Targeting c-MYC G-Quadruplexes for Cancer Treatment with Small Molecules
Abstract
1. Introduction
2. G-Quadruplex Topologies
Ligand Selectivity and G-Quadruplex Topology
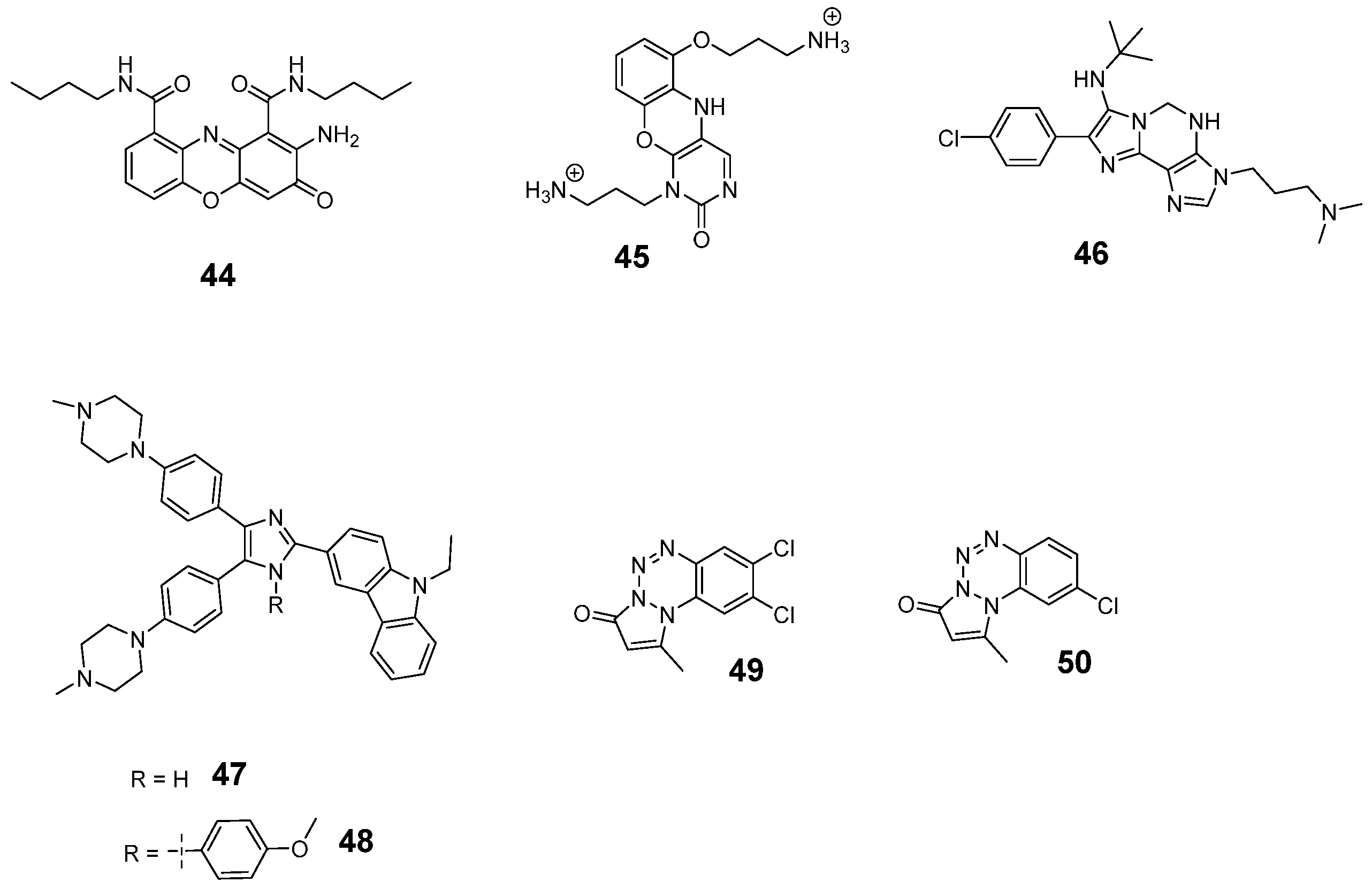
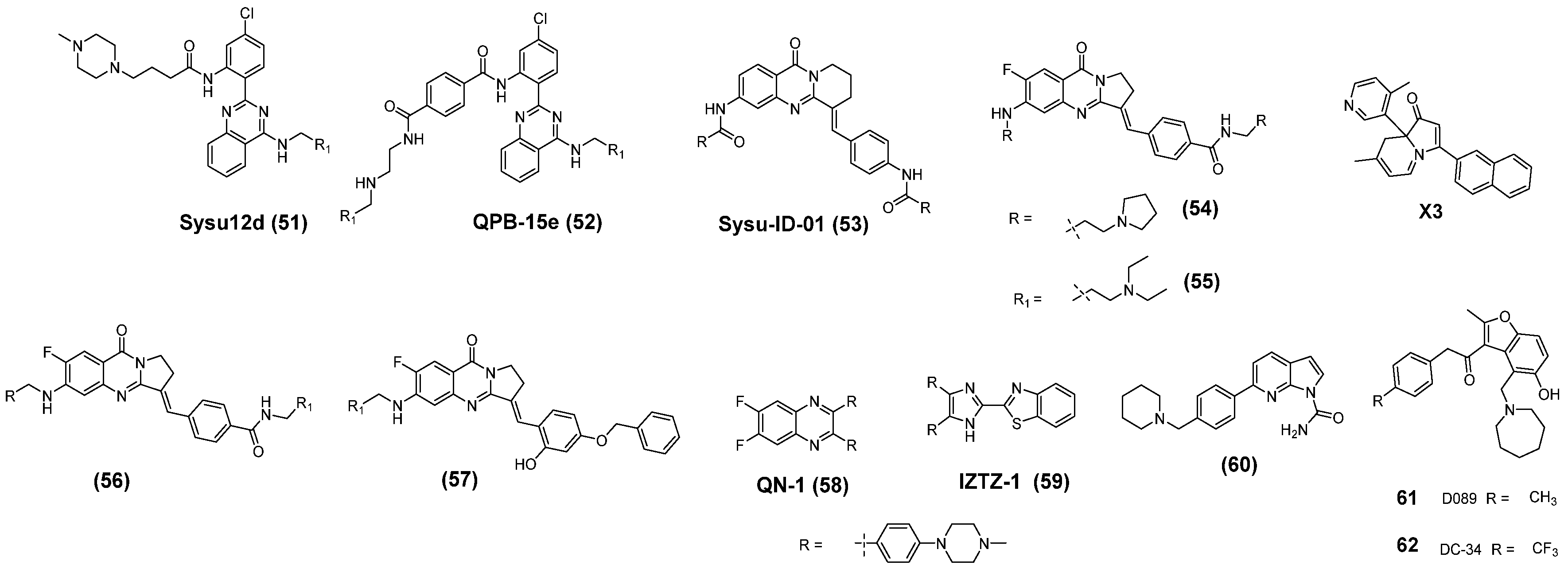
3. c-MYC G-Quadruplexes Stabilizing Small Molecules
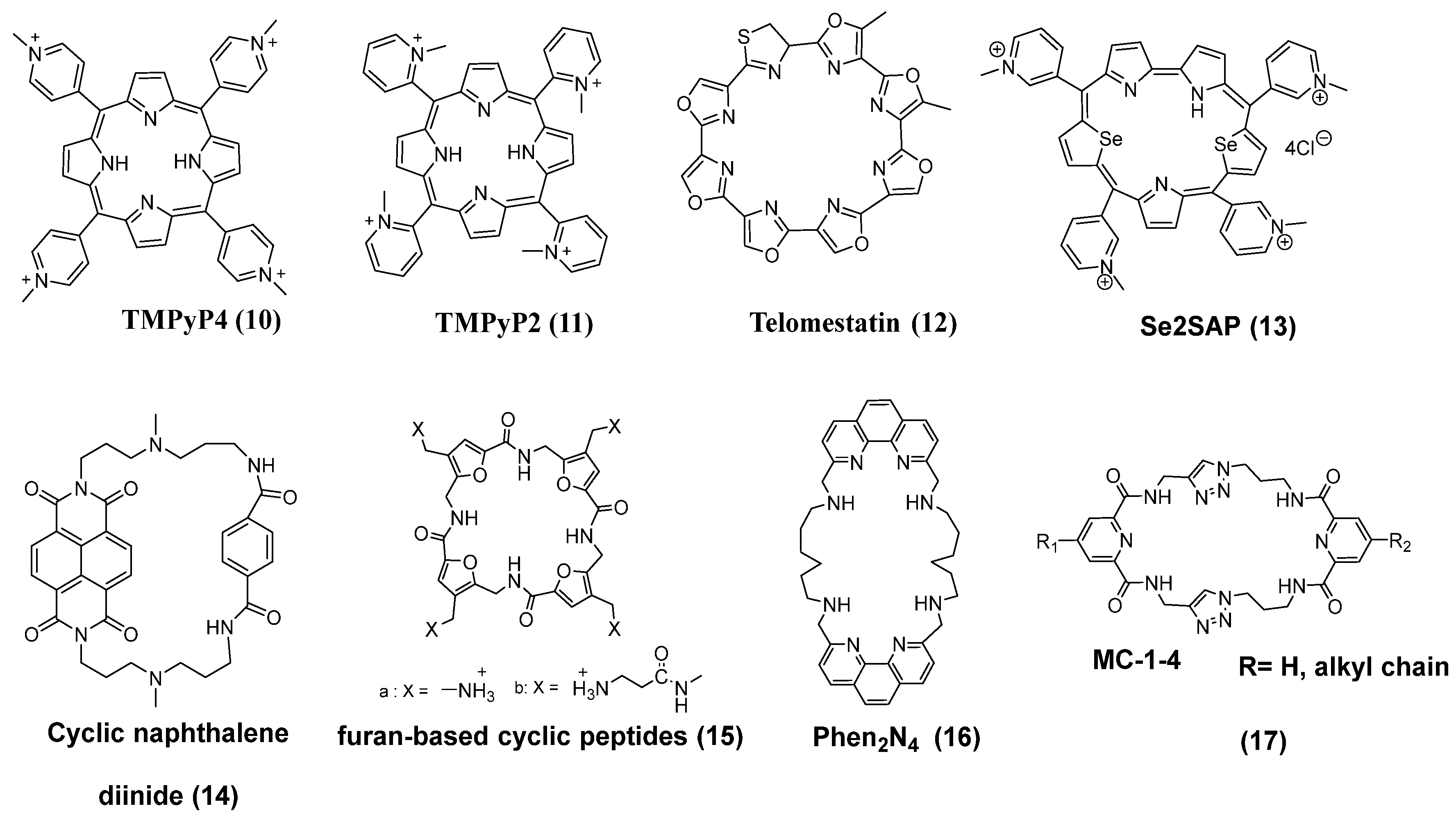
3.1. Macrocycles
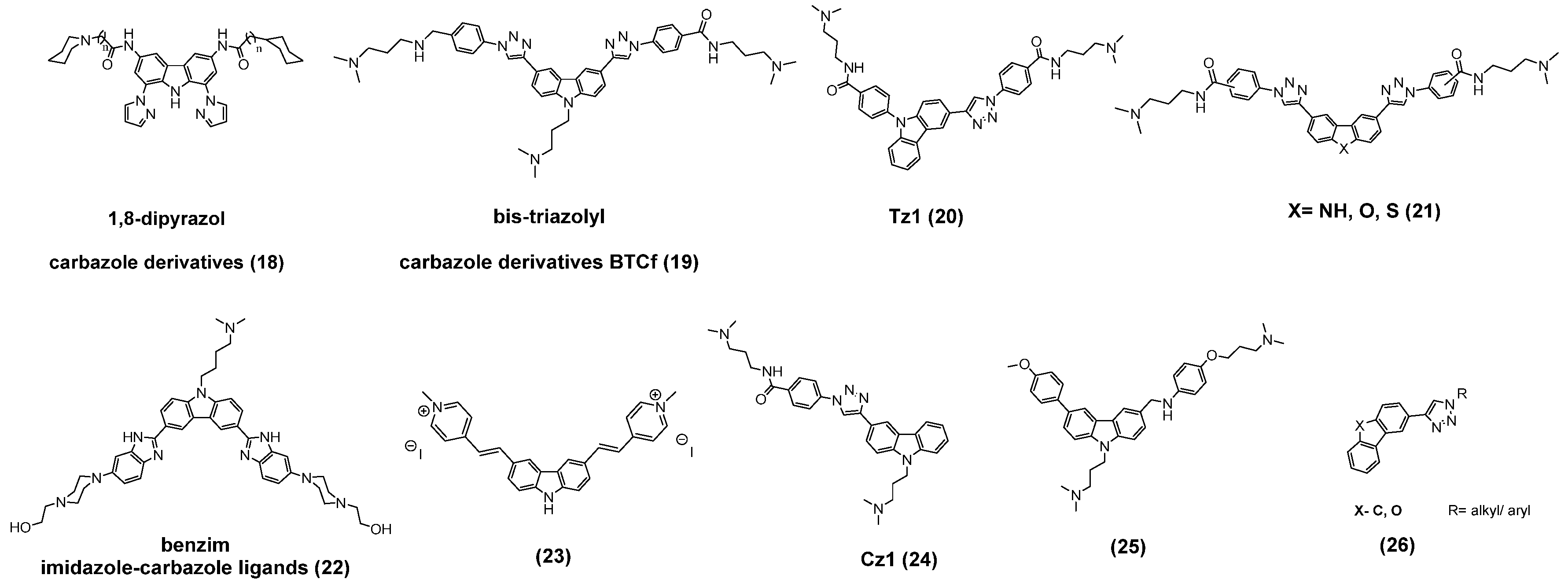
3.2. Carbazole Derivatives/Four-Membered Heterocycles
3.3. Ligands with Four or More Fused Aromatic Rings
3.4. Ligands with Three Fused Aromatic Rings
3.5. Ligands with Two Fused Aromatic Rings
3.6. Flexible G-Quadruplex Ligands
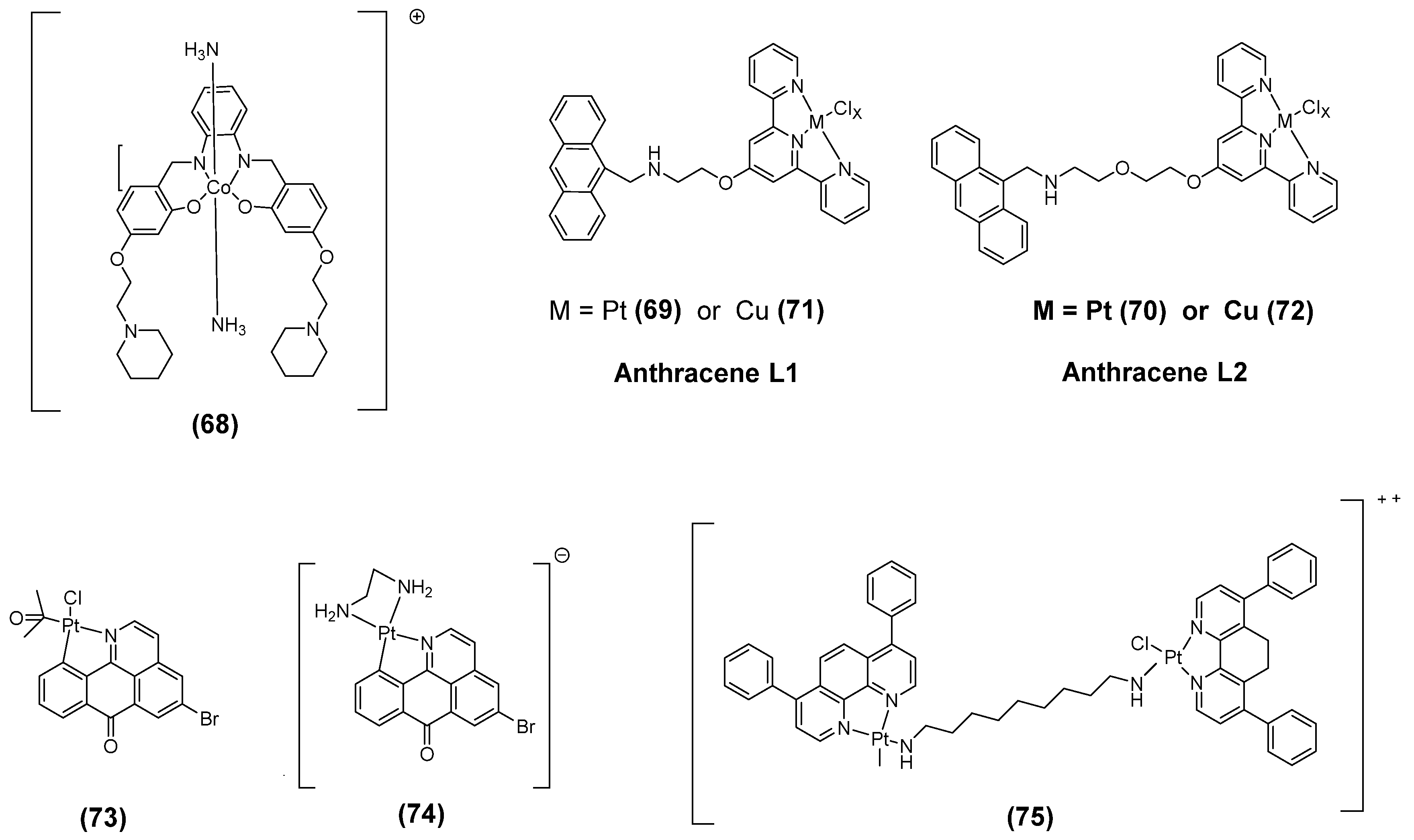
3.7. Metal Ligand Complex
4. Discussion


5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- Yahya, E.B.; Alqadhi, A.M. Recent Trends in Cancer Therapy: A Review on the Current State of Gene Delivery. Life Sci. 2021, 269, 119087. [Google Scholar] [CrossRef] [PubMed]
- Ciriello, G.; Miller, M.L.; Aksoy, B.A.; Senbabaoglu, Y.; Schultz, N.; Sander, C. Emerging Landscape of Oncogenic Signatures across Human Cancers. Nat. Genet. 2013, 45, 1127–1133. [Google Scholar] [CrossRef]
- Mendes, E.; Aljnadi, I.M.; Bahls, B.; Victor, B.L.; Paulo, A. Major Achievements in the Design of Quadruplex-Interactive Small Molecules. Pharmaceuticals 2022, 15, 300. [Google Scholar] [CrossRef] [PubMed]
- Bidzińska, J.; Cimino-Reale, G.; Zaffaroni, N.; Folini, M. G-Quadruplex Structures in the Human Genome as Novel Therapeutic Targets. Molecules 2013, 18, 12368–12395. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.; Cadoni, E.; Ressurreição, A.; Moreira, R.; Paulo, A. Design of Modular G-quadruplex Ligands. ChemMedChem 2018, 13, 869–893. [Google Scholar] [CrossRef]
- Chen, L.; Dickerhoff, J.; Sakai, S.; Yang, D. DNA G-Quadruplex in Human Telomeres and Oncogene Promoters: Structures, Functions, and Small Molecule Targeting. Acc. Chem. Res. 2022, 55, 18. [Google Scholar] [CrossRef] [PubMed]
- Boxer, L.; Dang, C. Translocations involving c-myc and c-myc function. Oncogene 2001, 20, 5595–5610. [Google Scholar] [CrossRef]
- Pelengaris, S.; Khan, M. The many faces of c-MYC. Arch. Biochem. Biophys. 2003, 416, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Pelengaris, S.; Khan, M.; Evan, G. c-MYC: More than just a matter of life and death. Nat. Rev. Cancer 2002, 2, 764–776. [Google Scholar] [CrossRef]
- Dalla-Favera, R.; Bregni, M.; Erikson, J.; Patterson, D.; Gallo, R.C.; Croce, C.M. Human C-Myc Onc Gene Is Located on the Region of Chromosome 8 That Is Translocated in Burkitt Lymphoma Cells. Proc. Proc. Natl. Acad. Sci. USA 1982, 79, 7824–7827. [Google Scholar] [CrossRef]
- Chen, B.J.; Wu, Y.L.; Tanaka, Y.; Zhang, W. Small Molecules Targeting C-Myc Oncogene: Promising Anti-Cancer Therapeutics. Int. J. Biol. Sci. 2014, 10, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Wolf, E.; Eilers, M. Targeting MYC Proteins for Tumor Therapy. Annu. Rev. Cancer Biol. 2020, 4, 61–75. [Google Scholar] [CrossRef]
- Chen, H.; Liu, H.; Qing, G. Targeting Oncogenic Myc as a Strategy for Cancer Treatment. Signal Transduct. Target. Ther. 2018, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; O’Grady, S.; Tang, M.; Crown, J. MYC as a Target for Cancer Treatment. Cancer Treat. Rev. 2021, 94, 102154. [Google Scholar] [CrossRef]
- Yue, M.; Iida, K.; Nagasawa, K. Topologies of G-quadruplex: Biological functions and regulation by ligands. Biochem. Biophys. Res. Commun. 2020, 531, 3–17. [Google Scholar]
- Kolesnikova, S.; Curtis, E. Structure and Function of Multimeric G-Quadruplexes. Molecules 2019, 24, 3074. [Google Scholar] [CrossRef] [PubMed]
- Collie, G.; Parkinson, G. The application of DNA and RNA G-quadruplexes to therapeutic medicines. Chem. Soc. Rev. 2011, 40, 5867–5892. [Google Scholar] [CrossRef]
- Takahashi, S.; Kotar, A.; Tateishi-Karimata, H.; Bhowmik, S.; Wang, Z.; Chang, T.; Sato, S.; Takenaka, S.; Plavec, J.; Sugimoto, N. Chemical Modulation of DNA Replication along G-Quadruplex Based on Topology-Dependent Ligand Binding. J. Am. Chem. Soc. 2021, 143, 16458–16469. [Google Scholar] [CrossRef]
- Robinson, J.; Raguseo, F.; Nuccio, S.P.; Liano, D.; Di Antonio, M. DNA G-Quadruplex Structures: More than Simple Roadblocks to Transcription? Nucleic Acids Res. 2021, 49, 8419–8431. [Google Scholar] [CrossRef]
- Burge, S.; Parkinson, G.N.; Hazel, P.; Todd, A.K.; Neidle, S. Quadruplex DNA: Sequence, Topology, and Structure. Nucleic Acids Res. 2006, 34, 5402–5415. [Google Scholar] [CrossRef]
- Spiegel, J.; Adhikari, S.; Balasubramanian, S. The Structure and Function of DNA G-Quadruplexes. Trends Chem. 2020, 2, 123–136. [Google Scholar] [CrossRef]
- Kosiol, N.; Juranek, S.; Brossart, P.; Heine, A.; Paeschke, K. G-Quadruplexes: A Promising Target for Cancer Therapy. Mol. Cancer 2021, 20, 40. [Google Scholar] [CrossRef]
- Mendes, E.; Bahls, B.; Aljnadi, I.M.; Paulo, A. Indoloquinolines as Scaffolds for the Design of Potent G-Quadruplex Ligands. Bioorganic Med. Chem. Lett. 2022, 72, 128862. [Google Scholar] [CrossRef]
- Francisco, A.P.; Paulo, A. Oncogene Expression Modulation in Cancer Cell Lines by DNA G-Quadruplex-Interactive Small Molecules. Curr. Med. Chem. 2017, 24, 4873–4904. [Google Scholar] [CrossRef]
- Awadasseid, A.; Ma, X.; Wu, Y.; Zhang, W. G-Quadruplex Stabilization via Small-Molecules as a Potential Anti-Cancer Strategy. Biomed. Pharmacother. 2021, 139, 111550. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, R.; Deutzmann, A.; Mahauad-Fernandez, W.D.; Hansen, A.S.; Gouw, A.M.; Felsher, D.W. The MYC Oncogene the Grand Orchestrator of Cancer Growth and Immune Evasion. Nat. Rev. Clin. Oncol. 2022, 19, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Simonsson, T.; Pecinka, P.; Kubista, M. DNA Tetraplex Formation in the Control Region of C-Myc. Nucleic Acids Res. 1998, 26, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Fedoroff, O.Y.; Salazar, M.; Han, H.; Chemeris, V.V.; Kerwin, S.M.; Hurley, L.H. NMR-based model of a telomerase-inhibiting compound bound to G-quadruplex DNA. Biochemistry 1998, 37, 12367–12374. [Google Scholar] [CrossRef]
- Rangan, A.; Fedoroff, O.Y.; Hurley, L.H. Induction of duplex to G-quadruplex transition in the c-myc promoter region by a small molecule. J. Biol. Chem. 2001, 276, 4640–4646. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Huang, J.; Ren, L.; Weng, X.; Zhou, Y.; Du, Y.; Wu, X.; Zhou, X.; Yang, G. Cationic corrole derivatives: A new family of G-quadruplex inducing and stabilizing ligands. Chem. Commun. 2007, 31, 3264–3266. [Google Scholar] [CrossRef]
- Casagrande, V.; Alvino, A.; Bianco, A.; Ortaggi, G.; Franceschin, M. Study of binding affinity and selectivity of perylene and coronene derivatives towards duplex and quadruplex DNA by ESI-MS. J. Mass Spectrom. 2009, 44, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Read, M.; Harrison, R.J.; Romagnoli, B.; Tanious, F.A.; Gowan, S.H.; Reszka, A.P.; Kelland, L.R.; Neidle, S. Structure-based design of selective and potent Gquadruplex-mediated telomerase inhibitors. Proc. Natl. Acad. Sci. USA 2001, 98, 4844–4849. [Google Scholar] [CrossRef] [PubMed]
- Burger, A.M.; Dai, F.; Schultes, C.M.; Reszka, A.P.; Moore, M.J.; Double, J.A.; Neidle, S. The G-quadruplex-interactive molecule BRACO-19 inhibits tumor growth, consistent with telomere targeting and interference with telomerase function. Cancer Res. 2005, 65, 1489–1496. [Google Scholar] [CrossRef]
- Harrison, R.J.; Gowan, S.M.; Kelland, L.R.; Neidle, S. Human telomerase inhibition by substituted acridine derivatives. Bioorganic Med. Chem. Lett. 1999, 9, 2463–2468. [Google Scholar] [CrossRef]
- Sun, D.; Thompson, B.; Cathers, E.; Salazar, M.; Kerwin, S.M.; Trent, J.O.; Jenkins, T.C.; Neidle, S.; Hurley, L.H. Inhibition of human telomerase by a G-quadruplex-interactive compound. J. Med. Chem. 1997, 40, 2113–2116. [Google Scholar] [CrossRef] [PubMed]
- Wheelhouse, R.T.; Sun, D.; Han, H.; Han, F.X.; Hurley, L.H. Cationic porphyrins as telomerase inhibitors: The interaction of tetra-(N-methyl-4-pyridyl) porphine with quadruplex DNA. J. Am. Chem. Soc. 1998, 120, 3261–3262. [Google Scholar] [CrossRef]
- Seenisamy, J.; Bashyam, S.; Gokhale, V.; Vankayalapati, H.; Sun, D.; Siddiqui-Jain, A.; Streiner, N.; Shin-Ya, K.; White, E.; Wilson, W.D.; et al. Design and synthesis of an expanded porphyrin that has selectivity for the c-MYC G-quadruplex structure. J. Am. Chem. Soc. 2005, 127, 2944–2959. [Google Scholar] [CrossRef]
- Shin-ya, K.; Wierzba, K.; Matsuo, K.-I.; Ohtani, T.; Yamada, Y.; Furihata, K.; Hayakawa, Y.; Seto, H. Telomestatin, a novel telomerase inhibitor from Streptomyces anulatus. J. Am. Chem. Soc. 2001, 123, 1262–1263. [Google Scholar] [CrossRef]
- Rodriguez, R.; Müller, S.; Yeoman, J.A.; Trentesaux, C.; Riou, J.F.; Balasubramanian, S. A novel small molecule that alters shelterin integrity and triggers a DNA-damage response at telomeres. J. Am. Chem. Soc. 2008, 130, 15758–15759. [Google Scholar] [CrossRef]
- Cocco, M.J.; Hanakahi, L.A.; Huber, M.D.; Maizels, N. Specific interactions of distamycin with G-quadruplex DNA. Nucleic Acids Res. 2003, 31, 2944–2951. [Google Scholar] [CrossRef]
- Drygin, D.; Siddiqui-Jain, A.; O’Brien, S.; Schwalbe, M.; Lin, A.; Bliesath, J.; Ho, C.B.; Proffitt, C.; Trent, K.; Whitten, J.P.; et al. Anticancer activity of CX-3543: A direct inhibitor of rRNA biogenesis. Cancer Res. 2009, 69, 7653–7661. [Google Scholar] [CrossRef]
- Alessandrini, I.; Recagni, M.; Zaffaroni, N.; Folini, M. On the Road to Fight Cancer: The Potential of G-Quadruplex Ligands as Novel Therapeutic Agents. Int. J. Mol. Sci. 2021, 22, 5947. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, L.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Asamitsu, S.; Obata, S.; Yu, Z.; Bando, T.; Sugiyama, H. Recent Progress of Targeted G-Quadruplex-Preferred Ligands Toward Cancer Therapy. Molecules 2019, 24, 429. [Google Scholar] [CrossRef]
- Bahls, B.; Aljnadi, I.; Emídio, R.; Mendes, E.; Paulo, A. G-Quadruplexes in c-MYC Promoter as Targets for Cancer Therapy. Biomedicines 2023, 11, 969. [Google Scholar] [CrossRef]
- Salerno, S.; Barresi, E.; Baglini, E.; Poggetti, V.; Taliani, S.; Da Settimo, F. Dual Targeting Topoisomerase/G-Quadruplex Agents in Cancer Therapy—An Overview. Biomedicines 2022, 10, 2932. [Google Scholar] [CrossRef]
- Ohnmacht, S.A.; Neidle, S. Small-Molecule Quadruplex-Targeted Drug Discovery. Bioorganic Med. Chem. Lett. 2014, 24, 2602–2612. [Google Scholar] [CrossRef] [PubMed]
- Paulo, A.; Castillo, C.C.; Neidle, S. Targeting Promoter Quadruplex Nucleic Acids for Cancer Therapy. In Comprehensive Medicinal Chemistry III; Elsevier: Amsterdam, The Netherlands, 2017; pp. 308–340. [Google Scholar]
- Neidle, S. Structured Waters Mediate Small Molecule Binding to G-Quadruplex Nucleic Acids. Pharmaceuticals 2021, 15, 7. [Google Scholar] [CrossRef]
- Grand, C.L.; Han, H.; Muñoz, R.M.; Weitman, S.; Von Hoff, D.D.; Hurley, L.H.; Bearss, D.J. The Cationic Porphyrin TMPyP4 Down-Regulates c-MYC and Human Telomerase Reverse Transcriptase Expression and Inhibits Tumor Growth in Vivo. Mol. Cancer Ther. 2002, 1, 565–573. [Google Scholar] [PubMed]
- Mikami-Terao, Y.; Akiyama, M.; Yuza, Y.; Yanagisawa, T.; Yamada, O.; Yamada, H. Antitumor Activity of G-Quadruplex-Interactive Agent TMPyP4 in K562 Leukemic Cells. Cancer Lett. 2008, 261, 226–234. [Google Scholar] [CrossRef]
- Di Antonio, M.; Chan, L.; Balasubramanian, S. G-quadruplex ligands exhibit differential G-tetrad selectivity. Chem. Commun. 2015, 51, 8048–8050. [Google Scholar]
- Cruz, C.; Cairrao, E.; Silvestre, S.; Breitenfeld, L.; Almeida, P.; Queiroz, J.A. Targeting of Mitochondria-Endoplasmic Reticulum by Fluorescent Macrocyclic Compounds. PLoS ONE 2011, 6, e27078. [Google Scholar] [CrossRef]
- Carvalho, J.; Quintela, T.; Gueddouda, N.M.; Bourdoncle, A.; Mergny, J.-L.; Salgado, G.F.; Queiroz, J.A.; Cruz, C. Phenanthroline polyazamacrocycles as G-quadruplex DNA binders. Org. Biomol. Chem. 2018, 16, 2776–2786. [Google Scholar] [CrossRef]
- Chaudhuri, R.; Prasanth, T.; Dash, J. Expanding the toolbox of target-directed bio-orthogonal synthesis: In situ direct macrocyclization by DNA templates. Angew. Chem. Int. 2023, 135, 7. [Google Scholar]
- Lala, B.; Chaudhuri, R.; Prasanth, T.; Burkhart, I.; Schwalbeb, H.; Dash, J. Guanosine-Based hydrogel as a supramolecular scaffold for template assisted macrocyclization. Chem. Commun. 2024, 60, 3433–3436. [Google Scholar] [CrossRef]
- Głuszynska, A. Biological potential of carbazole derivatives. Eur. J. Med. Chem. 2015, 94, 405–426. [Google Scholar] [CrossRef]
- Bashir, M.; Bano, A.; Ijaz, A.S.; Chaudhary, B.A. Recent developments and biological activities of N-substituted carbazole derivatives: A review. Molecules 2015, 20, 13496–13517. [Google Scholar] [CrossRef]
- Chang, C.-C.; Wu, J.-Y.; Chien, C.-W.; Wu, W.-S.; Liu, H.; Kang, C.-C.; Yu, L.-J.; Chang, T.-C. A fluorescent carbazole derivative: High sensitivity for quadruplex DNA. Anal. Chem. 2003, 75, 6177–6183. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Zhang, X.; Zheng, W.; Liu, X.; Qi, C.; Wang, F.; Shangguan, D. Fluorescence light-up probe for parallel G-quadruplexes. Anal. Chem. 2014, 86, 943–952. [Google Scholar] [CrossRef]
- Chen, W.-J.; Zhou, C.-X.; Yao, P.-F.; Wang, X.-X.; Tan, J.-H.; Li, D.; Ou, T.-M.; Gu, L.-Q.; Huang, Z.-S. Disubstituted 1, 8-dipyrazolcarbazole derivatives as a new type of C-myc G-quadruplex binding ligands. Bioorganic Med. Chem. 2012, 20, 2829–2836. [Google Scholar] [CrossRef] [PubMed]
- Panda, D.; Debnath, M.; Mandal, S.; Bessi, I.; Schwalbe, H.; Dash, J. A nucleus-imaging probe that selectively stabilizes a minor conformation of c-MYC G-quadruplex and down-regulates c-MYC transcription in human cancer cells. Sci. Rep. 2015, 5, 13183. [Google Scholar] [CrossRef]
- Das, T.; Panda, D.; Saha, P.; Dash, J. Small molecule driven stabilization of promoter G-quadruplexes and transcriptional regulation of c-MYC. Bioconjugate Chem. 2018, 29, 2636–2645. [Google Scholar] [CrossRef]
- Panda, D.; Saha, P.; Chaudhuri, R.; Prasanth, T.; Ravichandiran, V.; Dash, J. A competitive pull-down assay using G-quadruplex DNA linked magnetic nanoparticles to determine specificity of G-quadruplex ligands. Anal. Chem. 2019, 91, 7705–7711. [Google Scholar] [CrossRef] [PubMed]
- Maji, B.; Kumar, K.; Kaulage, M.; Muniyappa, K.; Bhattacharya, S. Design and synthesis of new benzimidazole-carbazole conjugates for the stabilization of human telomeric DNA, telomerase inhibition, and their selective action on cancer cells. J. Med. Chem. 2014, 57, 6973–6988. [Google Scholar] [CrossRef] [PubMed]
- Kaulage, M.H.; Maji, B.; Pasadi, S.; Ali, A.; Bhattacharya, S.; Muniyappa, K. Targeting G-quadruplex DNA structures in the telomere and oncogene promoter regions by benzimidazole−carbazole ligands. Eur. J. Med. Chem. 2018, 148, 178–194. [Google Scholar] [CrossRef]
- Liu, W.; Lin, C.; Wu, G.; Dai, J.; Chang, T.C.; Yang, D. Structures of 1:1 and 2:1 complexes of BMVC and MYC promoter G-quadruplex reveal a mechanism of ligand conformation adjustment for G4-recognition. Nucleic Acids Res. 2019, 47, 11931–11942. [Google Scholar] [CrossRef]
- Lewis, W.G.; Green, L.G.; Grynszpan, F.; Radić, Z.; Carlier, P.R.; Taylor, P.; Finn, M.; Sharpless, K.B. Click chemistry in situ: Acetylcholinesterase as a reaction vessel for the selective assembly of a femtomolar inhibitor from an array of building blocks. Angew. Chem. Int. 2002, 41, 1053–10577. [Google Scholar] [CrossRef]
- Mamidyala, S.K.; Finn, M. In situ click chemistry: Probing the binding landscapes of biological molecules. Chem. Soc. Rev. 2010, 39, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Di Antonio, M.; Biffi, G.; Mariani, A.; Raiber, E.A.; Rodriguez, R.; Balasubramanian, S. Selective RNA versus DNA G-quadruplex targeting by in situ click chemistry. Angew. Chem. Int. 2012, 124, 11235–11240. [Google Scholar] [CrossRef]
- Panda, D.; Saha, P.; Das, T.; Dash, J. Target guided synthesis using DNA nano-templates for selectively assembling a G-quadruplex binding c-MYC inhibitor. Nat. Commun. 2017, 8, 16103. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Panda, D.; Saha, P.; Pantos, G.D.; Dash, J. Dynamic Generation of G-Quadruplex DNA Ligands by Target-Guided Combinatorial Chemistry on a Magnetic Nanoplatform. J. Med. Chem. 2019, 62, 762–773. [Google Scholar] [CrossRef]
- Fatma, K.; Thumpati, P.; Panda, D.; Velayutham, R.; Dash, J. Selective recognition of c-KIT 1 G-quadruplex by structural tuning of heteroaromatic scaffolds and side chains. ACS Med. Chem. Lett. 2024, 15, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Riechert-Krause, F.; Weisz, K. Indoloquinolines as DNA Binding Ligands. Heterocycl. Commun. 2013, 19, 145–166. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Chen, A.-C.; Yin, Q.-K.; Li, Z.; Huang, S.-M.; Du, G.; He, J.-H.; Zan, L.-P.; Wang, S.-K.; Xu, Y.-H.; et al. New Disubstituted Quindoline Derivatives Inhibiting Burkitt’s Lymphoma Cell Proliferation by Impeding c-MYC Transcription. J. Med. Chem. 2017, 60, 5438–5454. [Google Scholar] [CrossRef]
- Vianney, Y.M.; Weisz, K. Indoloquinoline Ligands Favor Intercalation at Quadruplex-Duplex Interfaces. Chem. A Eur. J. 2022, 28, e202103718. [Google Scholar] [CrossRef] [PubMed]
- Ou, T.M.; Lin, J.; Lu, Y.J.; Hou, J.Q.; Tan, J.H.; Chen, S.H.; Li, Z.; Li, Y.P.; Li, D.; Gu, L.Q.; et al. Inhibition of Cell Proliferation by Quindoline Derivative (SYUIQ-05) through Its Preferential Interaction with c-Myc Promoter G-Quadruplex. Y.M. Vianney, K. Weisz, Indoloquinoline Ligands Favor Intercalation at Quadruplex-Duplex Interfaces. J. Med. Chem. 2011, 54, 5671–5679. [Google Scholar] [CrossRef]
- Boddupally, P.V.L.; Hahn, S.; Beman, C.; De, B.; Brooks, T.A.; Gokhale, V.; Hurley, L.H. Anticancer Activity and Cellular Repression of C-MYC by the G-Quadruplex-Stabilizing 11-Piperazinylquindoline Is Not Dependent on Direct Targeting of the G-Quadruplex in the c-MYC Promoter. J. Med. Chem. 2012, 55, 6076–6086. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.-Y.; Kuang, G.-T.; Wang, S.-K.; Peng, W.; Lin, S.-L.; Zhang, Q.; Su, X.-X.; Hu, M.-H.; Wang, H.; Tan, J.-H.; et al. Discovery of Novel 11-Triazole Substituted Benzofuro [3,2-b]Quinolone Derivatives as c-Myc G-Quadruplex Specific Stabilizers via Click Chemistry. J. Med. Chem. 2017, 60, 5407–5423. [Google Scholar] [CrossRef]
- Funke, A.; Dickerhoff, J.; Weisz, K. Towards the Development of Structure-Selective G-Quadruplex-Binding Indolo [3,2-b] Quinolines. Chem. A Eur. J. 2016, 22, 3170–3181. [Google Scholar] [CrossRef]
- Funke, A.; Karg, B.; Dickerhoff, J.; Balke, D.; Müller, S.; Weisz, K. Ligand-Induced Dimerization of a Truncated Parallel MYC G-Quadruplex. Chembiochem 2018, 19, 505–512. [Google Scholar] [CrossRef]
- Montoya, J.J.; Turnidge, M.A.; Wai, D.H.; Patel, A.R.; Lee, D.W.; Gokhale, V.; Hurley, L.H.; Arceci, R.J.; Wetmore, C.; Azorsa, D.O. In Vitro Activity of a G-Quadruplex-Stabilizing Small Molecule That Synergizes with Navitoclax to Induce Cytotoxicity in Acute Myeloid Leukemia Cells. BMC Cancer 2019, 19, 1251. [Google Scholar] [CrossRef]
- Brown, R.V.; Danford, F.L.; Gokhale, V.; Hurley, L.H.; Brooks, T.A. Demonstration That Drug-Targeted down-Regulation of MYC in Non-Hodgkins Lymphoma Is Directly Mediated through the Promoter G-Quadruplex. J. Biol. Chem. 2011, 286, 41018–41027. [Google Scholar] [CrossRef] [PubMed]
- Anuar, N.N.M.; Hisam, N.S.N.; Liew, S.L.; Ugusman, A. Clinical Review: Navitoclax as a pro-Apoptotic and Anti-Fibrotic Agent. Front. Pharmacol. 2020, 11, 564108. [Google Scholar]
- Kaiser, C.E.; Gokhale, V.; Yang, D.; Hurley, L.H. Gaining Insights into the Small Molecule Targeting of the G-Quadruplex in the c-MYC Promoter Using NMR and an Allele-Specific Transcriptional Assay. Top. Curr. Chem. 2013, 330, 1–21. [Google Scholar] [PubMed]
- Micheli, E.; Altieri, A.; Cianni, L.; Cingolani, C.; Iachettini, S.; Bianco, A.; Leonetti, C.; Cacchione, S.; Biroccio, A.; Franceschin, M.; et al. Perylene and Coronene Derivatives Binding to G-Rich Promoter Oncogene Sequences Efficiently Reduce Their Expression in Cancer Cells. Biochimie 2016, 125, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Porru, M.; Zizza, P.; Franceschin, M.; Leonetti, C.; Biroccio, A. EMICORON: A Multi-Targeting G4 Ligand with a Promising Preclinical Profile. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2017, 1861, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Tawani, A.; Mishra, S.K.; Kumar, A. Structural insight for the recognition of G-quadruplex structure at human c-MYC promoter sequence by flavonoid quercetin. Sci. Rep. 2017, 7, 3600. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ou, T.-M.; Tan, J.-H.; Hou, J.-Q.; Huang, S.-L.; Gu, L.Q.; Huang, Z.-S. Quinolino-benzo-[5,6]-dihydroisoquindolium compounds derived from berberine: A new class of highly selective ligands for G-quadruplex DNA in c-myc oncogene. Eur. J. Med. Chem. 2011, 46, 1906–1913. [Google Scholar] [CrossRef]
- Zhang, W.-J.; Ou, T.-M.; Lu, Y.-J.; Huang, Y.-Y.; Wu, W.-B.; Huang, Z.-S.; Zhou, J.-L.; Wong, K.-Y.; Gu, L.-Q. 9-Substituted berberine derivatives as G-quadruplex stabilizing ligands in telomeric DNA. Bioorganic Medicional Chem. 2007, 15, 5493–5501. [Google Scholar] [CrossRef]
- Hilton, J.; Gelmon, K.; Bedard, P.L.; Tu, D.; Xu, H.; Tinker, A.V.; Goodwin, R.; Laurie, S.A.; Hansen, A.R. Results of the Phase I CCTG IND.231 Trial of CX-5461 in Patients with Advanced Solid Tumors Enriched for DNA-Repair Deficiencies. Nat. Commun. 2022, 13, 3607. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Hurley, L.H. A First-in-Class Clinical G-Quadruplex-Targeting Drug. The Bench-to-Bedside Translation of the Fluoroquinolone QQ58 to CX-5461 (Pidnarulex). Bioorg. Med. Chem. Lett. 2022, 77, 129016. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Balasubramanian, C.; Kumar, N.; Agrawal, S.; Ojha, R.P.; Maiti, S. Binding of berberine to human telomeric quadruplex−spectroscopic, calorimetric and molecular modeling studies. FEBS J. 2008, 275, 3971–3983. [Google Scholar] [CrossRef]
- Wu, Q.; Song, Y.; Liu, R.; Wang, R.; Mei, W.; Chen, W.; Yang, H.; Wang, X. Synthesis, Docking Studies and Antitumor Activity of Phenanthroimidazole D erivatives as Promising c-Myc G-Quadruplex DNA Stabilizers. Bioorganic Chem. 2020, 102, 104074. [Google Scholar] [CrossRef] [PubMed]
- Aptose Biosciences Inc. A Study of APTO-253 in Patients with Relapsed or Refractory AML or MDS. Available online: https://clinicaltrials.gov/ct2/show/NCT02267863 (accessed on 30 September 2024).
- Ohanian, M.; Arellano, M.L.; Levy, M.Y.; O’Dwyer, K.; Babiker, H.; Mahadevan, D.; Zhang, H.; Rastgoo, N.; Jin, Y.; Marango, J.; et al. A Phase 1a/b Dose Escalation Study of the MYC Repressor Apto-253 in Patients with Relapsed or Refractory AML or High-Risk MDS. Blood 2021, 138, 3411. [Google Scholar] [CrossRef]
- Cercek, A.; Wheeler, J.; Murray, P.E.; Zhou, S.; Saltz, L. Phase 1 Study of APTO-253 HCl, an Inducer of KLF4, in Patients with Advanced or Metastatic Solid Tumors. Investig. New Drugs 2015, 33, 1086–1092. [Google Scholar] [CrossRef]
- Local, A.; Zhang, H.; Benbatoul, K.D.; Folger, P.; Sheng, X.; Tsai, C.-Y.; Howell, S.B.; Rice, W.G. APTO-253 Stabilizes G-Quadruplex DNA, Inhibits MYC Expression, and Induces DNA Damage in Acute Myeloid Leukemia Cells. Mol. Cancer Ther. 2018, 17, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Dickerhoff, J.; Brundridge, N.; McLuckey, S.A.; Yang, D. Berberine Molecular Recognition of the Parallel MYC G-Quadruplex in Solution. J. Med. Chem. 2021, 64, 16205–16212. [Google Scholar] [CrossRef]
- Wen, L.; Han, Z.; Li, J.Y. C-MYC and HIF1 Promoter G-Quadruplexes Dependent Metabolic Regulation Mechanism of Berberine in Colon Cancer. J. Gastrointest. Oncol. 2022, 13, 1152–1168. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, V.B.; Varizhuk, A.M.; Lizunova, S.A.; Nikolenko, T.A.; Ivanov, I.A.; Severov, V.V.; Belyaev, E.S.; Shitikov, E.A.; Pozmogova, G.E.; Aralov, A.V. Phenoxazine-Based Scaffold for Designing G4-Interacting Agents. Org. Biomol. Chem. 2020, 18, 6147–6154. [Google Scholar] [CrossRef]
- Tsvetkov, V.B.; Turaev, A.V.; Petrunina, N.A.; Melnik, D.M.; Khodarovich, Y.M.; Pozmogova, G.E.; Zatsepin, T.S.; Varizhuk, A.M.; Aralov, A.V. Phenoxazine Pseudonucleotides in DNA I-Motifs Allow Precise Profiling of Small Molecule Binders by Fluorescence Monitoring. Analyst 2021, 146, 4436–4440. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-J.; Park, H.-J. In Silico Identification of Novel Ligands for G-Quadruplex in the c-MYC Promoter. J. Comput.—Aided Mol. Des. 2015, 29, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, S.; Amato, J.; Capasso, D.; Di Gaetano, S.; Massarotti, A.; Piccolo, M.; Irace, C.; Tron, G.C.; Pagano, B.; Randazzo, A.; et al. Bio-Inspired Dual-Selective BCL-2/c-MYC G-Quadruplex Binders: Design, Synthesis, and Anticancer Activity of Drug-like Imidazo [2,1-i] Purine Derivatives. J. Med. Chem. 2020, 63, 2035–2050. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.-H.; Wang, Y.-Q.; Yu, Z.-Y.; Hu, L.-N.; Ou, T.-M.; Chen, S.-B.; Huang, Z.-S.; Tan, J.-H. Discovery of a New Four-Leaf Clover-like Ligand as a Potent c-MYC Transcription Inhibitor Specifically Targeting the Promoter G-Quadruplex. J. Med. Chem. 2018, 61, 2447–2459. [Google Scholar] [CrossRef] [PubMed]
- Mulliri, S.; Laaksonen, A.; Spanu, P.; Farris, R.; Farci, M.; Mingoia, F.; Roviello, G.N.; Mocci, F. Spectroscopic and in Silico Studies on the Interaction of Substituted Pyrazolo [1,2-a]Benzo [1,2,3,4]Tetrazine-3-One Derivatives with c-Myc G4-DNA. Int. J. Mol. Sci. 2021, 22, 6028. [Google Scholar] [CrossRef]
- Mingoia, F.; Di Sano, C.; Di Blasi, F.; Fazzari, M.; Martorana, A.; Almerico, A.M.; Lauria, A. Exploring the Anticancer Potential of Pyrazolo [1,2-a]Benzo [1,2,3,4]Tetrazin-3-One Derivatives: The Effect on Apoptosis Induction, Cell Cycle and Proliferation. Eur. J. Med. Chem. 2013, 64, 345–356. [Google Scholar] [CrossRef]
- Wang, D.; Gao, F. Quinazoline Derivatives: Synthesis and Bioactivities. Chem. Cent. J. 2013, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Theivendren, P. Quinazoline Marketed Drugs—A Review. Res. Pharm. 2011, 1, 1–21. [Google Scholar]
- Su, L.; Zheng, H.; Li, Z.; Qiu, J.; Chen, S.; Liu, J.; Ou, T.-M.; Tan, J.-H.; Gu, L.-Q.; Huang, Z.-S.; et al. Mechanistic Studies on the Anticancer Activity of 2,4-Disubstituted Quinazoline Derivative. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2014, 1840, 3123–3130. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, C.; Huang, C.; Meng, X.; Zhang, L.; He, J.; Li, J. Quinazoline Derivative QPB-15e Stabilizes the c-Myc Promoter G-Quadruplex and Inhibits Tumor Growth in Vivo. Oncotarget 2016, 7, 34266–34276. [Google Scholar] [CrossRef]
- Shan, C.; Lin, J.; Hou, J.-Q.; Liu, H.-Y.; Chen, S.-B.; Chen, A.-C.; Ou, T.-M.; Tan, J.-H.; Li, D.; Gu, L.-Q.; et al. Chemical Intervention of the NM23-H2 Transcriptional Programme on c-MYC via a Novel Small Molecule. Nucleic Acids Res. 2015, 43, 6677–6691. [Google Scholar] [CrossRef]
- Shan, C.; Yan, J.-W.; Wang, Y.-Q.; Che, T.; Huang, Z.-L.; Chen, A.-C.; Yao, P.-F.; Tan, J.-H.; Li, D.; Ou, T.-M.; et al. Design, Synthesis, and Evaluation of Isaindigotone Derivatives to Downregulate c-Myc Transcription via Disrupting the Interaction of NM23-H2 with G-Quadruplex. J. Med. Chem. 2017, 60, 1292–1308. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Q.; Huang, Z.-L.; Chen, S.-B.; Wang, C.-X.; Shan, C.; Yin, Q.-K.; Ou, T.-M.; Li, D.; Gu, L.-Q.; Tan, J.-H.; et al. Design, Synthesis, and Evaluation of New Selective NM23-H2 Binders as c-MYC Transcription Inhibitors via Disruption of the NM23-H2/G-Quadruplex Interaction. J. Med. Chem. 2017, 60, 6924–6941. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.A.; Cordeiro, M.N.D.S.; Fernandes, R.; Prudêncio, C.; Noronha, J.P.; Vieira, M. Quinoxaline, Its Derivatives and Applications: A State of the Art Review. Eur. J. Med. Chem. 2015, 97, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.; Somakala, K.; Amir, M. Quinoxaline: An Insight into the Recent Pharmacological Advances. Eur. J. Med. Chem. 2018, 143, 542–557. [Google Scholar] [CrossRef]
- Hu, M.-H.; Wu, T.-Y.; Huang, Q.; Jin, G. New Substituted Quinoxalines Inhibit Triple-Negative Breast Cancer by Specifically Downregulating the c-MYC Transcription. Nucleic Acids Res. 2019, 47, 10529–10542. [Google Scholar] [CrossRef]
- Wu, T.-Y.; Huang, Q.; Huang, Z.-S.; Hu, M.-H.; Tan, J.-H. A Drug-like Imidazole-Benzothiazole Conjugate Inhibits Malignant Melanoma by Stabilizing the c-MYC G-Quadruplex. Bioorganic Chem. 2020, 99, 103866. [Google Scholar] [CrossRef] [PubMed]
- Dallavalle, S.; Musso, L.; Artali, R.; Aviñó, A.; Scaglioni, L.; Eritja, R.; Gargallo, R.; Mazzini, S. G-Quadruplex Binding Properties of a Potent PARP-1 Inhibitor Derived from 7-Azaindole-1-Carboxamide. Sci. Rep. 2021, 11, 3869. [Google Scholar] [CrossRef]
- Cincinelli, R.; Musso, L.; Merlini, L.; Giannini, G.; Vesci, L.; Milazzo, F.M.; Carenini, N.; Perego, P.; Penco, S.; Artali, R.; et al. 7-Azaindole-1-Carboxamides as a New Class of PARP-1 Inhibitors. Bioorganic Med. Chem. 2014, 22, 1089–1103. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, S.M.; Phyo, Z.; Arteaga, A.Q.; Gorjifard, S.; Calabrese, D.R.; Connors, D.; Huang, J.; Michalowski, A.M.; Zhang, S.; Liu, Z.-G.; et al. A Small Molecule Stabilizer of the MYC G4-Quadruplex Induces Endoplasmic Reticulum Stress, Senescence and Pyroptosis in Multiple Myeloma. Cancers 2020, 12, 2952. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, K.M.; Saunders, L.B.; Simmons, J.K.; Leon, E.; Calabrese, D.R.; Zhang, S.; Michalowski, A.; Gareiss, P.; Mock, B.A.; Schneekloth, J.S., Jr. Small Molecule Microarrays Enable the Identification of a Selective, Quadruplex-Binding Inhibitor of MYC Expression. ACS Chem. Biol. 2016, 11, 139–148. [Google Scholar] [CrossRef]
- Calabrese, D.R.; Chen, X.; Leon, E.C.; Gaikwad, S.M.; Phyo, Z.; Hewitt, W.M.; Alden, S.; Hilimire, T.A.; He, F.; Michalowski, A.M.; et al. Chemical and Structural Studies Provide a Mechanistic Basis for Recognition of the MYC G-Quadruplex. Nat. Commun. 2018, 9, 4229. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Debnath, M.; Müller, D.; Paul, R.; Das, T.; Bessi, I.; Schwalbe, H.; Dash, J. Cell Penetrating Thiazole Peptides Inhibit C-MYC Expression via Site-Specific Targeting of c-MYC G-Quadruplex. Nucleic Acids Res. 2018, 46, 5355–5365. [Google Scholar] [CrossRef]
- Meunier, B. Hybrid Molecules with a Dual Mode of Action: Dream or Reality? Acc. Chem. Res. 2008, 41, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Debnath, M.; Ghosh, S.; Chauhan, A.; Paul, R.; Bhattacharyya, K.; Dash, J. Preferential Targeting of I-Motifs and G-Quadruplexes by Small Molecules. Chem. Sci. 2017, 8, 7448–7456. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.; Das, T.; Debnath, M.; Chauhan, A.; Dash, J. G-Quadruplex-Binding Small Molecule Induces Synthetic Lethality in Breast Cancer Cells by Inhibiting c-MYC and BCL2 Expression. Chembiochem 2020, 21, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Vilar, R. Interaction of Metal Complexes with G-Quadruplex DNA. In Medicinal Chemistry: Advances in Inorganic Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 425–445. [Google Scholar]
- Palma, E.; Carvalho, J.; Cruz, C.; Paulo, A. Metal-Based G-Quadruplex Binders for Cancer Theranostics. Pharmaceuticals 2021, 14, 605. [Google Scholar] [CrossRef]
- Gama, S.; Rodrigues, I.; Mendes, F.; Santos, I.C.; Gabano, E.; Klejevskaja, B.; Gonzalez-Garcia, J.; Ravera, M.; Vilar, R.; Paulo, A. Anthracene-Terpyridine Metal Complexes as New G-Quadruplex DNA Binders. J. Inorg. Biochem. 2016, 160, 275–286. [Google Scholar] [CrossRef]
- Wei, Z.-Z.; Qin, Q.-P.; Meng, T.; Deng, C.-X.; Liang, H.; Chen, Z.-F. 5-Bromo-Oxoisoaporphine Platinum (II) Complexes Exhibit Tumor Cell Cytotoxcicity via Inhibition of Telomerase Activity and Disruption of c-Myc G-Quadruplex DNA and Mitochondrial Functions. Eur. J. Medicianl Chem. 2018, 145, 360–369. [Google Scholar] [CrossRef]
- He, L.; Meng, Z.; Xu, D.; Shao, F. Dual Functional Dinuclear Platinum Complex with Selective Reactivity towards C-Myc G-Quadruplex. Sci. Rep. 2018, 8, 767. [Google Scholar] [CrossRef] [PubMed]
- Ritapa, C.; Prasanth, T.; Biswas, D.; Mandal, S.; Dash, J. Combating multidrug-resistance in S. pneumoniae: A G-quadruplex binding inhibitor of efflux pump and its bio-orthogonal assembly. Nar Mol. Med. 2024, 1, 2. [Google Scholar]
- Biswa, R.S.; Ranjan, J.S.; Beriha, S.K.; Mahanta, C.S.; Jena, B.B.; Prasanth, T.; Samanta, L.; Satapathy, R.; Dash, B.P. Synthesis and anticancer properties of dendritic glycoconjugates containing multiple o-carborane clusters. New J. Chem. 2023, 47, 10. [Google Scholar]
- Priya, D.; Gumpu, M.B.; Thumpati, P.; Rayappan, J.B.B.; Ravichandiran, V.; Pazhani, G.P.; Veerapandian, M. Chemically synthesized butein and butin: Optical, structure and electrochemical redox functionality at the electrode interface. J. Photochem. Photobiol. B Biol. 2018, 122–129. [Google Scholar]
- Prasanth, T.; Chakraborti, G.; Ravichandiran, T.M.V.; Dash, J. Cycloaddition of N-sulfonyl and N-sulfamoyl azides with alkynes in aqueous media for the selective synthesis of 1,2,3-triazoles. Green Chem. 2022, 24, 911. [Google Scholar] [CrossRef]
- Sagini, S.; Urbanelli, L.; Buratta, S.; Leonardi, L.; Emiliani, C. Nanovesicals from plants as edible carriers of bioactive compounds. AgroLife Sci. J. 2017, 6. [Google Scholar]
- Utoiu, E.; Oancea, A.; Gaspar, A.; Seciu, A.M.; Ștefan, L.M.; Coroiu, V.; Craciunescu, O.; Badiu, C.M.; Oancea, F. Selenium biofortification treatment of cauliflower enhances their content in chemopreventive compounds and in vitro antitumoral activity. Sci. Bull. Ser. F. Biotechnol. 2017, 111. [Google Scholar]
- Iordache, G.G.; Babeanu, N.E. Classification and industrial applications of biosurfactants. Sci. Bull. Ser. F. Biotechnol. 2024, 1, 139–148. [Google Scholar]
- Wang, W.; Hu, S.; Gu, Y.; Yan, Y.; Stovall, D.; Li, D.; Sui, G. Human MYC G-quadruplex: From discovery to a cancer therapeutic target. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188410. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, T.; Roy, S.; Chakraborty, T.; Maiti, S. Selective targeting of G-quadruplex using furan-based cyclic homooligopeptides: Effect on c-MYC expression. Biochemistry 2010, 49, 8388–8397. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Su, Q.; Chen, Y.; Wang, M.; Xu, Y.; Wang, L.; Lu, Y.; Li, J.; Liu, J.; Hong, X.; et al. Molecular Insights into the Specific Targeting of c-MYC G-Quadruplex by Thiazole Peptides. Int. J. Mol. Sci. 2024, 25, 623. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.L.J.Y.H.; Wu, B.; Huang, S.; Li, Q.; Ou, T.; Wang, H.; Tan, J.; Li, D.; Chen, S.; Huang, Z. Design, Synthesis, and Evaluation of New Sugar-Substituted Imidazole Derivatives as Selective c-MYC Transcription Repressors Targeting the Promoter G-Quadruplex. J. Med. Chem. 2022, 65, 12675–12700. [Google Scholar]
- Merlino, F.; Marzano, S.; Zizza, P.; D’Aria, F.; Grasso, N.; Carachino, A.; Iachettini, S.; Biroccio, A.; Fonzo, S.; Grieco, P.; et al. Unlocking the potential of protein-derived peptides to target G-quadruplex DNA: From recognition to anticancer activity. Nucleic Acids Res. 2024, 52, 6748–6762. [Google Scholar] [CrossRef]
- Liu, H.H.; Zheng, K.W.; Chen, Q.; Hao, Y.H.; Tan, Z. RNA G-Quadruplex formation in defined sequence in living cells detected by bimolecular fluorescence complementation. Chem. Sci. 2016, 7, 4573–4581. [Google Scholar] [CrossRef]
- Qin, G.; Liu, Z.; Yang, J.; Liao, X.; Zhao, C.; Ren, J.; Qu, X. Targeting specific DNA G-quadruplexes with CRISPR-guided G-quadruplex-binding proteins and ligands. Nat. Cell. Biol. 2024, 26, 1212–1224. [Google Scholar] [CrossRef] [PubMed]
- Youness, L.; Aline, P.; Hicham, W.; Mounia, O.; Bertrand, L.; Alain, S.; Raphaël, E.D. Ursolic Acid’s Alluring Journey: One Triterpenoid vs. Cancer Hallmarks. Molecules 2023, 28, 7897. [Google Scholar] [CrossRef] [PubMed]
- Hicham, W.; Ibtissam, Y.M.; Bertrand, L.; Vincent, S.; Mounia, O.; Raphaël, E.D.; Youness, L. Shining a light on prostate cancer: Photodynamic therapy and combination approaches. Pharmaceutics 2023, 15, 1767. [Google Scholar] [CrossRef] [PubMed]
- Hicham, W.; Youness, L.; Mounia, O. Flavonoids, and flavonoid-based nanoparticles for osteoarthritis and rheumatoid arthritis management. BioChem 2024, 4, 38–61. [Google Scholar] [CrossRef]
- Mishra, D.; Mishra, A.; Rai, S.N.; Vamanu, E.; Singh, M.P. In Silico Insight to Identify Potential Inhibitors of BUB1B from Mushroom Bioactive Compounds to Prevent Breast Cancer Metastasis. Front. Biosci. 2023, 28, 151. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.; Mishra, A.; Rai, S.N.; Vamanu, E.; Singh, M.P. Identification of Prognostic Biomarkers for Suppressing Tumorigenesis and Metastasis of Hepatocellular Carcinoma through Transcriptome Analysis. Diagnostics 2023, 13, 965. [Google Scholar] [CrossRef]
- Mishra, D.; Mishra, A.; Rai, S.N.; Vamanu, E.; Singh, M.P. Demystifying the Role of Prognostic Biomarkers in Breast Cancer through Integrated Transcriptome and Pathway Enrichment Analyses. Diagnostics 2023, 13, 1142. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, N.V.; Villuri, B.K.; Nagarajan, B.; Lewicki, S.; Das, S.K.; Fisher, P.B.; Desai, U.R. Design and Synthesis of Small Molecule Probes of MDA-9/Syntenin. Biomolecules 2024, 14, 1287. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thumpati, P.; Rai, S.N.; Prajapati, C.; Ramakrishna, K.; Singh, S.K. Targeting c-MYC G-Quadruplexes for Cancer Treatment with Small Molecules. Sci. Pharm. 2025, 93, 6. https://doi.org/10.3390/scipharm93010006
Thumpati P, Rai SN, Prajapati C, Ramakrishna K, Singh SK. Targeting c-MYC G-Quadruplexes for Cancer Treatment with Small Molecules. Scientia Pharmaceutica. 2025; 93(1):6. https://doi.org/10.3390/scipharm93010006
Chicago/Turabian StyleThumpati, Prasanth, Sachchida Nand Rai, Chandrabhan Prajapati, Kakarla Ramakrishna, and Santosh Kumar Singh. 2025. "Targeting c-MYC G-Quadruplexes for Cancer Treatment with Small Molecules" Scientia Pharmaceutica 93, no. 1: 6. https://doi.org/10.3390/scipharm93010006
APA StyleThumpati, P., Rai, S. N., Prajapati, C., Ramakrishna, K., & Singh, S. K. (2025). Targeting c-MYC G-Quadruplexes for Cancer Treatment with Small Molecules. Scientia Pharmaceutica, 93(1), 6. https://doi.org/10.3390/scipharm93010006






