Dynamic Changes in Serum Metabolomic Profiles of Growing Pigs Induced by Intravenous Infusion of Sodium Butyrate
Abstract
:1. Introduction
2. Results
2.1. Effects of Intravenous Infusion of SB on Serum Biochemical Parameters
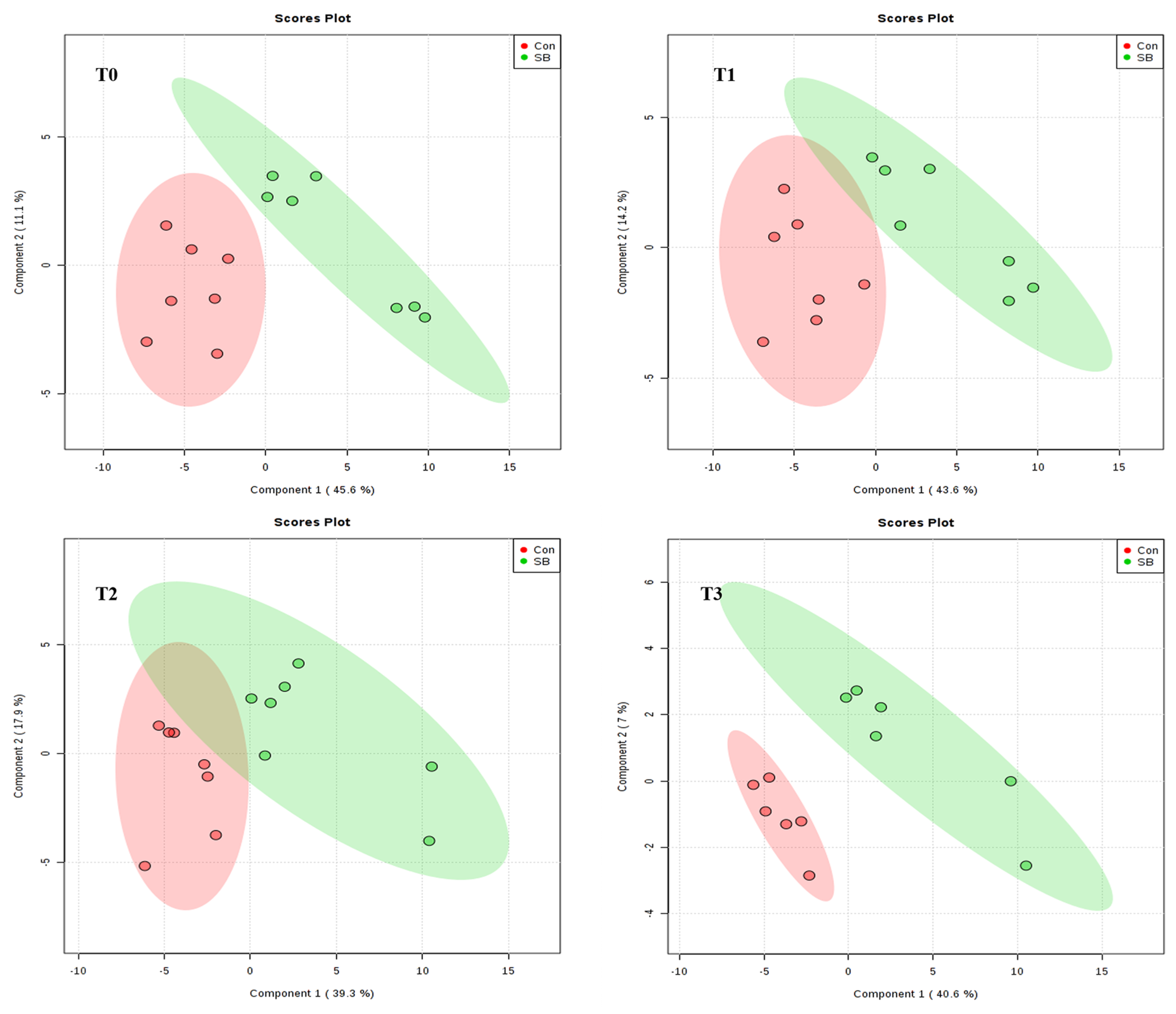
2.2. Global Overview of the Serum Metabolomic Profile
2.3. Effects of Intravenous Infusion of SB on Serum Metabolomics of Growing Pigs
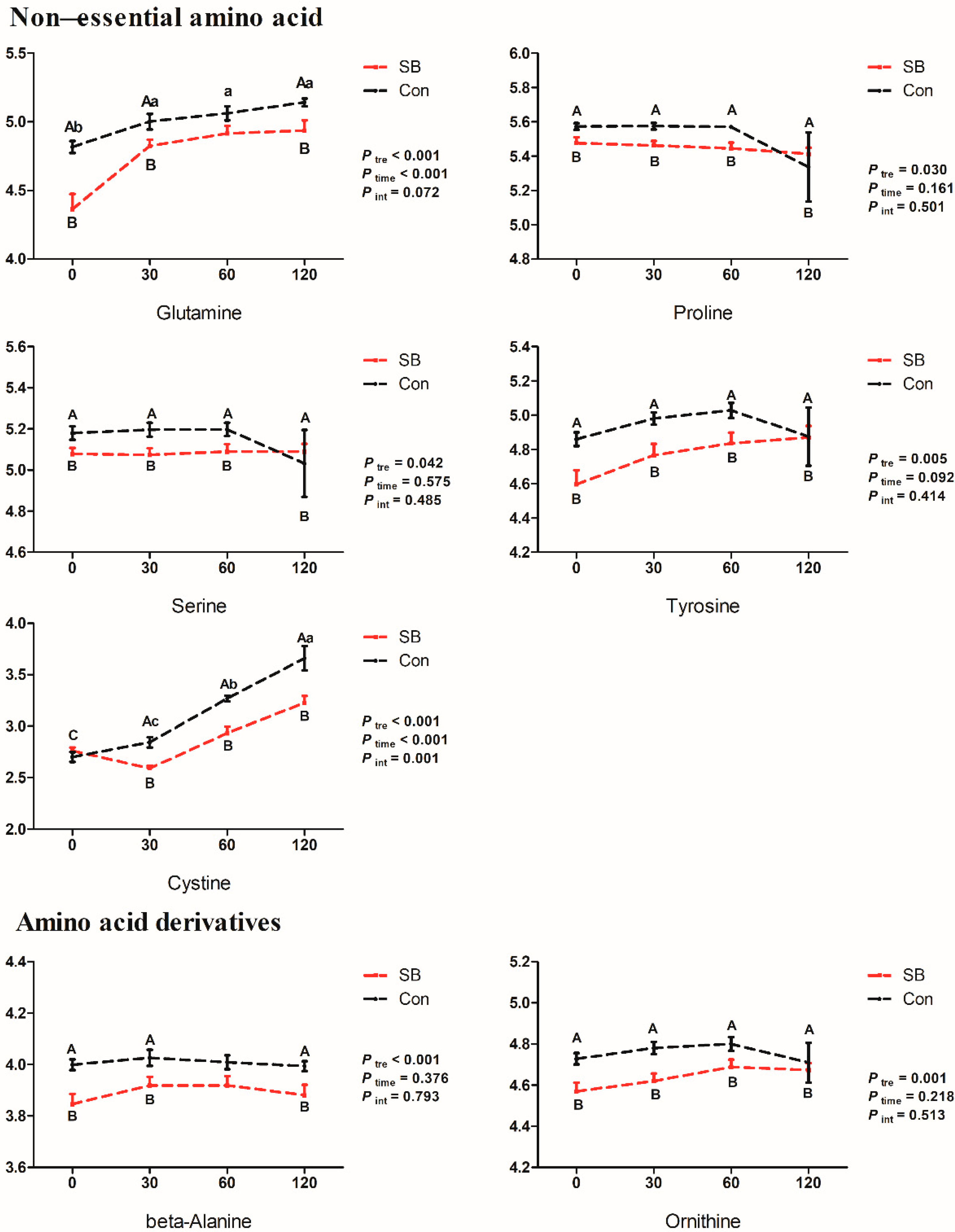
2.4. Effects of Intravenous Infusion of SB on the Dynamic Changes of Differential Metabolites
3. Discussion
3.1. Effects of Intravenous Infusion of SB on Amino Acid Metabolism of Growing Pigs
3.2. Effects of Intravenous Infusion of SB on Fat Metabolism of Growing Pigs
3.3. Effects of Intravenous Infusion of SB on the TCA Cycle of Growing Pigs
4. Material and Methods
4.1. Ethics Statement
4.2. Animals, Housing, and Experimental Design
4.3. Sampling
4.4. Serum Biochemical Analysis
4.5. Gas Chromatograph-Mass Spectrometry Based Metabolites Profiling
4.5.1. Serum Samples Preparation and GC-MS Analysis
4.5.2. Data Acquisition and Processing
4.5.3. Univariate, Multivariate, and Pathway Analysis
4.6. Data Analysis
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fu, X.; Liu, Z.; Zhu, C.; Mou, H.; Kong, Q. Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Crit. Rev. Food Sci. Nutr. 2019, 59 (Suppl. S1), S130–S152. [Google Scholar] [CrossRef]
- Hu, J.; Lin, S.; Zheng, B.; Cheung, P.C. Short-chain fatty acids in control of energy metabolism. Crit. Rev. Food Sci. Nutr. 2018, 58, 1243–1249. [Google Scholar] [CrossRef]
- Wang, C.C.; Wu, H.; Lin, F.H.; Gong, R.; Xie, F.; Peng, Y.; Hu, C.H. Sodium butyrate enhances intestinal integrity, inhibits mast cell activation, inflammatory mediator production and JNK signaling pathway in weaned pigs. Innate Immun. 2018, 24, 40–46. [Google Scholar] [CrossRef]
- Vieira, E.L.; Leonel, A.J.; Sad, A.P.; Beltrão, N.R.; Costa, T.F.; Ferreira, T.M.; Alvarez-Leite, J.I. Oral administration of sodium butyrate attenuates inflammation and mucosal lesion in experimental acute ulcerative colitis. J. Nutr. Biochem. 2012, 23, 430–436. [Google Scholar] [CrossRef]
- Huang, C.; Song, P.; Fan, P.; Hou, C.; Thacker, P.; Ma, X. Dietary sodium butyrate decreases postweaning diarrhea by modulating intestinal permeability and changing the bacterial communities in weaned piglets. J. Nutr. 2015, 145, 2774–2780. [Google Scholar] [CrossRef]
- Xu, J.; Chen, X.; Yu, S.; Su, Y.; Zhu, W. Effects of early intervention with sodium butyrate on gut microbiota and the expression of inflammatory cytokines in neonatal piglets. PLoS ONE 2016, 11, e0162461. [Google Scholar] [CrossRef] [PubMed]
- Thormar, H.; Hilmarsson, H.; Bergsson, G. Stable concentrated emulsions of the 1-monoglyceride of capric acid (monocaprin) with microbicidal activities against the food-borne bacteria Campylobacter jejuni, Salmonella spp., and Escherichia coli. Appl. Environ. Microbiol. 2006, 72, 522–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, Y.D.; Lindemann, M.D.; Monegue, H.J.; Monegue, J.S. The effect of coated sodium butyrate supplementation in sow and nursery diets on lactation performance and nursery pig growth performance. Livest. Sci. 2017, 195, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Feng, W.; Wu, Y.; Chen, G.; Fu, S.; Li, B.; Huang, B.; Liu, J. Sodium butyrate attenuates diarrhea in weaned piglets and promotes tight junction protein expression in colon in a GPR109A-dependent manner. Cell. Physiol. Biochem. 2018, 47, 1617–1629. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Wang, M.; Guo, H.; Jia, Y.; Yang, X.; Zhao, R. Effects of sodium butyrate supplementation on reproductive performance and colostrum composition in gilts. Animal 2016, 10, 1722–1727. [Google Scholar] [CrossRef]
- Song, B.; Li, H.; Wu, Y.; Zhen, W.; Wang, Z.; Xia, Z.; Guo, Y. Effect of microencapsulated sodium butyrate dietary supplementation on growth performance and intestinal barrier function of broiler chickens infected with necrotic enteritis. Anim. Feed Sci. Technol. 2017, 232, 6–15. [Google Scholar] [CrossRef]
- Ahsan, U.; Cengiz, Ö.; Raza, I.; Kuter, E.; Chacher, M.F.A.; Iqbal, Z.; Cakir, S. Sodium butyrate in chicken nutrition: The dynamics of performance, gut microbiota, gut morphology, and immunity. World’s Poult. Sci. J. 2016, 72, 265–275. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.; Zhou, X.Q.; Jiang, W.D.; Liu, Y.; Wu, P.; Jiang, J.; Xie, F. Sodium butyrate improved intestinal immune function associated with NF-κB and p38MAPK signalling pathways in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2017, 66, 548–563. [Google Scholar] [CrossRef]
- Liu, M.; Guo, W.; Wu, F.; Qu, Q.; Tan, Q.; Gong, W. Dietary supplementation of sodium butyrate may benefit growth performance and intestinal function in juvenile grass carp (Ctenopharyngodon idellus). Aquac. Res. 2017, 48, 4102–4111. [Google Scholar] [CrossRef]
- Qiu, Y.; Ma, X.; Yang, X.; Wang, L.; Jiang, Z. Effect of sodium butyrate on cell proliferation and cell cycle in porcine intestinal epithelial (IPEC-J2) cells. In Vitro Cell. Dev. Biol. Anim. 2017, 53, 304–311. [Google Scholar] [CrossRef]
- Miletta, M.C.; Petkovic, V.; Eblé, A.; Ammann, R.A.; Flück, C.E.; Mullis, P.E. Butyrate increases intracellular calcium levels and enhances growth hormone release from rat anterior pituitary cells via the G-protein-coupled receptors GPR41 and 43. PLoS ONE 2014, 9, e107388. [Google Scholar] [CrossRef] [Green Version]
- Davie, J.R. Inhibition of histone deacetylase activity by butyrate. J. Nutr. 2003, 133, 2485S–2493S. [Google Scholar] [CrossRef]
- Walsh, M.E.; Bhattacharya, A.; Sataranatarajan, K.; Qaisar, R.; Sloane, L.; Rahman, M.M.; Van Remmen, H. The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging. Aging Cell 2015, 14, 957–970. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.M.A.E.; Venema, K.; Vanhoutvin, S.A.L.W.; Troost, F.J.; Brummer, R.J. The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef]
- Li, G.; Yao, W.; Jiang, H. Short-Chain Fatty Acids Stimulate Porcine Adipocyte Differentiation Probably through Inhibition of Histone Deacetylases; Endocrine Society: Washington, DC, USA, 2014; Volume 35. [Google Scholar]
- Lu, C.; Thompson, C.B. Metabolic regulation of epigenetics. Cell Metab. 2012, 16, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Miyamoto, N.; Shibata, K.; Valasek, M.A.; Motoike, T.; Kedzierski, R.M.; Yanagisawa, M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc. Natl. Acad. Sci. USA 2004, 101, 1045–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoh, K.; Tsuda, T. Effects of intravenous injection of butyrate on the exocrine pancreatic secretion in guinea pigs. J. Comp. Physiol. Psychol. 1987, 87, 569–572. [Google Scholar] [CrossRef]
- Bartholome, A.L.; Albin, D.M.; Baker, D.H.; Holst, J.J.; Tappenden, K.A. Supplementation of total parenteral nutrition with butyrate acutely increases structural aspects of intestinal adaptation after an 80% jejunoileal resection in neonatal piglets. JPEN-Parenter. Enter. 2004, 28, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Drozdowski, L.A.; Dixon, W.T.; McBurney, M.I.; Thomson, A.B. Short-chain fatty acids and total parenteral nutrition affect intestinal gene expression. JPEN-Parenter. Enter. 2002, 26, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Ren, E.; Chen, X.; Yu, S.; Xu, J.; Su, Y.; Zhu, W. Transcriptomic and metabolomic responses induced in the livers of growing pigs by a short-term intravenous infusion of sodium butyrate. Animal 2018, 12, 2318–2326. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, J.; Su, Y.; Zhu, W.Y. Effects of intravenous infusion with sodium butyrate on colonic microbiota, intestinal development-and mucosal immune-related gene expression in normal growing pigs. Front. Microbiol. 2018, 9, 1652. [Google Scholar] [CrossRef]
- Sakdee, J.; Poeikhampha, T.; Rakangthong, C.; Poungpong, K.; Bunchasak, C. Effect of Tributyrin Supplementation in Diet on Production Performance and Gastrointestinal Tract of Healthy Nursery Pigs. Pak. J. Nutr. 2016, 15, 954–962. [Google Scholar]
- Grilli, E.; Tugnoli, B.; Foerster, C.J.; Piva, A. Butyrate modulates inflammatory cytokines and tight junctions components along the gut of weaned pigs. J. Anim. Sci. 2016, 94 (Suppl. S3), 433–436. [Google Scholar] [CrossRef]
- Casanova-Higes, A.; Andrés-Barranco, S.; Mainar-Jaime, R.C. Effect of the addition of protected sodium butyrate to the feed on Salmonella spp. infection dynamics in fattening pigs. Anim. Feed Sci. Technol. 2017, 231, 12–18. [Google Scholar] [CrossRef] [Green Version]
- Val-Laillet, D.; Guérin, S.; Coquery, N.; Nogret, I.; Formal, M.; Romé, V.; Malbert, C.H. Oral sodium butyrate impacts brain metabolism and hippocampal neurogenesis, with limited effects on gut anatomy and function in pigs. FASEB J. 2018, 32, 2160–2171. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Ren, E.; Xu, J.; Su, Y.; Zhu, W. Effects of early intervention with sodium butyrate on lipid metabolism-related gene expression and liver metabolite profiles in neonatal piglets. Livest. Sci. 2017, 195, 80–86. [Google Scholar] [CrossRef]
- Rader, D.J.; Hovingh, G.K. HDL and cardiovascular disease. Lancet 2014, 384, 618–625. [Google Scholar] [CrossRef]
- Hong, J.; Jia, Y.; Pan, S.; Jia, L.; Li, H.; Han, Z.; Zhao, R. Butyrate alleviates high fat diet-induced obesity through activation of adiponectin-mediated pathway and stimulation of mitochondrial function in the skeletal muscle of mice. Oncotarget 2016, 7, 56071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masud, F.; Shakoori, T.A.; Khawaja, K.I.; Ali, M.; Ameer, F. Changes in body weight and lipid profile of mice treated with sodium butyrate and metformin. Pak. J. Zool. 2016, 48, 1343–1348. [Google Scholar]
- Yin, F.; Yu, H.; Lepp, D.; Shi, X.; Yang, X.; Hu, J.; Gong, J. Transcriptome analysis reveals regulation of gene expression for lipid catabolism in young broilers by butyrate glycerides. PLoS ONE 2016, 11, e0160751. [Google Scholar]
- Naveenkumar, S.; Karthikeyan, N.; Narendra Babu, R.; Veeramani, P.; Sivarama Krishnani, S.; Srinivasan, G. Effect of calcium propionate and coated sodium butyrate as an alternative to antibiotic growth promoters on the serum profile of commercial broiler chicken. Int. J. Chem. Stud. 2018, 6, 36–39. [Google Scholar]
- Pamir, N.; Hutchins, P.; Ronsein, G.; Vaisar, T.; Reardon, C.A.; Getz, G.S.; Heinecke, J.W. Proteomic analysis of HDL from inbred mouse strains implicates APOE associated with HDL in reduced cholesterol efflux capacity via the ABCA1 pathway. J. Lipid Res. 2016, 57, 246–257. [Google Scholar] [CrossRef] [Green Version]
- Marcil, V.; Delvin, E.; Seidman, E.; Poitras, L.; Zoltowska, M.; Garofalo, C.; Levy, E. Modulation of lipid synthesis, apolipoprotein biogenesis, and lipoprotein assembly by butyrate. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G340–G346. [Google Scholar] [CrossRef] [Green Version]
- Tabuchi, Y.; Takasaki, I.; Doi, T.; Ishii, Y.; Sakai, H.; Kondo, T. Genetic networks responsive to sodium butyrate in colonic epithelial cells. FEBS Lett. 2006, 580, 3035–3041. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.H.; Ou, H.C.; Jang, T.H.; Lin, L.B.; Huang, H.M. Altered phospholipid metabolism in sodium butyrate-induced differentiation of C6 glioma cells. Lipids 1997, 32, 273–282. [Google Scholar] [CrossRef]
- Mailloux, R.J.; Bériault, R.; Lemire, J.; Singh, R.; Chénier, D.R.; Hamel, R.D.; Appanna, V.D. The tricarboxylic acid cycle, an ancient metabolic network with a novel twist. PLoS ONE 2007, 2, e690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, D.B.; Vagelos, P.R. The mechanism of tricarboxylic acid cycle regulation of fatty acid synthesis. J. Biol. Chem. 1962, 237, 1787–1792. [Google Scholar] [CrossRef]
- M Astbury, S.; M Corfe, B. Uptake and metabolism of the short-chain fatty acid butyrate, a critical review of the literature. Curr. Drug Metab. 2012, 13, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Item | Con | SB | SEM | p Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 30 | 60 | 120 | 0 | 30 | 60 | 120 | Time | SB | Interaction | ||
| Total protein | 49.83 | 53.17 | 50.13 | 50.79 | 54.43 | 51.5 | 51.49 | 53.03 | 2.14 | 0.787 | 0.161 | 0.293 |
| Triglyceride | 0.37 | 0.36 | 0.34 | 0.40 | 0.43 | 0.34 | 0.4 | 0.37 | 0.05 | 0.608 | 0.588 | 0.573 |
| Cholesterol | 1.94 | 1.93 | 1.91 | 1.96 | 2.15 | 2.01 | 2.04 | 2.09 | 0.19 | 0.930 | 0.176 | 0.973 |
| HDL-C | 0.90 | 0.94 | 0.93 | 0.95 | 1.13 | 1.05 | 1.02 | 1.06 | 0.08 | 0.939 | 0.005 | 0.699 |
| LDL-C | 1.14 | 1.19 | 1.14 | 1.17 | 1.22 | 1.16 | 1.13 | 1.19 | 0.13 | 0.969 | 0.972 | 0.927 |
| Glucose | 4.61 | 4.69 | 4.57 | 4.30 | 5.05 | 4.91 | 4.86 | 4.93 | 0.42 | 0.923 | 0.101 | 0.928 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Ren, E.; Xiang, X.; Su, Y.; Zhu, W. Dynamic Changes in Serum Metabolomic Profiles of Growing Pigs Induced by Intravenous Infusion of Sodium Butyrate. Metabolites 2020, 10, 20. https://doi.org/10.3390/metabo10010020
Wang H, Ren E, Xiang X, Su Y, Zhu W. Dynamic Changes in Serum Metabolomic Profiles of Growing Pigs Induced by Intravenous Infusion of Sodium Butyrate. Metabolites. 2020; 10(1):20. https://doi.org/10.3390/metabo10010020
Chicago/Turabian StyleWang, Hongyu, Erdu Ren, Xiaoe Xiang, Yong Su, and Weiyun Zhu. 2020. "Dynamic Changes in Serum Metabolomic Profiles of Growing Pigs Induced by Intravenous Infusion of Sodium Butyrate" Metabolites 10, no. 1: 20. https://doi.org/10.3390/metabo10010020
APA StyleWang, H., Ren, E., Xiang, X., Su, Y., & Zhu, W. (2020). Dynamic Changes in Serum Metabolomic Profiles of Growing Pigs Induced by Intravenous Infusion of Sodium Butyrate. Metabolites, 10(1), 20. https://doi.org/10.3390/metabo10010020






