Medication Safety in Intravenous Therapy: A Compatibility Study and Analysis of Reaction Products of Dihydralazine and Metamizole
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. HPLC-UV Analysis
2.3. HPLC-MS Analysis
3. Results
3.1. HPLC-UV Analysis
3.2. HPLC-MS Analysis
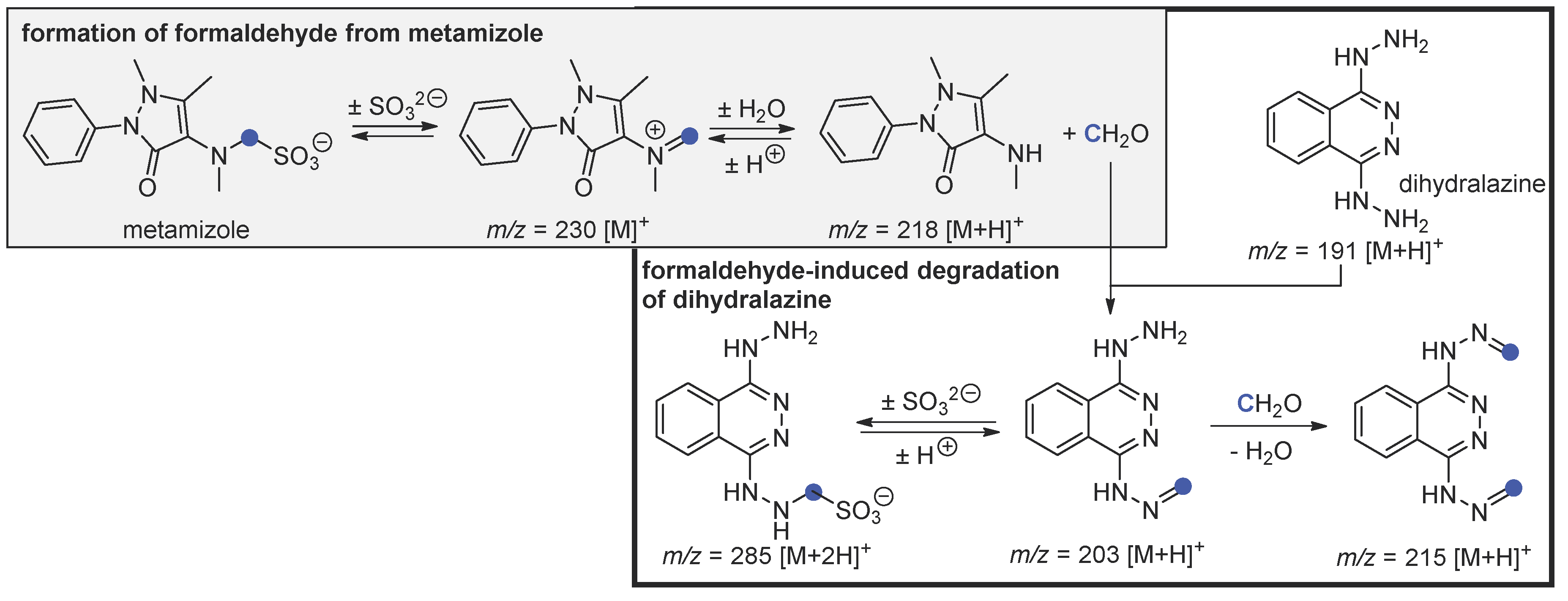
4. Discussion
4.1. Independent Degradation of Metamizole—Background Reaction
4.2. Hydrolysis of Metamizole and Degradation of Dihydralazine with Formaldehyde
Author Contributions
Funding
Conflicts of Interest
References
- Asif, M. Pharmacological activities of various phthalazine and phthalazinone derivatives. Chem. Int. 2019, 5, 97–108. [Google Scholar]
- Del Olmo, E.; Barboza, B.; Ybarra, M.I.; López-Pérez, J.L.; Carrón, R.; Sevilla, M.A.; Boselli, C.; San Feliciano, A. Vasorelaxant activity of phthalazinones and related compounds. Bioorganic Med. Chem. Lett. 2006, 16, 2786–2790. [Google Scholar] [CrossRef] [PubMed]
- Tissot, E.; Cornette, C.; Demoly, P.; Jacquet, M.; Barale, F.; Capellier, G. Medication errors at the administration stage in an intensive care unit. Intensive Care Med. 1999, 25, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Newton, D.W. Drug incompatibility chemistry. Am. J. Health Syst. Pharm. 2009, 66, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Kanji, S.; Lam, J.; Johanson, C.; Singh, A.; Goddard, R.; Fairbairn, J.; Lloyd, T.; Monsour, D.; Kakal, J. Systematic review of physical and chemical compatibility of commonly used medications administered by continuous infusion in intensive care units. Crit. Care Med. 2010, 38, 1890–1898. [Google Scholar] [CrossRef] [PubMed]
- Marsilio, N.R.; da Silva, D.; Bueno, D. Drug incompatibilities in the adult intensive care unit of a university hospital. Rev. Bras. de Ter. Intensiva 2016, 28, 147. [Google Scholar] [CrossRef] [PubMed]
- Machotka, O.; Manak, J.; Kubena, A.; Vlcek, J. Incidence of intravenous drug incompatibilities in intensive care units. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech. Repub. 2015, 159, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Trissel, L.A. Avoiding Common Flaws in Stability and Compatibility Studies of Injectable Drugs; Oxford University Press: Oxford, UK, 1983. [Google Scholar]
- Trissel, L.A.; Flora, K.P. Stability studies: Five years later. Am. J. Hosp. Pharm. 1988, 45, 1569–1571. [Google Scholar] [CrossRef] [PubMed]
- Research Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. Q2B Validation of Analytical Procedures: Methodology. U.S. Food & Drug Administration, 2018. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q2b-validation-analytical-procedures-methodology (accessed on 28 February 2020).
- European Medicines Agency. Specifications and Control Tests on the Finished Product. Guideline 3AQ11a. 1991. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/specifications-control-tests-finished-product_en.pdf (accessed on 22 April 2020).
| Time Elapsed After Mixing (h), % Initial Concentration Remaining | |||||||
|---|---|---|---|---|---|---|---|
| Drug combinations. nominal initial concentration (mg/mL) | 0 | 0.5 | 1 | 2 | 4 | 6 | 24 |
| Dihydralazine 0.5 mg/mL | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Metamizole 33.3 mg/mL | 100.00 | 91.79 | 92.10 | 92.40 | 92.40 | 91.49 | 91.19 |
| Dihydralazine 0.25 mg/Ml, | immediate major decline, | not detectable, | not detectable | not detectable, | not detectable, | not detectable, | not detectable, |
| Metamizole 25 mg/mL | 100.00 | 1.96; 91.81 | 91.07; 89.22 | 91.07; 89.66 | 90.18; 87.07 | 89.73; 87.07 | 89.73; 85.34 |
| Dihydralazine 0.75 mg/mL, | immediate major decline, | not detectable, | not detectable, | not detectable, | not detectable, | not detectable, | not detectable, |
| Metamizole 25 mg/mL | 100.00 | 92.96; 89.80 | 88.73; 86.27 | 88.73; 85.10 | 89.67; 83.14 | 87.79; 82.75 | 85.45; 81.57 |
| Dihydralazine 0.25 mg/mL, | immediate major decline, | not detectable, | not detectable, | not detectable, | not detectable, | not detectable, | not detectable, |
| Metamizole 50 mg/mL | 100.00 | 97.95; 95.78 | 95.69; 97.11 | 94.66; 95.11 | 95.28; 95.33 | 91.79; 93.33 | 89.94; 89.78 |
| Dihydralazine 0.75 mg/mL, | immediate major decline, | not detectable, | not detectable, | not detectable, | not detectable, | not detectable, | not detectable, |
| Metamizole 50 mg/mL | 100.00 | 94.32; 93.00 | 95.54; 92.00 | 93.71; 91.40 | 91.28; 88.80 | 88.84; 87.00 | 84.99; 80.80 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koller, A.K.; Fischer, O.; Krebs, S.; Heinrich, M.R.; Dörje, F. Medication Safety in Intravenous Therapy: A Compatibility Study and Analysis of Reaction Products of Dihydralazine and Metamizole. Sci. Pharm. 2020, 88, 25. https://doi.org/10.3390/scipharm88020025
Koller AK, Fischer O, Krebs S, Heinrich MR, Dörje F. Medication Safety in Intravenous Therapy: A Compatibility Study and Analysis of Reaction Products of Dihydralazine and Metamizole. Scientia Pharmaceutica. 2020; 88(2):25. https://doi.org/10.3390/scipharm88020025
Chicago/Turabian StyleKoller, Anna Katharina, Oliver Fischer, Sabine Krebs, Markus R. Heinrich, and Frank Dörje. 2020. "Medication Safety in Intravenous Therapy: A Compatibility Study and Analysis of Reaction Products of Dihydralazine and Metamizole" Scientia Pharmaceutica 88, no. 2: 25. https://doi.org/10.3390/scipharm88020025
APA StyleKoller, A. K., Fischer, O., Krebs, S., Heinrich, M. R., & Dörje, F. (2020). Medication Safety in Intravenous Therapy: A Compatibility Study and Analysis of Reaction Products of Dihydralazine and Metamizole. Scientia Pharmaceutica, 88(2), 25. https://doi.org/10.3390/scipharm88020025





