12, 6575. [Google Scholar] [CrossRef] [PubMed]
- Blinov, A.V.; Nagdalian, A.A.; Povetkin, S.N.; Gvozdenko, A.A.; Verevkina, M.N.; Rzhepakovsky, I.V.; Lopteva, M.S.; Maglakelidze, D.G.; Kataeva, T.S.; Blinova, A.A.; et al. Surface-Oxidized Polymer-Stabilized Silver Nanoparticles as a Covering Component of Suture Materials. Micromachines 2022, 13, 1105. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Fernandez, B.; Castaño, O.; Mateos-Timoneda, M.; Engel, E.; Pérez-Amodio, S. Nanotechnology Approaches in Chronic Wound Healing. Adv. Wound Care 2021, 10, 234–256. [Google Scholar] [CrossRef] [PubMed]
- Pati, R.; Mehta, R.K.; Mohanty, S.; Padhi, A.; Sengupta, M.; Vaseeharan, B.; Goswami, C.; Sonawane, A. Topical application of zinc oxide nanoparticles reduces bacterial skin infection in mice and exhibits antibacterial activity by inducing oxidative stress response and cell membrane disintegration in macrophages. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1195–1208. [Google Scholar] [CrossRef]
- Vimala, K.; Shanthi, K.; Sundarraj, S.; Kannan, S. Synergistic effect of chemo-photothermal for breast cancer therapy using folic acid (FA) modified zinc oxide nanosheet. J. Colloid Interface Sci. 2017, 488, 92–108. [Google Scholar] [CrossRef]
- Sadhukhan, P.; Kundu, M.; Chatterjee, S.; Ghosh, N.; Manna, P.; Das, J.; Sil, P.C. Targeted delivery of quercetin via pH-responsive zinc oxide nanoparticles for breast cancer therapy. Mater. Sci. Eng. C 2019, 100, 129–140. [Google Scholar] [CrossRef]
- Nagajyothi, P.; Cha, S.J.; Yang, I.J.; Sreekanth, T.; Kim, K.J.; Shin, H.M. Antioxidant and anti-inflammatory activities of zinc oxide nanoparticles synthesized using Polygala tenuifolia root extract. J. Photochem. Photobiol. B Biol. 2015, 146, 10–17. [Google Scholar] [CrossRef]
- Nazarizadeh, A.; Asri-Rezaie, S. Comparative Study of Antidiabetic Activity and Oxidative Stress Induced by Zinc Oxide Nanoparticles and Zinc Sulfate in Diabetic Rats. AAPS PharmSciTech 2015, 17, 834–843. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Wang, H.; Wang, X. Facile and mild preparation of fluorescent ZnO nanosheets and their bioimaging applications. Appl. Surf. Sci. 2011, 257, 6991–6995. [Google Scholar] [CrossRef]
- Sruthi, S.; Ashtami, J.; Mohanan, P. Biomedical application and hidden toxicity of Zinc oxide nanoparticles. Mater. Today Chem. 2018, 10, 175–186. [Google Scholar] [CrossRef]
- Gupta, M.; Mahajan, V.K.; Mehta, K.S.; Chauhan, P.S. Zinc Therapy in Dermatology: A Review. Dermatol. Res. Pract. 2014, 2014, 709152. [Google Scholar] [CrossRef]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a Gatekeeper of Immune Function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef] [Green Version]
- Mishra, P.K.; Mishra, H.; Ekielski, A.; Talegaonkar, S.; Vaidya, B. Zinc oxide nanoparticles: A promising nanomaterial for biomedical applications. Drug Discov. Today 2017, 22, 1825–1834. [Google Scholar] [CrossRef]
- Sengul, A.B.; Asmatulu, E. Toxicity of metal and metal oxide nanoparticles: A review. Environ. Chem. Lett. 2020, 18, 1659–1683. [Google Scholar] [CrossRef]
- Alavi, M.; Nokhodchi, A. An overview on antimicrobial and wound healing properties of ZnO nanobiofilms, hydrogels, and bionanocomposites based on cellulose, chitosan, and alginate polymers. Carbohydr. Polym. 2019, 227, 115349. [Google Scholar] [CrossRef]
- Zahran, M.; Marei, A.H. Innovative natural polymer metal nanocomposites and their antimicrobial activity. Int. J. Biol. Macromol. 2019, 136, 586–596. [Google Scholar] [CrossRef]
- Selvan, N.K.; Shanmugarajan, T.; Uppuluri, V.N.V.A. Hydrogel based scaffolding polymeric biomaterials: Approaches towards skin tissue regeneration. J. Drug Deliv. Sci. Technol. 2019, 55, 101456. [Google Scholar] [CrossRef]
- Jayakumar, R.; Sudheesh Kumar, P.; Mohandas, A.; Lakshmanan, V.-K.; Biswas, R. Exploration of alginate hydrogel/nano zinc oxide composite bandages for infected wounds. Int. J. Nanomed. 2015, 10, 53. [Google Scholar] [CrossRef] [Green Version]
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Wang, H.; Gong, X.; Guo, X.; Liu, C.; Fan, Y.-Y.; Zhang, J.; Niu, B.; Li, W. Characterization, release, and antioxidant activity of curcumin-loaded sodium alginate/ZnO hydrogel beads. Int. J. Biol. Macromol. 2018, 121, 1118–1125. [Google Scholar] [CrossRef]
- Lu, Z.; Gao, J.; He, Q.; Wu, J.; Liang, D.; Yang, H.; Chen, R. Enhanced antibacterial and wound healing activities of microporous chitosan-Ag/ZnO composite dressing. Carbohydr. Polym. 2017, 156, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Dananjaya, S.H.; Kumar, R.S.; Yang, M.; Nikapitiya, C.; Lee, J.; De Zoysa, M. Synthesis, characterization of ZnO-chitosan nanocomposites and evaluation of its antifungal activity against pathogenic Candida albicans. Int. J. Biol. Macromol. 2018, 108, 1281–1288. [Google Scholar] [CrossRef]
- Kumar, P.T.S.; Lakshmanan, V.-K.; Anilkumar, T.; Ramya, C.; Reshmi, P.; Unnikrishnan, A.; Nair, S.V.; Jayakumar, R. Flexible and Microporous Chitosan Hydrogel/Nano ZnO Composite Bandages for Wound Dressing: In Vitro and In Vivo Evaluation. ACS Appl. Mater. Interfaces 2012, 4, 2618–2629. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, M.T.; Siyahi, V.; Ashassi-Sorkhabi, H.; Zarrini, G. ZnO, AgCl and AgCl/ZnO nanocomposites incorporated chitosan in the form of hydrogel beads for photocatalytic degradation of MB, E. coli and S. aureus. Int. J. Biol. Macromol. 2019, 147, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshi, R.; Negi, Y.S. Effect of Varying Filler Concentration on Zinc Oxide Nanoparticle Embedded Chitosan Films as Potential Food Packaging Material. J. Polym. Environ. 2016, 25, 1087–1098. [Google Scholar] [CrossRef]
- Zhang, M.; Qiao, X.; Han, W.; Jiang, T.; Liu, F.; Zhao, X. Alginate-chitosan oligosaccharide-ZnO composite hydrogel for accelerating wound healing. Carbohydr. Polym. 2021, 266, 118100. [Google Scholar] [CrossRef]
- Khalid, A.; Khan, R.; Ul-Islam, M.; Khan, T.; Wahid, F. Bacterial cellulose-zinc oxide nanocomposites as a novel dressing system for burn wounds. Carbohydr. Polym. 2017, 164, 214–221. [Google Scholar] [CrossRef]
- George, D.; Maheswari, P.U.; Begum, K.M.S. Chitosan-cellulose hydrogel conjugated with L-histidine and zinc oxide nanoparticles for sustained drug delivery: Kinetics and in-vitro biological studies. Carbohydr. Polym. 2020, 236, 116101. [Google Scholar] [CrossRef]
- Azizi, S.; Ahmad, M.B.; Ibrahim, N.A.; Hussein, M.Z.; Namvar, F. Cellulose nanocrystals/ZnO as a bifunctional reinforcing nanocomposite for poly(vinyl alcohol)/chitosan blend films: Fabrication, characterization and properties. Int. J. Mol. Sci. 2014, 15, 11040–11053. [Google Scholar] [CrossRef] [Green Version]
- Jebel, F.S.; Almasi, H. Morphological, physical, antimicrobial and release properties of ZnO nanoparticles-loaded bacterial cellulose films. Carbohydr. Polym. 2016, 149, 8–19. [Google Scholar] [CrossRef]
- Hashem, M.; Sharaf, S.; Abd El-Hady, M.M.; Hebeish, A. Synthesis and characterization of novel carboxymethylcellulose hydrogels and carboxymethylcellulolse-hydrogel-ZnO-nanocomposites. Carbohydr. Polym. 2013, 95, 421–427. [Google Scholar] [CrossRef]
- Zare-Akbari, Z.; Farhadnejad, H.; Furughi-Nia, B.; Abedin, S.; Yadollahi, M.; Khorsand-Ghayeni, M. PH-sensitive bionanocomposite hydrogel beads based on caborboxymethyl cellulose/ZnO nanoparticle as drug carrier. Int. J. Biol. Macromol. 2016, 93, 1317–1327. [Google Scholar] [CrossRef]
- Shankar, S.; Teng, X.; Li, G.; Rhim, J.-W. Preparation, characterization, and antimicrobial activity of gelatin/ZnO nanocomposite films. Food Hydrocoll. 2015, 45, 264–271. [Google Scholar] [CrossRef]
- Naserian, F.; Mesgar, A.S. Development of antibacterial and superabsorbent wound composite sponges containing carboxymethyl cellulose/gelatin/Cu-doped ZnO nanoparticles. Colloids Surf. B Biointerfaces 2022, 218, 112729. [Google Scholar] [CrossRef]
- Wahid, F.; Yin, J.-J.; Xue, D.-D.; Xue, H.; Lu, Y.-S.; Zhong, C.; Chu, L.-Q. Synthesis and characterization of antibacterial carboxymethyl Chitosan/ZnO nanocomposite hydrogels. Int. J. Biol. Macromol. 2016, 88, 273–279. [Google Scholar] [CrossRef]
- Noshirvani, N.; Ghanbarzadeh, B.; Mokarram, R.R.; Hashemi, M.; Coma, V. Preparation and characterization of active emulsified films based on chitosan-carboxymethyl cellulose containing zinc oxide nano particles. Int. J. Biol. Macromol. 2017, 99, 530–538. [Google Scholar] [CrossRef]
- Hu, T.; Wu, G.-P.; Bu, H.; Zhang, H.; Li, W.-X.; Song, K.; Jiang, G.-B. An injectable, adhesive, and self-healable composite hydrogel wound dressing with excellent antibacterial activity. Chem. Eng. J. 2022, 450, 138201. [Google Scholar] [CrossRef]
- Bajpai, S.; Jadaun, M.; Tiwari, S. Synthesis, characterization and antimicrobial applications of zinc oxide nanoparticles loaded gum acacia/poly(SA) hydrogels. Carbohydr. Polym. 2016, 153, 60–65. [Google Scholar] [CrossRef]
- Nafchi, A.M.; Alias, A.K.; Mahmud, S.; Robal, M. Antimicrobial, rheological, and physicochemical properties of sago starch films filled with nanorod-rich zinc oxide. J. Food Eng. 2012, 113, 511–519. [Google Scholar] [CrossRef]
- El-Nahhal, I.M.; Salem, J.; Anbar, R.; Kodeh, F.S.; Elmanama, A. Preparation and antimicrobial activity of ZnO-NPs coated cotton/starch and their functionalized ZnO-Ag/cotton and Zn(II) curcumin/cotton materials. Sci. Rep. 2020, 10, 5410. [Google Scholar] [CrossRef] [Green Version]
- Namazi, H.; Hasani, M.; Yadollahi, M. Antibacterial oxidized starch/ZnO nanocomposite hydrogel: Synthesis and evaluation of its swelling behaviours in various pHs and salt solutions. Int. J. Biol. Macromol. 2018, 126, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Peighambardoust, S.J.; Peighambardoust, S.H.; Pournasir, N.; Pakdel, P.M. Properties of active starch-based films incorporating a combination of Ag, ZnO and CuO nanoparticles for potential use in food packaging applications. Food Packag. Shelf Life 2019, 22, 100420. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Carrageenan-based antimicrobial bionanocomposite films incorporated with ZnO nanoparticles stabilized by melanin. Food Hydrocoll. 2019, 90, 500–507. [Google Scholar] [CrossRef]
- Saputri, A.E.; Praseptiangga, D.; Rochima, E.; Panatarani, C.; Joni, I.M. Mechanical and solubility properties of bio-nanocomposite film of semi refined kappa carrageenan/ZnO nanoparticles. In AIP Conference Proceedings; American Institute of Physics Inc.: College Park, MD, USA, 2018; Volume 1927, p. 030040. [Google Scholar]
- Kanmani, P.; Rhim, J.-W. Properties and characterization of bionanocomposite films prepared with various biopolymers and ZnO nanoparticles. Carbohydr. Polym. 2014, 106, 190–199. [Google Scholar] [CrossRef]
- Pittarate, C.; Yoovidhya, T.; Srichumpuang, W.; Intasanta, N.; Wongsasulak, S. Effects of poly(ethylene oxide) and ZnO nanoparticles on the morphology, tensile and thermal properties of cellulose acetate nanocomposite fibrous film. Polym. J. 2011, 43, 978–986. [Google Scholar] [CrossRef] [Green Version]
- Aly, A.A.; Ahmed, M. Nanofibers of cellulose acetate containing ZnO nanoparticles/graphene oxide for wound healing applications. Int. J. Pharm. 2021, 598, 120325. [Google Scholar] [CrossRef]
- Indumathi, M.; Sarojini, K.S.; Rajarajeswari, G. Antimicrobial and biodegradable chitosan/cellulose acetate phthalate/ZnO nano composite films with optimal oxygen permeability and hydrophobicity for extending the shelf life of black grape fruits. Int. J. Biol. Macromol. 2019, 132, 1112–1120. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, L.; Wei, C.; Guo, R. A bioactive dextran-based hydrogel promote the healing of infected wounds via antibacterial and immunomodulatory. Carbohydr. Polym. 2022, 291, 119558. [Google Scholar] [CrossRef]
- Sudheesh Kumar, P.T.; Lakshmanan, V.K.; Raj, M.; Biswas, R.; Hiroshi, T.; Nair, S.V.; Jayakumar, R. Evaluation of wound healing potential of β-chitin hydrogel/nano zinc oxide composite bandage. Pharm. Res. 2013, 30, 523–537. [Google Scholar]
- Razzaq, H.A.; D’Ayala, G.G.; Santagata, G.; Bosco, F.; Mollea, C.; Larsen, N.; Duraccio, D. Bioactive films based on barley β-glucans and ZnO for wound healing applications. Carbohydr. Polym. 2021, 272, 118442. [Google Scholar] [CrossRef]
- Rao, K.M.; Suneetha, M.; Zo, S.; Duck, K.H.; Han, S.S. One-pot synthesis of ZnO nanobelt-like structures in hyaluronan hydrogels for wound dressing applications. Carbohydr. Polym. 2019, 223, 115124. [Google Scholar] [CrossRef]
- Sani, E.S.; Portillo-Lara, R.; Spencer, A.; Yu, W.; Geilich, B.M.; Noshadi, I.; Webster, T.J.; Annabi, N. Engineering Adhesive and Antimicrobial Hyaluronic Acid/Elastin-like Polypeptide Hybrid Hydrogels for Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2018, 4, 2528–2540. [Google Scholar] [CrossRef] [Green Version]
- Păunica-Panea, G.; Ficai, A.; Marin, M.M.; Marin, Ş.; Albu, M.G.; Constantin, V.D.; Dinu-Pîrvu, C.; Vuluga, Z.; Corobea, M.C.; Ghica, M.V. New Collagen-Dextran-Zinc Oxide Composites for Wound Dressing. J. Nanomater. 2016, 2016, 34. [Google Scholar] [CrossRef] [Green Version]
- Zhai, M.; Xu, Y.; Zhou, B.; Jing, W. Keratin-chitosan/n-ZnO nanocomposite hydrogel for antimicrobial treatment of burn wound healing: Characterization and biomedical application. J. Photochem. Photobiol. B 2018, 180, 253–258. [Google Scholar] [CrossRef]
- Villanueva, M.E.; Cuestas, M.L.; Pérez, C.J.; Campo Dall′ Orto, V.; Copello, G.J. Smart release of antimicrobial ZnO nanoplates from a pH-responsive keratin hydrogel. J. Colloid. Interface Sci. 2019, 536, 372–380. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Benjakul, S.; Prodpran, T.; Sumpavapol, P.; Songtipya, P. Properties and antimicrobial activity of fish protein isolate/fish skin gelatin film containing basil leaf essential oil and zinc oxide nanoparticles. Food Hydrocoll. 2014, 41, 265–273. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Benjakul, S.; Prodpran, T.; Sumpavapol, P.; Songtipya, P. Physico-Mechanical Characterization and Antimicrobial Properties of Fish Protein Isolate/Fish Skin Gelatin-Zinc Oxide (ZnO) Nanocomposite Films. Food Bioprocess Technol. 2015, 9, 101–112. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Benjakul, S.; Vongkamjan, K.; Sumpavapol, P.; Yarnpakdee, S. Shelf-life extension of refrigerated sea bass slices wrapped with fish protein isolate/fish skin gelatin-ZnO nanocomposite film incorporated with basil leaf essential oil. J. Food Sci. Technol. 2015, 52, 6182–6193. [Google Scholar] [CrossRef] [Green Version]
- Thanusha, A.V.; Dinda, A.K.; Koul, V. Evaluation of nano hydrogel composite based on gelatin/HA/CS suffused with Asiatic acid/ZnO and CuO nanoparticles for second degree burns. Mater. Sci. Eng. C 2018, 89, 378–386. [Google Scholar]
- Amjadi, S.; Emaminia, S.; Nazari, M.; Davudian, S.H.; Roufegarinejad, L.; Hamishehkar, H. Application of Reinforced ZnO Nanoparticle-Incorporated Gelatin Bionanocomposite Film with Chitosan Nanofiber for Packaging of Chicken Fillet and Cheese as Food Models. Food Bioprocess Technol. 2019, 12, 1205–1219. [Google Scholar] [CrossRef]
- Azari, S.S.; Alizadeh, A.; Roufegarinejad, L.; Asefi, N.; Hamishehkar, H. Preparation and characterization of gelatin/β-glucan nanocomposite film incorporated with ZnO nanoparticles as an active food packaging system. J. Polym. Environ. 2020, 29, 1143–1152. [Google Scholar] [CrossRef]
- Jaberifard, F.; Ramezani, S.; Ghorbani, M.; Arsalani, N.; Moghadam, F.M. Investigation of wound healing efficiency of multifunctional eudragit/soy protein isolate electrospun nanofiber incorporated with ZnO loaded halloysite nanotubes and allantoin. Int. J. Pharm. 2023, 630, 122434. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Zeng, B.; Zhu, Y.; Yu, F.; Wang, M.; Song, X.; Cheng, X.; Chen, L.; Wang, X. Porous ZnO modified silk sutures with dual light defined antibacterial, healing promotion and controlled self-degradation capabilities. Biomater. Sci. 2019, 8, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- El-Hack, M.E.A.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; El-Hakim, Y.M.A.; Al-Sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef]
- Costa, E.; Silva, S.; Vicente, S.; Neto, C.; Castro, P.; Veiga, M.; Madureira, R.; Tavaria, F.; Pintado, M. Chitosan nanoparticles as alternative anti-staphylococci agents: Bactericidal, antibiofilm and antiadhesive effects. Mater. Sci. Eng. C 2017, 79, 221–226. [Google Scholar] [CrossRef]
- Torkaman, S.; Rahmani, H.; Ashori, A.; Najafi, S.H.M. Modification of chitosan using amino acids for wound healing purposes: A review. Carbohydr. Polym. 2021, 258, 117675. [Google Scholar] [CrossRef]
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; Gomez d’Ayala, G. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: A review. Carbohydr. Polym. 2020, 233, 115839. [Google Scholar] [CrossRef]
- Deng, P.; Jin, W.; Liu, Z.; Gao, M.; Zhou, J. Novel multifunctional adenine-modified chitosan dressings for promoting wound healing. Carbohydr. Polym. 2021, 260, 117767. [Google Scholar] [CrossRef]
- Miguel, S.P.; Moreira, A.F.; Correia, I.J. Chitosan based-asymmetric membranes for wound healing: A review. Int. J. Biol. Macromol. 2019, 127, 460–475. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Eini, M.; Rastegari, A.; Tehrani, M.R. Chitosan as a machine for biomolecule delivery: A review. Carbohydr. Polym. 2020, 256, 117414. [Google Scholar] [CrossRef]
- Shahrousvand, M.; Haddadi-Asl, V.; Shahrousvand, M. Step-by-step design of poly (ε-caprolactone)/chitosan/Melilotus officinalis extract electrospun nanofibers for wound dressing applications. Int. J. Biol. Macromol. 2021, 180, 36–50. [Google Scholar] [CrossRef]
- Ho, T.T.-P.; Doan, V.K.; Tran, N.M.-P.; Nguyen, L.K.-K.; Le, A.N.-M.; Ho, M.H.; Trinh, N.-T.; Van Vo, T.; Tran, L.D.; Nguyen, T.-H. Fabrication of chitosan oligomer-coated electrospun polycaprolactone membrane for wound dressing application. Mater. Sci. Eng. C 2020, 120, 111724. [Google Scholar] [CrossRef]
- Saral Sarojini, K.; Indumathi, M.P.; Rajarajeswari, G.R. Mahua oil-based polyurethane/chitosan/nano ZnO composite films for biodegradable food packaging applications. Int. J. Biol. Macromol. 2019, 124, 163–174. [Google Scholar] [CrossRef]
- Hezma, A.; Rajeh, A.; Mannaa, M.A. An insight into the effect of zinc oxide nanoparticles on the structural, thermal, mechanical properties and antimicrobial activity of Cs/PVA composite. Colloids Surf. A Physicochem. Eng. Asp. 2019, 581, 123821. [Google Scholar] [CrossRef]
- Ahmed, R.; Tariq, M.; Ali, I.; Asghar, R.; Khanam, P.N.; Augustine, R.; Hasan, A. Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing. Int. J. Biol. Macromol. 2018, 120, 385–393. [Google Scholar] [CrossRef]
- Zhou, L.; Cai, L.; Ruan, H.; Zhang, L.; Wang, J.; Jiang, H.; Wu, Y.; Feng, S.; Chen, J. Electrospun chitosan oligosaccharide/polycaprolactone nanofibers loaded with wound-healing compounds of Rutin and Quercetin as antibacterial dressings. Int. J. Biol. Macromol. 2021, 183, 1145–1154. [Google Scholar] [CrossRef]
- Cacicedo, M.L.; Castro, M.C.; Servetas, I.; Bosnea, L.; Boura, K.; Tsafrakidou, P.; Dima, A.; Terpou, A.; Koutinas, A.; Castro, G.R. Progress in bacterial cellulose matrices for biotechnological applications. Bioresour. Technol. 2016, 213, 172–180. [Google Scholar] [CrossRef]
- Zhong, C. Industrial-Scale Production and Applications of Bacterial Cellulose. Front. Bioeng. Biotechnol. 2020, 8, 605374. [Google Scholar] [CrossRef]
- Klemm, D.; Petzold-Welcke, K.; Kramer, F.; Richter, T.; Raddatz, V.; Fried, W.; Nietzsche, S.; Bellmann, T.; Fischer, D. Biotech nanocellulose: A review on progress in product design and today’s state of technical and medical applications. Carbohydr. Polym. 2020, 254, 117313. [Google Scholar] [CrossRef] [PubMed]
- Raghav, N.; Sharma, M.R.; Kennedy, J.F. Nanocellulose: A mini-review on types and use in drug delivery systems. Carbohydr. Polym. Technol. Appl. 2021, 2, 100031. [Google Scholar] [CrossRef]
- Dhali, K.; Ghasemlou, M.; Daver, F.; Cass, P.; Adhikari, B. A review of nanocellulose as a new material towards environmental sustainability. Sci. Total Environ. 2021, 775, 145871. [Google Scholar] [CrossRef] [PubMed]
- Ho, N.A.D.; Leo, C. A review on the emerging applications of cellulose, cellulose derivatives and nanocellulose in carbon capture. Environ. Res. 2021, 197, 111100. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Keddie, D.; Kannappan, V.; Gibson, H.; Khalil, I.; Kowalczuk, M.; Martin, C.; Shuai, X.; Radecka, I. Production and characterisation of bacterial cellulose hydrogels loaded with curcumin encapsulated in cyclodextrins as wound dressings. Eur. Polym. J. 2019, 118, 437–450. [Google Scholar] [CrossRef]
- Wang, Q.; Barnes, L.-M.; Maslakov, K.I.; Howell, C.A.; Illsley, M.J.; Dyer, P.; Savina, I.N. In situ synthesis of silver or selenium nanoparticles on cationized cellulose fabrics for antimicrobial application. Mater. Sci. Eng. C 2021, 121, 111859. [Google Scholar] [CrossRef]
- Jin, T.; Yan, L.; Liu, W.; Liu, S.; Liu, C.; Zheng, L. Preparation and physicochemical/antimicrobial characteristics of asparagus cellulose films containing quercetin. Food Sci. Hum. Wellness 2021, 10, 251–257. [Google Scholar] [CrossRef]
- Gopi, S.; Balakrishnan, P.; Chandradhara, D.; Poovathankandy, D.; Thomas, S. General scenarios of cellulose and its use in the biomedical field. Mater. Today Chem. 2019, 13, 59–78. [Google Scholar] [CrossRef]
- Park, S.U.; Lee, B.K.; Kim, M.S.; Park, K.K.; Sung, W.J.; Kim, H.Y.; Gil Han, D.; Shim, J.S.; Lee, Y.J.; Kim, S.H.; et al. The possibility of microbial cellulose for dressing and scaffold materials. Int. Wound J. 2012, 11, 35–43. [Google Scholar] [CrossRef]
- Wen, X.; Zheng, Y.; Wu, J.; Yue, L.; Wang, C.; Luan, J.; Wu, Z.; Wang, K. In vitro and in vivo investigation of bacterial cellulose dressing containing uniform silver sulfadiazine nanoparticles for burn wound healing. Prog. Nat. Sci. 2015, 25, 197–203. [Google Scholar] [CrossRef] [Green Version]
- Tavakolian, M.; Gil Munguia-Lopez, J.; Valiei, A.; Islam, S.; Kinsella, J.M.; Tufenkji, N.; van de Ven, T.G.M. Highly absorbent antibacterial and biofilm-disrupting hydrogels from cellulose for wound dressing application. ACS Appl. Mater. Interfaces 2020, 12, 39991–40001. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, X.; Tang, Z.; Xiao, H.; Zhang, M.; Liu, K.; Chen, L.; Huang, L.; Ni, Y.; Wu, H. Mussel-inspired blue-light-activated cellulose-based adhesive hydrogel with fast gelation, rapid haemostasis and antibacterial property for wound healing. Chem. Eng. J. 2021, 417, 129329. [Google Scholar] [CrossRef]
- Sze, J.H.; Brownlie, J.C.; Love, C.A. Biotechnological production of hyaluronic acid: A mini review. 3 Biotech 2016, 6, 67. [Google Scholar] [CrossRef] [Green Version]
- Cerminati, S.; Leroux, M.; Anselmi, P.; Peirú, S.; Alonso, J.C.; Priem, B.; Menzella, H.G. Low cost and sustainable hyaluronic acid production in a manufacturing platform based on Bacillus subtilis 3NA strain. Appl. Microbiol. Biotechnol. 2021, 105, 3075–3086. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef]
- Graça, M.F.P.; Miguel, S.P.; Cabral, C.S.D.; Correia, I.J. Hyaluronic acid—Based wound dressings: A review. Carbohydr. Polym. 2020, 241, 116364. [Google Scholar] [CrossRef]
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic acid and its biomedical applications: A review. Eng. Regen. 2020, 1, 102–113. [Google Scholar] [CrossRef]
- Agarwal, G.; Agiwal, S.; Srivastava, A. Hyaluronic acid containing scaffolds ameliorate stem cell function for tissue repair and regeneration. Int. J. Biol. Macromol. 2020, 165, 388–401. [Google Scholar] [CrossRef]
- Ucm, R.; Aem, M.; Lhb, Z.; Kumar, V.; Taherzadeh, M.J.; Garlapati, V.K.; Chandel, A.K. Comprehensive review on biotechnological production of hyaluronic acid: Status, innovation, market and applications. Bioengineered 2022, 13, 9645–9661. [Google Scholar] [CrossRef]
- Reig-Vano, B.; Tylkowski, B.; Montané, X.; Giamberini, M. Alginate-based hydrogels for cancer therapy and research. Int. J. Biol. Macromol. 2020, 170, 424–436. [Google Scholar] [CrossRef]
- Urtuvia, V.; Maturana, N.; Acevedo, F.; Peña, C.; Díaz-Barrera, A. Bacterial alginate production: An overview of its biosynthesis and potential industrial production. World J. Microbiol. Biotechnol. 2017, 33, 198. [Google Scholar] [CrossRef] [PubMed]
- Raus, R.A.; Nawawi, W.M.F.W.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2020, 16, 280–306. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. State-of-the-art of 3D printing technology of alginate-based hydrogels—An emerging technique for industrial applications. Adv. Colloid Interface Sci. 2021, 293, 102436. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, L.; McClements, D.J.; Yang, T.; Zhang, Z.; Ren, F.; Miao, M.; Tian, Y.; Jin, Z. Starch-based biodegradable packaging materials: A review of their preparation, characterization and diverse applications in the food industry. Trends Food Sci. Technol. 2021, 114, 70–82. [Google Scholar] [CrossRef]
- Hemamalini, T.; Dev, V.R.G. Comprehensive review on electrospinning of starch polymer for biomedical applications. Int. J. Biol. Macromol. 2018, 106, 712–718. [Google Scholar] [CrossRef]
- Ma, J.; Underhill, D.M. β-glucan signaling connects phagocytosis to autophagy. Glycobiology 2013, 23, 1047–1051. [Google Scholar] [CrossRef] [Green Version]
- Wei, D.; Zhang, L.; Williams, D.L.; Browder, I.W. Glucan stimulates human dermal fibroblast collagen biosynthesis through a nuclear factor-1 dependent mechanism. Wound Repair Regen. 2002, 10, 161–168. [Google Scholar] [CrossRef]
- Majtan, J.; Jesenak, M. β-Glucans: Multi-Functional Modulator of Wound Healing. Molecules 2018, 23, 806. [Google Scholar] [CrossRef] [Green Version]
- Seo, G.; Hyun, C.; Choi, S.; Kim, Y.M.; Cho, M. The wound healing effect of four types of beta-glucan. Appl. Biol. Chem. 2019, 62, 20. [Google Scholar] [CrossRef] [Green Version]
- King, B.; Barrett, S.; Cutting, K. Clinical evaluation of a bioactive beta-glucan gel in the treatment of ‘hard-to-heal’ wounds. J. Wound Care 2017, 26, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Grip, J.; Engstad, R.E.; Skjæveland, I.; Škalko-Basnet, N.; Isaksson, J.; Basnet, P.; Holsæter, A.M. Beta-glucan-loaded nanofiber dressing improves wound healing in diabetic mice. Eur. J. Pharm. Sci. 2018, 121, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Yoon, D.; Yoon, D.; Cha, H.-J.; Lee, J.-S.; Chun, W. Enhancement of wound healing efficiency mediated by artificial dermis functionalized with EGF or NRG1. Biomed. Mater. 2018, 13, 045007. [Google Scholar] [CrossRef]
- Gopinath, D.; Ahmed, M.R.; Gomathi, K.; Chitra, K.; Sehgal, P.K.; Jayakumar, R. Dermal wound healing processes with curcumin incorporated collagen films. Biomaterials 2004, 25, 1911–1917. [Google Scholar] [CrossRef]
- Taraballi, F.; Zanini, S.; Lupo, C.; Panseri, S.; Cunha, C.; Riccardi, C.; Marcacci, M.; Campione, M.; Cipolla, L. Amino and carboxyl plasma functionalization of collagen films for tissue engineering applications. J. Colloid Interface Sci. 2012, 394, 590–597. [Google Scholar] [CrossRef]
- Irastorza, A.; Zarandona, I.; Andonegi, M.; Guerrero, P.; de la Caba, K. The versatility of collagen and chitosan: From food to biomedical applications. Food Hydrocoll. 2021, 116, 106633. [Google Scholar] [CrossRef]
- Tavassoli, M.; Sani, M.A.; Khezerlou, A.; Ehsani, A.; McClements, D.J. Multifunctional nanocomposite active packaging materials: Immobilization of quercetin, lactoferrin, and chitosan nanofiber particles in gelatin films. Food Hydrocoll. 2021, 118, 106747. [Google Scholar] [CrossRef]
- Wang, H.; Ding, F.; Ma, L.; Zhang, Y. Edible films from chitosan-gelatin: Physical properties and food packaging application. Food Biosci. 2021, 40, 100871. [Google Scholar] [CrossRef]
- Sarika, P.; Cinthya, K.; Jayakrishnan, A.; Anilkumar, P.; James, N.R. Modified gum arabic cross-linked gelatin scaffold for biomedical applications. Mater. Sci. Eng. C 2014, 43, 272–279. [Google Scholar] [CrossRef]
- Jalaja, K.; Kumar, P.A.; Dey, T.; Kundu, S.C.; James, N.R. Modified dextran cross-linked electrospun gelatin nanofibres for biomedical applications. Carbohydr. Polym. 2014, 114, 467–475. [Google Scholar] [CrossRef]
- Mao, Z.; Bai, J.; Jin, X.; Mao, W.; Dong, Y. Construction of a multifunctional 3D nanofiber aerogel loaded with ZnO for wound healing. Colloids Surf. B Biointerfaces 2021, 208, 112070. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.W.; Kim, J.E.; Lee, K.H. Green fabrication of antibacterial gelatin fiber for biomedical application. React. Funct. Polym. 2019, 136, 86–94. [Google Scholar] [CrossRef]
- Chilakamarry, C.R.; Mahmood, S.; Saffe, S.N.B.M.; Bin Arifin, M.A.; Gupta, A.; Sikkandar, M.Y.; Begum, S.S.; Narasaiah, B. Extraction and application of keratin from natural resources: A review. 3 Biotech 2021, 11, 220. [Google Scholar] [CrossRef] [PubMed]
- Suarato, G.; Bertorelli, R.; Athanassiou, A. Borrowing From Nature: Biopolymers and Biocomposites as Smart Wound Care Materials. Front. Bioeng. Biotechnol. 2018, 6, 137. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Gupta, A. Sustainable Management of Keratin Waste Biomass: Applications and Future Perspectives. Braz. Arch. Biol. Technol. 2016, 59. [Google Scholar] [CrossRef] [Green Version]
- Feroz, S.; Muhammad, N.; Ratnayake, J.; Dias, G. Keratin-Based materials for biomedical applications. Bioact. Mater. 2020, 5, 496–509. [Google Scholar] [CrossRef]
- McLellan, J.; Thornhill, S.G.; Shelton, S.; Kumar, M. Keratin-Based Biofilms, Hydrogels, and Biofibers. In Keratin as a Protein Biopolymer; Springer: Berlin/Heidelberg, Germany, 2018; pp. 187–200. [Google Scholar] [CrossRef]
- Pino, P.; Ronchetti, S.; Mollea, C.; Sangermano, M.; Onida, B.; Bosco, F. Whey Proteins–Zinc Oxide Bionanocomposite as Antibacterial Films. Pharmaceutics 2021, 13, 1426. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef]
- Zou, C.; Liang, F.; Xue, S. Synthesis and oxygen vacancy-related photocatalytic properties of ZnO nanotubes grown by thermal evaporation. Res. Chem. Intermed. 2014, 41, 5167–5176. [Google Scholar] [CrossRef]
- Khan, M.F.; Ansari, A.H.; Hameedullah, M.; Ahmad, E.; Husain, F.M.; Zia, Q.; Baig, U.; Zaheer, M.R.; Alam, M.M.; Khan, A.M.; et al. Sol-gel synthesis of thorn-like ZnO nanoparticles endorsing mechanical stirring effect and their antimicrobial activities: Potential role as nano-antibiotics. Sci. Rep. 2016, 6, 27689. [Google Scholar] [CrossRef] [Green Version]
- Paper, N.; Submission, P. Synthesis of Zinc Oxide Nanoparticles via Sol—Gel Route and Their Characterization. Nanosci. Nanotechnol. 2016, 5, 2010–2014. [Google Scholar]
- Espitia, P.J.P.; de Fátima Ferreira Soares, N.; dos Reis Coimbra, J.S.; De Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc Oxide Nanoparticles: Synthesis, Antimicrobial Activity and Food Packaging Applications. Food Bioprocess Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Ponnamma, D.; Cabibihan, J.-J.; Rajan, M.; Pethaiah, S.S.; Deshmukh, K.; Gogoi, J.P.; Pasha, S.K.K.; Ahamed, M.B.; Krishnegowda, J.; Chandrashekar, B.N.; et al. Synthesis, optimization and applications of ZnO/polymer nanocomposites. Mater. Sci. Eng. C 2019, 98, 1210–1240. [Google Scholar] [CrossRef]
- Zukas, B.G.; Gupta, N.R. Interphase Synthesis of Zinc Oxide Nanoparticles in a Droplet Flow Reactor. Ind. Eng. Chem. Res. 2017, 56, 7184–7191. [Google Scholar] [CrossRef]
- Garino, N.; Sanvitale, P.; Dumontel, B.; Laurenti, M.; Colilla, M.; Izquierdo-Barba, I.; Cauda, V.; Vallet-Regì, M. Zinc oxide nanocrystals as a nanoantibiotic and osteoinductive agent. RSC Adv. 2019, 9, 11312–11321. [Google Scholar] [CrossRef]
- Kajbafvala, A.; Zanganeh, S.; Kajbafvala, E.; Zargar, H.; Bayati, M.; Sadrnezhaad, S. Microwave-assisted synthesis of narcis-like zinc oxide nanostructures. J. Alloys Compd. 2010, 497, 325–329. [Google Scholar] [CrossRef]
- Garino, N.; Limongi, T.; Dumontel, B.; Canta, M.; Racca, L.; Laurenti, M.; Castellino, M.; Casu, A.; Falqui, A.; Cauda, V. A Microwave-Assisted Synthesis of Zinc Oxide Nanocrystals Finely Tuned for Biological Applications. Nanomaterials 2019, 9, 212. [Google Scholar] [CrossRef] [Green Version]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A Review of Microwave Synthesis of Zinc Oxide Nanomaterials: Reactants, Process Parameters and Morphologies. Nanomaterials 2020, 10, 1086. [Google Scholar] [CrossRef]
- Chittofrati, A.; Matijević, E. Uniform particles of zinc oxide of different morphologies. Colloids Surf. 1990, 48, 65–78. [Google Scholar] [CrossRef]
- Bharathi, D.; Ranjithkumar, R.; Chandarshekar, B.; Bhuvaneshwari, V. Preparation of chitosan coated zinc oxide nanocomposite for enhanced antibacterial and photocatalytic activity: As a bionanocomposite. Int. J. Biol. Macromol. 2019, 129, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Phan, D.-N.; Rebia, R.A.; Saito, Y.; Kharaghani, D.; Khatri, M.; Tanaka, T.; Lee, H.; Kim, I.-S. Zinc oxide nanoparticles attached to polyacrylonitrile nanofibers with hinokitiol as gluing agent for synergistic antibacterial activities and effective dye removal. J. Ind. Eng. Chem. 2020, 85, 258–268. [Google Scholar] [CrossRef]
- Kakiuchi, K.; Hosono, E.; Kimura, T.; Imai, H.; Fujihara, S. Fabrication of mesoporous ZnO nanosheets from precursor templates grown in aqueous solutions. J. Sol-Gel Sci. Technol. 2006, 39, 63–72. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Bi, S.; Luo, G. Preparation of ZnO nanoparticles using the direct precipitation method in a membrane dispersion micro-structured reactor. Powder Technol. 2010, 202, 130–136. [Google Scholar] [CrossRef]
- Gunalan, S.; Sivaraj, R.; Rajendran, V. Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Prog. Nat. Sci. 2012, 22, 693–700. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, H.; Nakara, A.; Menon, S.; Shanmugam, V. Eco-friendly synthesis of zinc oxide nanoparticles using Cinnamomum Tamala leaf extract and its promising effect towards the antibacterial activity. J. Drug Deliv. Sci. Technol. 2019, 53, 101212. [Google Scholar] [CrossRef]
- Dhandapani, K.V.; Anbumani, D.; Gandhi, A.D.; Annamalai, P.; Muthuvenkatachalam, B.S.; Kavitha, P.; Ranganathan, B. Green route for the synthesis of zinc oxide nanoparticles from Melia azedarach leaf extract and evaluation of their antioxidant and antibacterial activities. Biocatal. Agric. Biotechnol. 2020, 24, 101517. [Google Scholar] [CrossRef]
- Singhal, U.; Pendurthi, R.; Khanuja, M. Prunus: A natural source for synthesis of zinc oxide nanoparticles towards photocatalytic and antibacterial applications. Mater. Today Proc. 2020, 28, 261–265. [Google Scholar] [CrossRef]
- Darvishi, E.; Kahrizi, D.; Arkan, E. Comparison of different properties of zinc oxide nanoparticles synthesized by the green (using Juglans regia L. leaf extract) and chemical methods. J. Mol. Liq. 2019, 286, 110831. [Google Scholar] [CrossRef]
- Agarwal, H.; Menon, S.; Kumar, S.V.; RajeshKumar, S. Mechanistic study on antibacterial action of zinc oxide nanoparticles synthesized using green route. Chem. Interact. 2018, 286, 60–70. [Google Scholar] [CrossRef]
- Elumalai, K.; Velmurugan, S.; Ravi, S.; Kathiravan, V.; Raj, G.A. Bio-approach: Plant mediated synthesis of ZnO nanoparticles and their catalytic reduction of methylene blue and antimicrobial activity. Adv. Powder Technol. 2015, 26, 1639–1651. [Google Scholar] [CrossRef]
- Ahmed, B.; Solanki, B.; Zaidi, A.; Khan, M.S.; Musarrat, J. Bacterial toxicity of biomimetic green zinc oxide nanoantibiotic: Insights into ZnONP uptake and nanocolloid–bacteria interface. Toxicol. Res. 2019, 8, 246–261. [Google Scholar] [CrossRef] [Green Version]
- Zanni, E.; Chandraiahgari, C.R.; De Bellis, G.; Montereali, M.R.; Armiento, G.; Ballirano, P.; Polimeni, A.; Sarto, M.S.; Uccelletti, D. Zinc Oxide Nanorods-Decorated Graphene Nanoplatelets: A Promising Antimicrobial Agent against the Cariogenic Bacterium Streptococcus mutans. Nanomaterials 2016, 6, 179. [Google Scholar] [CrossRef]
- Li, M.; Li, G.; Jiang, J.; Tao, Y.; Mai, K. Preparation, antimicrobial, crystallization and mechanical properties of nano-ZnO-supported zeolite filled polypropylene random copolymer composites. Compos. Sci. Technol. 2013, 81, 30–36. [Google Scholar] [CrossRef]
- Tiwari, V.; Mishra, N.; Gadani, K.; Solanki, P.; Shah, N.A.; Tiwari, M. Mechanism of Anti-bacterial Activity of Zinc Oxide Nanoparticle Against Carbapenem-Resistant Acinetobacter baumannii. Front. Microbiol. 2018, 9, 1218. [Google Scholar] [CrossRef] [Green Version]
- Shaba, E.Y.; Jacob, J.O.; Tijani, J.O.; Suleiman, M.A.T. A critical review of synthesis parameters affecting the properties of zinc oxide nanoparticle and its application in wastewater treatment. Appl. Water Sci. 2021, 11, 48. [Google Scholar] [CrossRef]
- Singh, A.; Singh, N.B.; Afzal, S.; Singh, T.; Hussain, I. Zinc oxide nanoparticles: A review of their biological synthesis, antimicrobial activity, uptake, translocation and biotransformation in plants. J. Mater. Sci. 2017, 53, 185–201. [Google Scholar] [CrossRef]
- da Silva, B.L.; Abuçafy, M.P.; Manaia, E.B.; Junior, J.A.O.; Chiari-Andréo, B.G.; Pietro, R.C.R.; Chiavacci, L.A. Relationship Between Structure And Antimicrobial Activity Of Zinc Oxide Nanoparticles: An Overview. Int. J. Nanomed. 2019, 14, 9395–9410. [Google Scholar] [CrossRef] [Green Version]
- Matusoiu, F.; Negrea, A.; Nemes, N.S.; Ianasi, C.; Ciopec, M.; Negrea, P.; Duteanu, N.; Ianasi, P.; Duda-Seiman, D.; Muntean, D. Antimicrobial Perspectives of Active SiO2FexOy/ZnO Composites. Pharmaceutics 2022, 14, 2063. [Google Scholar] [CrossRef]
- Alwin, S.; Shajan, X.S. Facile synthesis of 3-D nanostructured zinc oxide aerogel and its application as photoanode material for dye-sensitized solar cells. Surf. Interfaces 2017, 7, 14–19. [Google Scholar] [CrossRef]
- Kairyte, K.; Kadys, A.; Luksiene, Z. Antibacterial and antifungal activity of photoactivated ZnO nanoparticles in suspension. J. Photochem. Photobiol. B Biol. 2013, 128, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.L.; Fang, C.M.; Pung, S.Y.; Ong, C.E.; Pung, Y.F.; Kong, C.; Pan, Y. Current Updates On the In vivo Assessment of Zinc Oxide Nanoparticles Toxicity Using Animal Models. Bionanoscience 2021, 11, 590–620. [Google Scholar] [CrossRef]
- Waghmode, B.J.; Husain, Z.; Joshi, M.; Sathaye, S.D.; Patil, K.R.; Malkhede, D.D. Synthesis and study of calixarene-doped polypyrrole-TiO2/ZnO composites: Antimicrobial activity and electrochemical sensors. J. Polym. Res. 2016, 23, 35. [Google Scholar] [CrossRef]
- Rahman, P.M.; Mujeeb, V.A.; Muraleedharan, K.; Thomas, S.K. Chitosan/nano ZnO composite films: Enhanced mechanical, antimicrobial and dielectric properties. Arab. J. Chem. 2018, 11, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Shankar, S.; Rhim, J.-W. Effect of types of zinc oxide nanoparticles on structural, mechanical and antibacterial properties of poly(lactide)/poly(butylene adipate-co-terephthalate) composite films. Food Packag. Shelf Life 2019, 21, 100327. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Khattak, W.A.; Ullah, M.W.; Khan, S.; Park, J.K. Synthesis of regenerated bacterial cellulose-zinc oxide nanocomposite films for biomedical applications. Cellulose 2013, 21, 433–447. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Joorabloo, A.; Moghaddam, A.; Shamsi, H.; MansooriMoghadam, Z. Incorporation of ZnO nanoparticles into heparinised polyvinyl alcohol/chitosan hydrogels for wound dressing application. Int. J. Biol. Macromol. 2018, 114, 1203–1215. [Google Scholar] [CrossRef]
- Kim, J.H.; Joshi, M.K.; Lee, J.; Park, C.H.; Kim, C.S. Polydopamine-assisted immobilization of hierarchical zinc oxide nanostructures on electrospun nanofibrous membrane for photocatalysis and antimicrobial activity. J. Colloid Interface Sci. 2018, 513, 566–574. [Google Scholar] [CrossRef]
- Augustine, R.; Malik, H.; Singhal, D.K.; Mukherjee, A.; Malakar, D.; Kalarikkal, N.; Thomas, S. Electrospun polycaprolactone/ZnO nanocomposite membranes as biomaterials with antibacterial and cell adhesion properties. J. Polym. Res. 2014, 21, 347. [Google Scholar] [CrossRef]
- Cordero-Arias, L.; Cabanas-Polo, S.; Goudouri, O.M.; Misra, S.K.; Gilabert, J.; Valsami-Jones, E.; Sanchez, E.; Virtanen, S.; Boccaccini, A.R. Electrophoretic deposition of ZnO/alginate and ZnO-bioactive glass/alginate composite coatings for antimicrobial applications. Mater. Sci. Eng. C 2015, 55, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Anagha, B.; George, D.; Maheswari, P.U.; Begum, K.M.M.S. Biomass Derived Antimicrobial Hybrid Cellulose Hydrogel with Green ZnO Nanoparticles for Curcumin Delivery and its Kinetic Modelling. J. Polym. Environ. 2019, 27, 2054–2067. [Google Scholar] [CrossRef]
- Rahman, M.M. Polyurethane/Zinc Oxide (PU/ZnO) Composite—Synthesis, Protective Propertyand Application. Polymers 2020, 12, 1535. [Google Scholar] [CrossRef]
- Pandiselvi, K.; Thambidurai, S. Synthesis, characterization, and antimicrobial activity of chitosan-zinc oxide/polyaniline composites. Mater. Sci. Semicond. Process 2015, 31, 573–581. [Google Scholar] [CrossRef]
- Zanini, N.C.; Souza, A.G.; Barbosa RF, S.; Rosa, D.S.; Mulinari, D.R. A novel hybrid polyurethane composites with ZnO particles and sheath palm residues: Synergistic effect. Polym. Compos. 2021, 42, 532–542. [Google Scholar] [CrossRef]
- Li, L.-H.; Deng, J.-C.; Deng, H.-R.; Liu, Z.-L.; Li, X.-L. Preparation, characterization and antimicrobial activities of chitosan/Ag/ZnO blend films. Chem. Eng. J. 2010, 160, 378–382. [Google Scholar] [CrossRef]
- Rahman, P.M.; Mujeeb, V.A.; Muraleedharan, K. Flexible chitosan-nano ZnO antimicrobial pouches as a new material for extending the shelf life of raw meat. Int. J. Biol. Macromol. 2017, 97, 382–391. [Google Scholar] [CrossRef]
- Gong, X.; Tang, C.Y.; Pan, L.; Hao, Z.; Tsui, C.P. Characterization of poly(vinyl alcohol) (PVA)/ZnO nanocomposites prepared by a one-pot method. Compos. B Eng. 2014, 60, 144–149. [Google Scholar] [CrossRef]
- Petchwattana, N.; Covavisaruch, S.; Wibooranawong, S.; Naknaen, P. Antimicrobial food packaging prepared from poly(butylene succinate) and zinc oxide. Measurement 2016, 93, 442–448. [Google Scholar] [CrossRef]
- Chu, Z.; Zhao, T.; Li, L.; Fan, J.; Qin, Y. Characterization of Antimicrobial Poly (Lactic Acid)/Nano-Composite Films with Silver and Zinc Oxide Nanoparticles. Materials 2017, 10, 659. [Google Scholar] [CrossRef] [Green Version]
- Steffy, K.; Shanthi, G.; Maroky Anson, S.; Selvakumar, S. Potential bactericidal activity of S. nux-vomica–ZnO nanocomposite against multidrug-resistant bacterial pathogens and wound-healing properties. J. Trace Elem. Med. Biol. 2018, 50, 229–239. [Google Scholar] [CrossRef]
- Prasert, A.; Sontikaew, S.; Sriprapai, D.; Chuangchote, S. Polypropylene/ZnO Nanocomposites: Mechanical Properties, Photocatalytic Dye Degradation, and Antibacterial Property. Materials 2020, 13, 914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Jia, X.; Zhi, C.; Jin, Z.; Miao, M. Improving the properties of starch-based antimicrobial composite films using ZnO-chitosan nanoparticles. Carbohydr. Polym. 2019, 210, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Dhapte, V.; Gaikwad, N.; More, P.V.; Banerjee, S.; Dhapte, V.V.; Kadam, S.; Khanna, P.K. Transparent ZnO/polycarbonate nanocomposite for food packaging application. Nanocomposites 2015, 1, 106–112. [Google Scholar] [CrossRef]
- El Saeed, A.M.; Abd El-Fattah, M.; Azzam, A.M. Synthesis of ZnO nanoparticles and studying its influence on the antimicrobial, anticorrosion and mechanical behavior of polyurethane composite for surface coating. Dye. Pigment. 2015, 121, 282–289. [Google Scholar] [CrossRef]
- Anitha, S.; Brabu, B.; Thiruvadigal, D.J.; Gopalakrishnan, C.; Natarajan, T. Optical, bactericidal and water repellent properties of electrospun nano-composite membranes of cellulose acetate and ZnO. Carbohydr. Polym. 2012, 87, 1065–1072. [Google Scholar] [CrossRef]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J. Chitosan-zinc oxide nanoparticle composite coating for active food packaging applications. Innov. Food Sci. Emerg. Technol. 2016, 38, 231–237. [Google Scholar] [CrossRef]
- Rath, G.; Hussain, T.; Chauhan, G.; Garg, T.; Goyal, A.K. Development and characterization of cefazolin loaded zinc oxide nanoparticles composite gelatin nanofiber mats for postoperative surgical wounds. Mater. Sci. Eng. C 2016, 58, 242–253. [Google Scholar] [CrossRef]
- Tan, S.T.; Winarto, N.; Dosan, R.; Aisyah, P.B. The Benefits Of Occlusive Dressings In Wound Healing. Open Dermatol. J. 2019, 13, 27–33. [Google Scholar] [CrossRef]
- Vaezi, K.; Asadpour, G.; Sharifi, H. Effect of ZnO nanoparticles on the mechanical, barrier and optical properties of thermoplastic cationic starch/montmorillonite biodegradable films. Int. J. Biol. Macromol. 2018, 124, 519–529. [Google Scholar] [CrossRef]
- George, D.; Maheswari, P.U.; Begum, K.M.S. Synergic formulation of onion peel quercetin loaded chitosan-cellulose hydrogel with green zinc oxide nanoparticles towards controlled release, biocompatibility, antimicrobial and anticancer activity. Int. J. Biol. Macromol. 2019, 132, 784–794. [Google Scholar] [CrossRef]
- Siyanbola, T.O.; Sasidhar, K.; Rao, B.V.; Narayan, R.; Olaofe, O.; Akintayo, E.T.; Raju, K.V. Development of functional polyurethane-ZnO hybrid nanocomposite coatings from Thevetia peruviana seed oil. JAOCS. J. Am. Oil Chem. Soc. 2015, 92, 267–275. [Google Scholar] [CrossRef]
- Ma, X.-Y.; Zhang, W.-D. Effects of flower-like ZnO nanowhiskers on the mechanical, thermal and antibacterial properties of waterborne polyurethane. Polym. Degrad. Stab. 2009, 94, 1103–1109. [Google Scholar] [CrossRef]
- Dural Erem, A.; Ozcan, G.; Skrifvars, M. Antibacterial activity of PA6/ZnO nanocomposite fibers. Text. Res. J. 2011, 81, 1638–1646. [Google Scholar] [CrossRef]
- Sirohi, S.; Singh, R.; Jain, N.; Pani, B.; Dutt, K.; Nain, R. Synthesis and characterization of multifunctional ZnO/polyester green composite films. J. Polym. Res. 2017, 24, 193. [Google Scholar] [CrossRef]
- Shekh, M.I.; Patel, K.P.; Patel, R.M. Electrospun ZnO Nanoparticles Doped Core–Sheath Nanofibers: Characterization and Antimicrobial Properties. J. Polym. Environ. 2018, 26, 4376–4387. [Google Scholar] [CrossRef]
- Li, X.-F.; Feng, X.-Q.; Yang, S.; Fu, G.-Q.; Wang, T.-P.; Su, Z.-X. Chitosan kills Escherichia coli through damage to be of cell membrane mechanism. Carbohydr. Polym. 2010, 79, 493–499. [Google Scholar] [CrossRef]
- Liu, H.; Du, Y.; Wang, X.; Sun, L. Chitosan kills bacteria through cell membrane damage. Int. J. Food Microbiol. 2004, 95, 147–155. [Google Scholar] [CrossRef]
- Helander, I.; Nurmiaho-Lassila, E.-L.; Ahvenainen, R.; Rhoades, J.; Roller, S. Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. Int. J. Food Microbiol. 2001, 71, 235–244. [Google Scholar] [CrossRef]
- Zhao, G.; Usui, M.L.; Lippman, S.I.; James, G.A.; Stewart, P.S.; Fleckman, P.; Olerud, J.E. Biofilms and Inflammation in Chronic Wounds. Adv. Wound Care 2013, 2, 389–399. [Google Scholar] [CrossRef] [Green Version]
- El Fawal, G.; Hong, H.; Song, X.; Wu, J.; Sun, M.; He, C.; Mo, X.; Jiang, Y.; Wang, H. Fabrication of antimicrobial films based on hydroxyethylcellulose and ZnO for food packaging application. Food Packag. Shelf Life 2020, 23, 100462. [Google Scholar] [CrossRef]
- Yu, F.; Fei, X.; He, Y.; Li, H. Poly(lactic acid)-based composite film reinforced with acetylated cellulose nanocrystals and ZnO nanoparticles for active food packaging. Int. J. Biol. Macromol. 2021, 186, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Yadollahi, M.; Gholamali, I.; Namazi, H.; Aghazadeh, M. Synthesis and characterization of antibacterial carboxymethyl cellulose/ZnO nanocomposite hydrogels. Int. J. Biol. Macromol. 2015, 74, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, S.; Emaminia, S.; Davudian, S.H.; Pourmohammad, S.; Hamishehkar, H.; Roufegarinejad, L. Preparation and characterization of gelatin-based nanocomposite containing chitosan nanofiber and ZnO nanoparticles. Carbohydr. Polym. 2019, 216, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Lan, W.; Ji, T.; Sameen, D.E.; Ahmed, S.; Qin, W.; Liu, Y. Development of polylactic acid/ZnO composite membranes prepared by ultrasonication and electrospinning for food packaging. LWT 2020, 135, 110072. [Google Scholar] [CrossRef]
- Mocanu, A.; Isopencu, G.; Busuioc, C.; Popa, O.-M.; Dietrich, P.; Socaciu-Siebert, L. Bacterial cellulose films with ZnO nanoparticles and propolis extracts: Synergistic antimicrobial effect. Sci. Rep. 2019, 9, 17687. [Google Scholar] [CrossRef] [Green Version]
- Blinov, A.V.; Kachanov, M.D.; Gvozdenko, A.A.; Nagdalian, A.A.; Blinova, A.A.; Rekhman, Z.A.; Golik, A.B.; Vakalov, D.S.; Maglakelidze, D.G.; Nagapetova, A.G.; et al. Synthesis and Characterization of Zinc Oxide Nanoparticles Stabilized with Biopolymers for Application in Wound-Healing Mixed Gels. Gels 2023, 9, 57. [Google Scholar] [CrossRef]
- Hasanin, M.; Swielam, E.M.; Atwa, N.A.; Agwa, M.M. Novel design of bandages using cotton pads, doped with chitosan, glycogen and ZnO nanoparticles, having enhanced antimicrobial and wounds healing effects. Int. J. Biol. Macromol. 2021, 197, 121–130. [Google Scholar] [CrossRef]
- Rakhshaei, R.; Namazi, H. A potential bioactive wound dressing based on carboxymethyl cellulose/ZnO impregnated MCM-41 nanocomposite hydrogel. Mater. Sci. Eng. C 2017, 73, 456–464. [Google Scholar] [CrossRef]
- Raguvaran, R.; Manuja, B.K.; Chopra, M.; Thakur, R.; Anand, T.; Kalia, A.; Manuja, A. Sodium alginate and gum acacia hydrogels of ZnO nanoparticles show wound healing effect on fibroblast cells. Int. J. Biol. Macromol. 2017, 96, 185–191. [Google Scholar] [CrossRef]


| Biopolymer | Properties | Refs. |
|---|---|---|
| Polysaccharides | ||
| Alginate | Biocompatible, non-toxic, non-immunogenic, biodegradable, antimicrobial, and haemostatic | [39,41,42,43] |
| Chitosan | Non-toxic, biocompatible, biodegradable, moisture retentive, and haemostatic | [44,45,46,47,48] |
| Chitosan oligosaccharide | Antibacterial, anti-inflammatory, and immune-stimulating | [49] |
| Cellulose | Biocompatible, hydrophilic, microporous, transparent, and non-toxic | [50,51,52,53] |
| Carboxymethyl-cellulose | Biocompatible and biodegradable | [54,55,56,57] |
| Carboxymethyl-chitosan | Amphoteric and hydrophilic | [58,59,60] |
| Gum acacia | Biocompatible, nontoxic, and water-soluble | [61] |
| Starch | Low-cost, biocompatible, biodegradable, easy preparation, and good film-forming properties | [62,63,64,65] |
| Carrageenan | Water-soluble, and good film-forming abilities and mechanical properties | [66,67,68] |
| Cellulose acetate | Water-insoluble, high transparency, and good mechanical and chemical resistance | [69,70] |
| Cellulose acetate phthalate | Hydrophilic and biodegradable | [71] |
| Oxidised starch | Low viscosity, and high stability and transparency | [64] |
| Dextran | Active in wound healing and controls bacterial growth | [39,72] |
| Chitin | Biodegradable, haemostatic, and cytocompatible | [73] |
| β-glucans | Immunostimulatory, and biodegradable | [74] |
| Hyaluronic Acid | Primary component of connective tissue, safe long-term, and reduces bacterial adhesion | [75,76] |
| Proteins | ||
| Collagen | High biocompatibility and biodegradability. Bioactive | [77] |
| Keratin | Low-cost and highly suitable for hydrogels, but concerns about wastes | [78,79] |
| Fish protein isolate | High film-forming ability | [80,81,82] |
| Gelatin | Biodegradable, biocompatible, and cell-recognizable | [39,56,81,83,84,85] |
| Soy protein isolate | Biocompatible, non-immunogenic, non-toxic, low-cost, biodegradable, and highly stable | [86] |
| Silk fibroin | Biocompatible, biodegradable, and minimum inflammatory reactions | [87] |
| Matrix Type | EM (mPa) | TS (Mpa) | nZnO (% wt) | Refs. | ||
|---|---|---|---|---|---|---|
| Pure | Filled | Pure | Filled | |||
| Agar | 1004.9 | 109.8 | 34.6 | 13.0 | - | [68] |
| Carrageenan | 1112.1 | 130.4 | 44.6 | 12.3 | - | [68] |
| Carboxymethyl-cellulose | 20.5 | 14.0 | 6.4 | 5.1 | - | [68] |
| Gelatin | 1451.2 | 262.5–344.4 | 50.1 | 29.8–33.4 | - | [56] |
| Chitosan | 1821 | 3304 | 12.84 | 41.73 | 1–2 | [48,189] |
| Starch | 7.8 | 25.44 | 4.11 | 12.73 | 2–3 | [65,207] |
| Poly(lactic acid)/poly(butyleneadipate-co-terephthalate | 800 | 970–1220 | 14.5 | 16.8–26.9 | 3 | [190] |
| Bacterial cellulose | 93.8 | 132.11 | 26.3 | 45.21 | 5 | [53] |
| Poly(lactic acid) | 3118.8 | 2610.64 | 47.78 | 39.96 | 3 | [204] |
| Poly(caprolactone) nanofibers | 3.70 | 5.25–3.78 | 1.40 | 1.60–0.98 | 1–6 | [194] |
| Matrix Type | WCA (deg) | nZnO (% wt) | Refs. | |
|---|---|---|---|---|
| Pure | Filled | |||
| Agar | 66.5 | 68.8 | - | [68] |
| Carrageenan | 61.6 | 84.5 | - | [68] |
| Carboxymethyl-cellulose | 31.6 | 55.2 | - | [68] |
| Gelatin | 52.4 | 62.15 | - | [56] |
| Starch | 51 | 43 | 2–3 | [65,207] |
| Poly(lactic acid)/poly(butyleneadipate-co-terephthalate | 82 | 78.1–88.3 | 3 | [190] |
| Chitosan-cellulose acetate phthalate | 57 | 81 | 5 | [71] |
| Cellulose acetate nanofibers | 47 | 124 | - | [210] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pino, P.; Bosco, F.; Mollea, C.; Onida, B. Antimicrobial Nano-Zinc Oxide Biocomposites for Wound Healing Applications: A Review. Pharmaceutics 2023, 15, 970. https://doi.org/10.3390/pharmaceutics15030970
Pino P, Bosco F, Mollea C, Onida B. Antimicrobial Nano-Zinc Oxide Biocomposites for Wound Healing Applications: A Review. Pharmaceutics. 2023; 15(3):970. https://doi.org/10.3390/pharmaceutics15030970
Chicago/Turabian StylePino, Paolo, Francesca Bosco, Chiara Mollea, and Barbara Onida. 2023. "Antimicrobial Nano-Zinc Oxide Biocomposites for Wound Healing Applications: A Review" Pharmaceutics 15, no. 3: 970. https://doi.org/10.3390/pharmaceutics15030970
APA StylePino, P., Bosco, F., Mollea, C., & Onida, B. (2023). Antimicrobial Nano-Zinc Oxide Biocomposites for Wound Healing Applications: A Review. Pharmaceutics, 15(3), 970. https://doi.org/10.3390/pharmaceutics15030970






