Bioactive Components Analysis and Pharmacological Properties of Extracts and Metabolites of Lichen Umbilicaria crustulosa
Abstract
1. Introduction
2. Materials and Methods
2.1. The Process of Collecting and Identifying Lichen Samples
2.2. The Process of Preparing the Lichen Extracts
2.3. Isolation Process of Lichen Metabolites and Fractions
2.4. Analysis Using High-Performance Liquid Chromatography (HPLC)
2.5. Measurement of the Total Phenol Content
2.6. DPPH Method
2.7. ABTS Method
2.8. Reduction Capacity
2.9. Animal Studies–Acute Oral Toxicity and Anti-Inflammatory Activity
- Ctrl–rats were treated with 1% CMC solution in water, per os, 60 min before inflammation induction.
- 50 UCA–rats were treated with 50 mg/kg of the U. crustulosa acetone extract per os dissolved in 1% CMC solution in water, per os, 60 min before inflammation induction.
- 100 UCA–rats were treated with 100 mg/kg of the U. crustulosa acetone extract per os dissolved in 1% CMC solution in water, per os, 60 min before inflammation induction.
- 200 UCA–rats were treated with 200 mg/kg of the U. crustulosa acetone extract per os dissolved in 1% CMC solution in water, per os, 60 min before inflammation induction.
- 50 UCM–rats were treated with 50 mg/kg of the U. crustulosa methanolic extract per os dissolved in 1% CMC solution in water, per os, 60 min before inflammation induction.
- 100 UCM–rats were treated with 100 mg/kg of the U. crustulosa methanolic extract per os dissolved in 1% CMC solution in water, per os, 60 min before inflammation induction.
- 200 UCM–rats were treated with 200 mg/kg of the U. crustulosa methanolic extract per os dissolved in 1% CMC solution in water, per os, 60 min before inflammation induction.
- Ind–rats were treated with 10 mg/kg indomethacin, per os, 60 min before inflammation induction.
3. Results
3.1. HPLC Analysis
3.2. Total Phenolic Content
3.3. Antioxidant Activity
3.4. Animal Studies
3.4.1. Acute Oral Toxicity Study
3.4.2. Anti-Inflammatory Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanders, W.B.; Masumoto, H. Lichen algae: The photosynthetic partners in Lichen Symbioses. Lichenologist 2021, 53, 347–393. [Google Scholar] [CrossRef]
- DePriest, P.T. Early molecular investigations of lichen-forming symbionts: 1986–2001*. Annu. Rev. Microbiol. 2004, 58, 273–301. [Google Scholar] [CrossRef]
- Ranković, B.; Kosanić, M. Lichens as a Potential Source of Bioactive Secondary Metabolites. In Lichen Secondary Metabolites: Bioactive Properties and Pharmaceutical Potential, 2nd ed.; Ranković, B., Ed.; Springer Nature: Cham, Switzerland, 2019; Volume 1, pp. 1–29. [Google Scholar] [CrossRef]
- Nelsen, M.P.; Gargas, A. Phylogenetic distribution and evolution of secondary metabolites in the lichenized fungal genus Lepraria (Lecanorales: Stereocaulaceae). Nova Hedwig. 2008, 86, 115–131. [Google Scholar] [CrossRef]
- Nash, T.H. Lichen Biology, 2nd ed.; Cambridge University Press: Cambridge, UK, 2008; pp. 104–133. [Google Scholar] [CrossRef]
- Stocker-Wörgötter, E. Metabolic diversity of lichen-forming ascomycetous fungi: Culturing, polyketide and shikimate metabolite production, and PKS genes. Nat. Prod. Rep. 2008, 25, 188–200. [Google Scholar] [CrossRef]
- Ranković, B.; Kosanić, M.; Stanojković, T.; Vasiljević, P.; Manojlović, N. Biological activities of Toninia candida and Usnea barbata together with their norstictic acid and usnic acid constituents. Int. J. Mol. Sci. 2012, 13, 14707–14722. [Google Scholar] [CrossRef]
- Tomović, J.; Kosanić, M.; Ranković, B.; Vasiljević, P.; Najman, S.; Manojlović, N. Phytochemical analysis and biological activity of extracts of lichen Physcia semipinnata: As a new source of pharmacologically active compounds. Farmacia 2019, 67, 346–353. [Google Scholar] [CrossRef]
- Kari, P.R. Tanaina plantlore. In Dena’ina K’et’una: An Ethnobotany of the Dena’ina Indians of Southcentral Alaska, 2nd ed.; US National Park Service: Anchorage, AK, USA, 1987. [Google Scholar]
- Huneck, S. The significance of lichens and their metabolites. Naturwissenschaften 1999, 86, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Manojlovic, N.T.; Vasiljevic, P.J.; Maskovic, P.Z.; Juskovic, M.; Bogdanovic-Dusanovic, G. Chemical Composition, Antioxidant, and Antimicrobial Activities of Lichen Umbilicaria cylindrica (L.) Delise (Umbilicariaceae). Evid. Based Complement. Alternat. Med. 2012, 2012, 452431. [Google Scholar] [CrossRef] [PubMed]
- Zlatanović, I.; Stanković, M.; Stankov Jovanović, V.; Mitić, V.; Zrnzevic, I.; Đorđević, A.; Stojanović, G. Biological activities of Umbilicaria crustulosa (Ach.) Frey acetone extract. J. Serb. Chem. Soc. 2017, 82, 141–150. [Google Scholar] [CrossRef]
- Dobson, F.S. Lichens. In An Illustrated Guide to the British and Irish Species, 6th ed.; Richmond Publishing Co.: London, UK, 2011. [Google Scholar]
- Lukač, M.; Onderka, M. Aplicaton of DDQ (2,3-dichloro-5,6-dicyano-benzoquinone) in detection of lichen acids. Acta Fac. Pharm. Univ. Comen. 2006, 53, 160–166. [Google Scholar]
- Huneck, S.; Yoshimura, I. Identification of Lichen Substances, 1st ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 1996; pp. 125–446. [Google Scholar] [CrossRef]
- Manojlović, N.T.; Rančić, A.B.; Décor, R.; Vasiljević, P.; Tomović, J. Determination of chemical composition and antimicrobial, antioxidant and cytotoxic activities of lichens Parmelia conspersa and Parmelia Perlata. J. Food Meas. Charact. 2020, 15, 686–696. [Google Scholar] [CrossRef]
- Aoussar, N.; Achmit, M.; Es-Sadeqy, Y.; Vasiljević, P.; Rhallabi, N.; Ait Mhand, R.; Zerouali, K.; Manojlović, N.; Mellouki, F. Phytochemical constituents, antioxidant and antistaphylococcal activities of Evernia prunastri (L.) Ach., Pseudevernia furfuracea (L.) Zopf. and Ramalina farinacea (L.) Ach. from Morocco. Arch. Microbiol. 2021, 203, 2887–2894. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Dorman, H.; Bachmayer, O.; Kosar, M.; Hiltunen, R. Antioxidant Properties of Aqueous Extracts from Selected Lamiaceae Species Grown in Turkey. J. Agric. Food Chem. 2004, 52, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, S.; Ahmad, S.; Rehman Khan, K.U.; Tabassum, F.; Khursheed, A.; Zaman, Q.U.; Bukhari, N.A.; Alfagham, A.; Hatamleh, A.A.; Chen, Y. Phytochemical profiling, antioxidant, anti-inflammatory, thrombolytic, hemolytic activity in vitro and in silico potential of Portulacaria afra. Molecules 2022, 27, 2377. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Studies on products of browning reaction. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- The Organization for Economic Cooperation and Development (OECD). Acute Toxic Class Method, OECD Guidelines for the Testing of Chemicals; Test No. 423: Acute Oral toxicity; Section 4; OECD Publishing: Paris, France, 2002. [Google Scholar] [CrossRef]
- Mićović, T.; Katanić Stanković, J.S.; Bauer, R.; Nöst, X.; Marković, Z.; Milenković, D.; Jakovljević, V.; Tomović, M.; Bradić, J.; Stešević, D.; et al. In vitro, in vivo and in silico evaluation of the anti-inflammatory potential of Hyssopus officinalis L. subsp. aristatus (Godr.) Nyman (Lamiaceae). J. Ethnopharmacol. 2022, 293, 115201. [Google Scholar] [CrossRef] [PubMed]
- Posner, B.; Feige, G.B.; Huneck, S. Studies on the chemistry of the lichen genus Umbilicaria Hoffm. Z. Naturforsch. C 1992, 47, 1–9. [Google Scholar] [CrossRef]
- Elix, J.A.; Gaul, K.L. The interconversion of the lichen depsides para-and meta-scrobiculin, and the biosynthetic implications, Aust. J. Chem. 1986, 39, 613–624. [Google Scholar] [CrossRef]
- Ranković, B.; Kosanić, M.; Manojlović, N.; Rančić, A.; Stanojković, T. Chemical composition of Hypogymnia physodes lichen and biological activities of some its major metabolites. Med. Chem. 2014, 23, 408–416. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B.; Stanojković, T. Antioxidant, antimicrobial, and anticancer activity of 3 Umbilicaria species. J. Food Sci. 2012, 77, 20–25. [Google Scholar] [CrossRef]
- Mykhailenko, O.; Gudžinskas, Z.; Kovalyov, V.; Desenko, V.; Ivanauskas, L.; Bezruk, I.; Georgiyants, V. Effect of ecological factors on the accumulation of phenolic compounds in Iris species from Latvia, Lithuania and Ukraine. Phytochem. Anal. 2020, 31, 545–563. [Google Scholar] [CrossRef]
- Aslan, A.; Güllüce, M.; Sökmen, M.; Adιgüzel, A.; Sahin, F.; Özkan, H. Antioxidant and Antimicrobial Properties of the Lichens Cladonia foliacea., Dermatocarpon miniatum., Everinia divaricata., Evernia prunastri., and Neofuscella pulla. Pharm. Biol. 2006, 44, 247–252. [Google Scholar] [CrossRef]
- Kumar, J.; Dhar, P.; Tayade, A.B.; Gupta, D.; Chaurasia, O.P.; Upreti, D.K.; Arora, R.; Srivastava, R.B. Antioxidant capacities, phenolic profile and cytotoxic effects of saxicolous lichens from trans-Himalayan cold desert of Ladakh. PLoS ONE 2014, 9, e98696. [Google Scholar] [CrossRef] [PubMed]
- Maulidiyah, M.; Darmawan, A.; Usman, U.; Musdalifah, A.; Ode, L.; Salim, A.; Nurdin, M. Antioxidant activity of secondary metabolite compounds from lichen Teloschistes flavicans. Biointerface Res. Appl. Chem. 2021, 11, 13878–13884. [Google Scholar] [CrossRef]
- Elečko, J.; Vilková, M.; Frenák, R.; Routray, D.; Ručová, D.; Bačkor, M.; Goga, M. A comparative study of isolated secondary metabolites from lichens and their antioxidative properties. Plants 2022, 11, 1077. [Google Scholar] [CrossRef]
- Stanly, C.; Ali, D.M.H.; Keng, C.L.; Boey, P.L.; Bhatt, A. Comparative evaluation of antioxidant activity and total phenolic content of selected lichen species from Malaysia. J. Pharm. Res. 2011, 4, 2824–2827. [Google Scholar] [CrossRef]
- Odabasoglu, F.; Aslan, A.; Cakir, A.; Suleymand, H.; Karagoza, Y.; Bayira, Y.; Haliciaet, M. Antioxidant activity, reducing power phenolic content of some lichen species. Fitoterapia 2005, 76, 216–219. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B.; Stanojković, T.; Rančić, A.; Manojlović, N. Cladonia lichens and their major metabolites as possible natural antioxidant, antimicrobial and anticancer agents. LWT—Food Sci. Technol. 2014, 59, 518–525. [Google Scholar] [CrossRef]
- Thadhani, V.M.; Choudhary, M.I.; Ali, S.; Omar, I.; Siddique, H.; Karunaratne, V. Antioxidant activity of some lichen metabolites. Nat. Prod. Res. 2011, 25, 1827–1837. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.E.; Fernández, E.; Quilhot, W.; Lissi, E. Antioxidant activity of depsides and depsidones. Phytochemistry 1994, 37, 1585–1587. [Google Scholar] [CrossRef] [PubMed]
- William, C.M.; Rao, N.V.; Kumar, B.R.; Mohan, G.K. Anti-inflammatory and analgesic activities of methanolic extract of Kigelia pinnata DC flower. J. Ethnopharmacol. 2010, 130, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Morales, G.; Paredes, A.; Olivares, A.; Bravo, J. Acute oral toxicity and anti-inflammatory activity of hydroalcoholic extract from Lampaya medicinalis Phil in rats. Biol. Res. 2014, 47, 6. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Su, J.; Wang, G.; Zheng, L.; Wang, G.; Sun, Y.; Bao, Y.; Wang, S.; Huang, Y. Discovery of phenolic glycoside from Hyssopus cuspidatus attenuates LPS-induced inflammatory responses by inhibition of iNOS and COX-2 expression through suppression of NF-κB activation. Int. J. Mol. Sci. 2021, 22, 12128. [Google Scholar] [CrossRef] [PubMed]
- Komakech, R.; Kim, Y.G.; Matsabisa, G.M.; Kang, Y. Anti-inflammatory and analgesic potential of Tamarindus indica Linn.(Fabaceae): A narrative review. Integr. Med. Res. 2019, 8, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Studzinska-Sroka, E.; Galanty, A.; Bylka, W. Atranorin-an interesting lichen secondary metabolite. Mini-Rev. Med. Chem. 2017, 17, 1633–1645. [Google Scholar] [CrossRef] [PubMed]
- Ureña-Vacas, I.; González-Burgos, E.; Divakar, P.K.; Gómez-Serranillos, M.P. Lichen Depsides and Tridepsides: Progress in Pharmacological Approaches. J. Fungus 2023, 9, 116. [Google Scholar] [CrossRef]
- Joshi, T.; Sharma, P.; Joshi, T.; Chandra, S. In silico screening of anti-inflammatory compounds from Lichen by targeting cyclooxygenase-2. J. Biomol. Struct. Dyn. 2020, 38, 3544–3562. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Dubino, A. Lichens as a source of chemical compounds with anti-inflammatory activity. Herba Pol. 2018, 64, 56–64. [Google Scholar] [CrossRef]


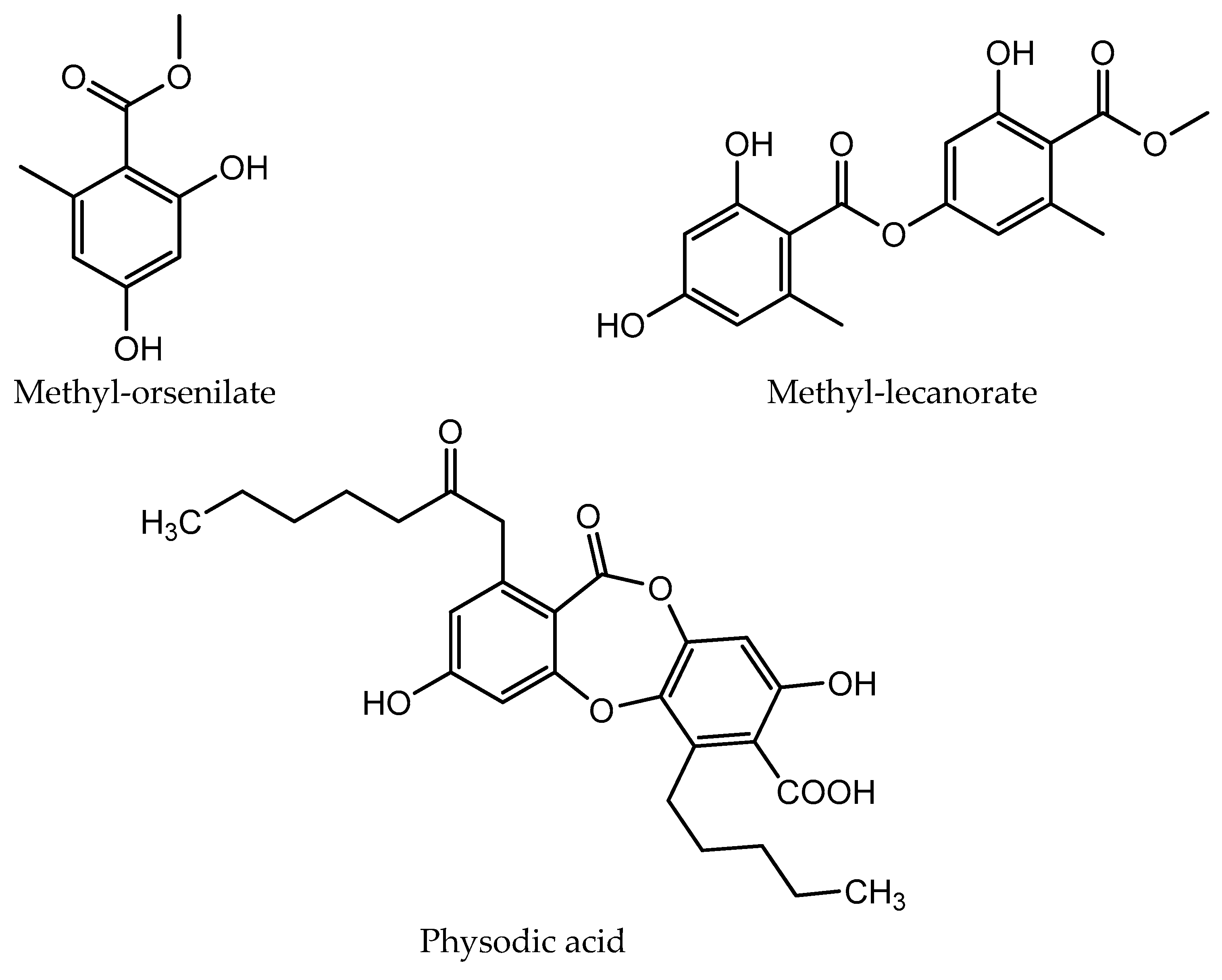
| Compound | Retention Time (tR ± SD) * (min) | Absorbance Maxima (nm) | Relative Abundance (%) | |
|---|---|---|---|---|
| UCA | UCM | |||
| Methyl orsellinate | 3.54 ± 0.02 | 218, 270, 308 | 2.89 ± 0.01 | 5.24 ± 0.03 |
| Lecanoric acid | 4.22 ± 0.04 | 220, 270, 312 | 7.41 ± 0.15 | 11.43 ± 0.07 |
| Crustinic acid | 4.51 ± 0.01 | 220, 268, 308 | 3.23 ± 0.27 | ND |
| Methyl lecanorate | 5.62 ± 0.01 | 228, 270, 308 | ND | 2.63 ± 0.22 |
| Haematommic acid | 6.13 ± 0.01 | 202, 236, 258, 280, 344 | ND | 0.49 ± 0.01 |
| Gyrophoric acid | 6.70 ± 0.10 | 214, 270, 304 | 59.27 ± 0.70 | 58.32 ± 0.45 |
| Physodic acid | 9.61 ± 0.18 | 212, 263, 314 | 1.95 ± 0.06 | 1.70 ± 0.01 |
| Atranorin | 20.43 ± 0.01 | 210, 252, 321 | 6.65 ± 0.40 | ND |
| Chloroatranorin | 25.90 ± 0.01 | 213, 252, 315 m, 350 | 3.34 ± 0.11 | ND |
| Lichen Extracts | Phenolics Content (mg GA/g) |
|---|---|
| UCA | 205.46 ± 0.39 |
| UCM | 156.36 ± 0.59 |
| Lichen Extracts/Compound | IC50 (μg/mL) | |
|---|---|---|
| DPPH Scavenging | ABTS Scavenging | |
| UCA | 390.10 ± 4.02 | 239.60 ± 3.45 |
| UCM | 245.69 ± 2.33 | 216.80 ± 3.52 |
| GYR | >500 | 246.99 ± 11.88 |
| PHY | 158.97 ± 2.02 | 107.58 ± 1.42 |
| ATR/CHL | 150.77 ± 2.46 | 43.94 ± 2.27 |
| Ascorbic acid | 9.08 ± 1.96 | 8.28 ± 0.24 |
| Trolox | 14.26 ± 3.81 | 12.40 ± 0.40 |
| Lichen Extract/ Compound | Absorbance (700 nm) | |||||
|---|---|---|---|---|---|---|
| 1000 μg/mL | 500 μg/mL | 250 μg/mL | 125 μg/mL | 62.5 μg/mL | 31.25 μg/mL | |
| UCA | 0.515 ± 0.003 | 0.333 ± 0.006 | 0.23 ± 0.002 | 0.0167 ± 0.006 | 0.013 ± 0.005 | 0.098 ± 0.002 |
| UCM | 0.701 ± 0.004 | 0.448 ± 0.004 | 0.287 ± 0.011 | 0.201 ± 0.007 | 0.015 ± 0.004 | 0.12 ± 0.006 |
| GYR | 0.237 ± 0.016 | 0.236 ± 0.018 | 0.193 ± 0.008 | 0.178 ± 0.004 | 0.133 ± 0.012 | 0.119 ± 0.003 |
| PHY | 0.786 ± 0.011 | 0.471 ± 0.009 | 0.3 ± 0.002 | 0.198 ± 0.002 | 0.152 ± 0.002 | 0.13 ± 0.003 |
| ATR/CHL | 0.637 ± 0.001 | 0.407 ± 0.010 | 0.198 ± 0.002 | 0.145 ± 0.003 | 0.112 ± 0.004 | 0.065 ± 0.004 |
| Ascorbic acid | 2.219 ± 0.012 | 2.147 ± 0.010 | 1.582 ± 0.008 | 1.17 ± 0.004 | 0.736 ± 0.002 | 0.281 ± 0.006 |
| Trolox | 1.96 ± 0.009 | 1.521 ± 0.020 | 0.904 ± 0.008 | 0.513 ± 0.001 | 0.312 ± 0.001 | 0.204 ± 0.018 |
| Rat Paw Thickness (mm) (% Inhibition) | |||||
|---|---|---|---|---|---|
| Experimental Groups | 0 h | 1 h | 2 h | 3 h | 4 h |
| Ctrl | 5.24 ± 0.12 | 7.25 ± 0.18 | 7.43 ± 0.4 | 7.15 ± 0.36 | 7.04 ± 0.36 |
| 50 UCA | 5.27 ± 0.18 | 6.82 ± 0.12 (22.89) | 7.05 ± 0.4 (18.72) | 6.57 ± 0.26 (31.94) | 6.34 ± 0.27 (40.56) |
| 100 UCA | 5.30 ± 0.17 | 6.72 ± 0.17 (29.35) | 6.50 ± 0.24 (45.21) * | 6.79 ± 0.20 (21.99) | 6.50 ± 0.23 (33.33) |
| 200 UCA | 5.43 ± 0.20 | 6.57 ± 0.13 (43.28) * | 6.69 ± 0.35 (42.47) * | 6.84 ± 0.40 (26.18) | 6.39 ± 0.29 (46.67) * |
| 50 UCM | 5.29 ± 0.14 | 7.00 ± 0.15 (14.93) | 6.57 ± 0.29 (41.55) * | 6.86 ± 0.21 (17.8) | 6.30 ± 0.22 (43.89) |
| 100 UCM | 5.44 ± 0.13 | 6.64 ± 0.19 (40.30) * | 6.93 ± 0.38 (31.96) | 6.58 ± 0.27 (40.31) | 6.54 ± 0.28 (38.89) |
| 200 UCM | 5.60 ± 0.10 | 6.60 ± 0.10 (50.25) * | 6.56 ± 0.20 (56.16) * | 6.53 ± 0.32 (51.31) * | 6.36 ± 0.20 (57.78) * |
| Ind | 5.33 ± 0.23 | 6.38 ± 0.15 (47.76) * | 6.44 ± 0.18 (49.32) * | 6.23 ± 0.26 (52.88) * | 6.01 ± 0.15 (62.22) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomović, J.; Kočović, A.; Anđić, M.; Bradić, J.; Zubić, N.; Jakovljević, V.; Sovrlić, M.; Vasiljević, P.; Manojlović, N. Bioactive Components Analysis and Pharmacological Properties of Extracts and Metabolites of Lichen Umbilicaria crustulosa. Sci. Pharm. 2024, 92, 27. https://doi.org/10.3390/scipharm92020027
Tomović J, Kočović A, Anđić M, Bradić J, Zubić N, Jakovljević V, Sovrlić M, Vasiljević P, Manojlović N. Bioactive Components Analysis and Pharmacological Properties of Extracts and Metabolites of Lichen Umbilicaria crustulosa. Scientia Pharmaceutica. 2024; 92(2):27. https://doi.org/10.3390/scipharm92020027
Chicago/Turabian StyleTomović, Jovica, Aleksandar Kočović, Marijana Anđić, Jovana Bradić, Nevena Zubić, Vladimir Jakovljević, Miroslav Sovrlić, Perica Vasiljević, and Nedeljko Manojlović. 2024. "Bioactive Components Analysis and Pharmacological Properties of Extracts and Metabolites of Lichen Umbilicaria crustulosa" Scientia Pharmaceutica 92, no. 2: 27. https://doi.org/10.3390/scipharm92020027
APA StyleTomović, J., Kočović, A., Anđić, M., Bradić, J., Zubić, N., Jakovljević, V., Sovrlić, M., Vasiljević, P., & Manojlović, N. (2024). Bioactive Components Analysis and Pharmacological Properties of Extracts and Metabolites of Lichen Umbilicaria crustulosa. Scientia Pharmaceutica, 92(2), 27. https://doi.org/10.3390/scipharm92020027








