Biodistribution and Anticancer Characteristics of Les-3833, A Novel 4-thiazolidinone-Based Lead Compound
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drug
2.2. Cytotoxicity In Vitro
2.3. In Vivo Biodistribution Study
2.3.1. Calibration Standards for Quantification of Les-3833 in Blood Plasma Samples
2.3.2. Calibration Standards for Quantification of Les-3833 in Brain Tissue
2.3.3. Calibration Standards for Quantification of Les-3833 in Liver and Kidney Tissues
2.4. Statistical Analysis
3. Results
3.1. Evaluation of Cytotoxic Effect of Les-3833 on Different Cell Lines In Vitro
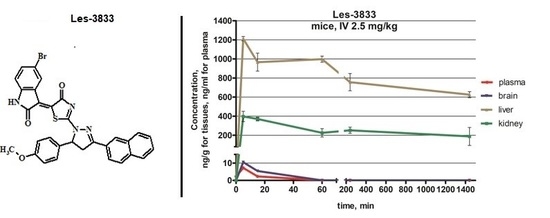
3.2. Biodistribution Study of Les-3833 In Vivo
3.3. Biodistribution Parameters for Les-3833 in Male Balb/c Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ali, R.; Mirza, Z.; Ashraf, G.M.; Kamal, M.A.; Ansari, S.A.; Damanhouri, G.A.; Abuzenadah, A.M.; Chaudhary, A.G.; Sheikh, I.A. New anticancer agents: Recent developments in tumor therapy. Anticancer Res. 2012, 32, 2999–3005. [Google Scholar] [PubMed]
- Prager, G.; Unseld, M.; Waneck, F.; Mader, R.; Wrba, F.; Raderer, M.; Fuereder, T.; Staber, P.; Jäger, U.; Kieler, M.; et al. Results of the extended analysis for cancer treatment (EXACT) trial: A prospective translational study evaluating individualized treatment regimens in oncology. Oncotarget 2019, 10, 942–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faqi, A.S. A Comprehensive Guide to Toxicology in Nonclinical Drug Development, 2nd ed.; Elsevier: London, UK, 2017; p. 657. [Google Scholar]
- Kobayashi, H.; Carrasquillo, J.A.; Paik, C.H.; Waldmann, T.A.; Tagaya, Y. Differences of biodistribution, pharmacokinetics, and tumor targeting between interleukins 2 and 15. Cancer Res. 2000, 60, 3577–3583. [Google Scholar] [PubMed]
- Xiao, W.; Luo, J.; Lam, K.; Henderson, P.; Jain, T.; Wriggs, J.; Tseng, H.P.; Henderson, P.T.; Cherry, S.R.; Rowland, D.; et al. Biodistribution and pharmacokinetics of a telodendrimer micellar paclitaxel nanoformulation in a mouse xenograft model of ovarian cancer. Int. J. Nanomed. 2012, 7, 1587–1597. [Google Scholar] [CrossRef] [Green Version]
- England, C.; Ehlerding, E.; Hernandez, R.; Rekoske, B.; Graves, S.; Sun, H.; Liu, G.; McNeel, D.G.; Barnhart, T.E.; Cai, W. Preclinical Pharmacokinetics and Biodistribution Studies of 89Zr-Labeled Pembrolizumab. J. Nucl. Med. 2016, 58, 162–168. [Google Scholar] [CrossRef] [Green Version]
- Matzneller, P.; Kussmann, M.; Eberl, S.; Maier-Salamon, A.; Jäger, W.; Bauer, M.; Langer, O.; Zeitlinger, M.; Poeppl, W. Pharmacokinetics of the P-gp Inhibitor Tariquidar in Rats After Intravenous, Oral, and Intraperitoneal Administration. Eur. J. Drug. Metab. Ph. 2018, 43, 599–606. [Google Scholar] [CrossRef] [Green Version]
- Wilsker, D.; Barrett, A.; Dull, A.; Lawrence, S.; Hollingshead, M.; Chen, A.; Kummar, S.; Parchment, R.E.; Doroshow, J.H.; Kinders, R.J. Evaluation of Pharmacodynamic Responses to Cancer Therapeutic Agents Using DNA Damage Markers. Clin. Cancer Res. 2019, 25, 3084–3095. [Google Scholar] [CrossRef] [Green Version]
- Kobylinska, L.; Klyuchivska, O.; Grytsyna, I.; Finiuk, N.; Panchuk, R.; Starykovych, M.; Lehka, L.; Lesyk, R.B.; Zimenkovsky, B.S.; Stoika, R.S. Differential pro-apoptotic effects of synthetic 4-thiazolidinone derivative Les-3288, doxorubicin and temozolomide in human glioma U251 cells. Croat. Med. J. 2017, 58, 150–159. [Google Scholar] [CrossRef] [Green Version]
- Kobylinska, L.; Klyuchivska, O.; Lesyk, R.; Stoika, R. Targeting of the pro-oxidant-antioxidant balance in vitro and in vivo by 4-thiazolidinone-based chemotherapeutics with anticancer potential. Ukr. Biochem. J. 2019, 91, 7–17. [Google Scholar] [CrossRef] [Green Version]
- Kobylinska, L.; Skorohyd, N.; Klyuchivska, O.; Mitina, N.; Zaichenko, A.; Lesyk, R.; Zimenkovsky, B.S.; Stoika, R.S. Increased antitumor efficiency and reduced negative side effects in laboratory mice of 4-thiazolidinone derivatives in complexes with PEG-containing polymeric nanocarrier. Biopolym. Cell. 2018, 34, 313–328. [Google Scholar] [CrossRef] [Green Version]
- Havrylyuk, D.; Zimenkovsky, B.; Vasylenko, O.; Day, C.; Smee, D.; Grellier, P.; Lesyk, R. Synthesis and biological activity evaluation of 5-pyrazoline substituted 4-thiazolidinones. Eur. J. Med. Chem. 2013, 66, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Havrylyuk, D.; Kovach, N.; Zimenkovsky, B.; Vasylenko, O.; Lesyk, R. Synthesis and Anticancer Activity of Isatin-Based Pyrazolines and Thiazolidines Conjugates. Arch. Pharm. 2011, 344, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Cutshall, N.; O’Day, C.; Prezhdo, M. Rhodanine derivatives as inhibitors of JSP-1. Bioorg. Med. Chem. Lett. 2005, 15, 3374–3379. [Google Scholar] [CrossRef] [PubMed]
- Carter, P.; Scherle, P.; Muckelbauer, J.; Voss, M.; Liu, R.; Thompson, L.; Tebben, A.J.; Solomon, K.A.; Lo, Y.C.; Li, Z.; et al. Photochemically enhanced binding of small molecules to the tumor necrosis factor receptor-1 inhibits the binding of TNF-α. Proc. Natl. Acad. Sci. USA 2001, 98, 11879–11884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manna, F.; Chimenti, F.; Fioravanti, R.; Bolasco, A.; Secci, D.; Chimenti, P.; Ferlini, C.; Scambia, G. Synthesis of some pyrazole derivatives and preliminary investigation of their affinity binding to P-glycoprotein. Bioorg. Med. Chem. Lett. 2005, 15, 4632–4635. [Google Scholar] [CrossRef]
- Kobylinska, L.; Ivasechko, I.; Skorokhyd, N.; Panchuk, R.; Riabtseva, A.; Mitina, N.; Zaichenko, A.; Lesyk, R.; Zimenkovsky, B.; Stoika, R.; et al. Enhanced Proapoptotic Effects of Water Dispersed Complexes of 4-Thiazolidinone-Based Chemotherapeutics with a PEG-Containing Polymeric Nanocarrier. Nanoscale Res. Lett. 2019, 14. [Google Scholar] [CrossRef] [Green Version]
- Havrylyuk, D.; Zimenkovsky, B.; Vasylenko, O.; Gzella, A.; Lesyk, R. Synthesis of New 4-Thiazolidinone-, Pyrazoline-, and Isatin-Based Conjugates with Promising Antitumor Activity. J. Med. Chem. 2012, 55, 8630–8641. [Google Scholar] [CrossRef]
- Marenberg, B. FDA Issues Final Rule on Patent Listing Requirements and 30-Month Stays of Approval Following Submission of Abbreviated New Drug Applications. Biotechnol. Law Rep. 2004, 23, 48–51. [Google Scholar] [CrossRef]
- Kobylinska, L.; Boiko, N.; Panchuk, R.; Grytsyna, I.; Klyuchivska, O.; Biletska, L.; Lesyk, R.B.; Zimenkovsky, B.S.; Stoika, R.S. Putative anticancer potential of novel 4-thiazolidinone derivatives: Cytotoxicity toward rat C6 glioma in vitro and correlation of general toxicity with the balance of free radical oxidation in rats. Croat. Med. J. 2016, 57, 151–163. [Google Scholar] [CrossRef] [Green Version]
- Kobylinska, L.; Havrylyuk, D.Y.; Ryabtseva, A.O.; Mitina, N.E.; Zaichenko, O.S.; Zimenkovsky, B.S.; Stoika, R.S. Study of rat blood serum biochemical indicators of cardiotoxic action of novel antitumor 4-thiazolidinone derivatives and doxorubicin in complexes with polyethylenglycol-containing polymeric carrier in the rat blood serum. Ukr. Biochem. J. 2014, 86, 84–95. [Google Scholar] [CrossRef] [Green Version]
- Chumak, V.; Panchuk, R.; Manko, N.; Havrylyuk, D.; Lesyk, R.; Kobylinska, L.; Zimenkovsky, B.; Stoika, R. Comparative study of the cytotoxic properties of isatin-containing derivatives of 4-thiazolidinone with different structure toward human tumor cells in vitro. Stud Biol. 2014, 8, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Kobylinska, L.; Havrylyuk, D.Y.; Ryabtseva, A.O.; Mitina, N.E.; Zaichenko, O.S.; Lesyk, R.B.; Zimenkovsky, B.S.; Stoika, R.S. Biochemical indicators of hepatotoxicity in blood serum of rats under the effect of novel 4-thiazolidinone derivatives and doxorubicin and their complexes with polyethyleneglycol-containing nanoscale polymeric carrier. Ukr. Biochem. J. 2015, 87, 122–132. [Google Scholar] [CrossRef] [Green Version]
- Kobylinska, L.; Havrylyuk, D.; Mitina, N.; Zichenko, A.S.; Lesyk, R.B.; Zimenkovsky, B.S.; Stoika, R.S. Biochemical indicators of nephrotoxicity in blood serum of rats treated with novel 4-thiazolidinone derivatives or their complexes with polyethylene glycol-containing nanoscale polymeric carrier. Ukr. Biochem. J. 2016, 88, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Rollerova, E.; Tulinska, J.; Liskova, A.; Kuricova, M.; Kovriznych, J.; Mlynarcikova, A.; Kiss, A.; Scsukova, S. Titanium dioxide nanoparticles: Some aspects of toxicity/focus on the development. Endocr. Regul. 2015, 49, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Carmichael, D.; Harris, M.; Roh, J.K. Comparative pharmacokinetics of free doxorubicin and doxorubicin entrapped in cardiolipin liposomes. Cancer Res. 1986, 46, 2295–2299. [Google Scholar] [PubMed]
- Beechinor, R.; Gonzalez, D. Book Review: Introduction to Drug Disposition and Pharmacokinetics. Clin. Pharmacol. Ther. 2017, 102, 893. [Google Scholar] [CrossRef]




| Number of Mice (Male) | Compound | Formulation | Delivery Route | Target Dose Level (mg/kg) | Target Dose Concentration (mg/mL) | Target Dose Volume (ml/kg) |
|---|---|---|---|---|---|---|
| 50 | Les-3833 | 20%:80% Captisol: physiological saline solution | IV | 2.5 | 0.5 | 5 |
| 5 | Vehicle | 20%:80% Captisol: physiological saline solution | IV | 0 | 0 | 5 |
| Cell Lines | Les-3833 µg/mL | Doxorubicin µg/mL |
|---|---|---|
| human acute promyelocytic leukemia HL-60 | 2.04 ± 0.21 | 0.09 ± 0.01 |
| breast adenocarcinoma cells MCF-7 | 4.75 ± 0.24 | 1.38 ± 0.12 |
| human glioma U251 cells | 0.84 ± 0.09 | 0.90 ± 0.11 |
| rat glioma C6 cells | 0.89 ± 0.12 | 0.84 ± 0.08 |
| human melanoma WM793 cells | 0.22 ± 0.03 | 0.24 ± 0.05 |
| human melanoma SK-MEL-28 cells | 0.30 ± 0.04 | 0.35 ± 0.06 |
| human lung carcinoma A549 cells | 2.50 ± 0.19 | 0.80 ± 0.05 |
| human colorectal carcinoma HCT-116 | 3.40 ± 0.32 | 1.10 ± 0.09 |
| human embryonic kidney HEK293 cells | >5 | 3.20 ± 0.42 |
| Sample | Administration Route | Dose, mg/kg | Tmax, min | Cmax, ng/mL, ng/g | AUC0→t min (AUClast) ng × min/mL | AUC0→∞ (AUCINF_obs) ng × min/mL | T1/2 (HL_ λ _z), min | Kel (λ_z), min-1 |
|---|---|---|---|---|---|---|---|---|
| Plasma | IV | 2.5 | 2.08 | 5.55 ± 0.25 | 119.9 | 119.9 | 15 | 1.10 × 10−1 |
| Brain | IV | 2.5 | 5.00 | 7.17 ± 3.58 | 205.2 | 205.2 | 15 | 2.74 × 10−2 |
| Liver | IV | 2.5 | 5.00 | 1246 ± 65 | 1,190,000 | 4,070,000 | 2880 | 2.40 × 10−4 |
| Kidney | IV | 2.5 | 15.0 | 404 ± 39 | 370,000 | 1,280,000 | 3090 | 2.24 × 10−4 |
| Plasma | Brain | Liver | Kidney | |
|---|---|---|---|---|
| Clearance | 2.09 × 10−2 | 13.97 × 10−2 | 2.10 × 10−6 | 6.8 × 10−6 |
| Varea | 1.90 × 10−1 | 4.45 × 10−1 | 8.75 × 10−3 | 3.02 × 10−2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobylinska, L.; Lozynskii, A.; Lesyk, R.; Stoika, R.; Vari, S.G. Biodistribution and Anticancer Characteristics of Les-3833, A Novel 4-thiazolidinone-Based Lead Compound. Sci. Pharm. 2020, 88, 18. https://doi.org/10.3390/scipharm88020018
Kobylinska L, Lozynskii A, Lesyk R, Stoika R, Vari SG. Biodistribution and Anticancer Characteristics of Les-3833, A Novel 4-thiazolidinone-Based Lead Compound. Scientia Pharmaceutica. 2020; 88(2):18. https://doi.org/10.3390/scipharm88020018
Chicago/Turabian StyleKobylinska, Lesya, Andrii Lozynskii, Roman Lesyk, Rostyslav Stoika, and Sandor G. Vari. 2020. "Biodistribution and Anticancer Characteristics of Les-3833, A Novel 4-thiazolidinone-Based Lead Compound" Scientia Pharmaceutica 88, no. 2: 18. https://doi.org/10.3390/scipharm88020018
APA StyleKobylinska, L., Lozynskii, A., Lesyk, R., Stoika, R., & Vari, S. G. (2020). Biodistribution and Anticancer Characteristics of Les-3833, A Novel 4-thiazolidinone-Based Lead Compound. Scientia Pharmaceutica, 88(2), 18. https://doi.org/10.3390/scipharm88020018