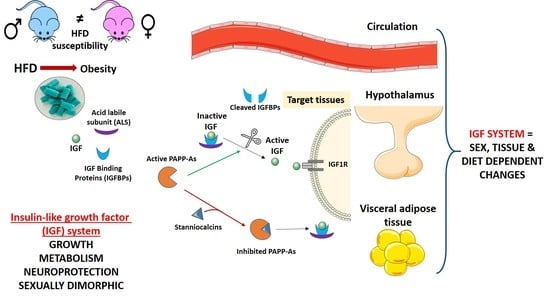
Impact of Long-Term HFD Intake on the Peripheral and Central IGF System in Male and Female Mice
Abstract
:1. Introduction
2. Results
2.1. Body Composition
2.2. Glucose Tolerance Test and Insulin Levels
2.3. Circulating Levels of the IGF System
2.4. The IGF System in Visceral Adipose Tissue (VAT)
2.5. Hypothalamic Response to Dietary Change
2.5.1. The IGF System in the Hypothalamus
2.5.2. Hypothalamic Neuropeptides
2.5.3. Gliosis and Hypothalamic Stress
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Animals and Diets
4.3. Glucose Tolerance Test (GTT)
4.4. Tissue Collection
4.5. ELISA Assays
4.6. RNA and Protein Extraction
4.7. Western Blotting
4.8. Real-Time qPCR
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yakar, S.; Rosen, C.J.; Beamer, W.G.; Ackert-Bicknell, C.L.; Wu, Y.; Liu, J.L.; Ooi, G.T.; Setser, J.; Frystyk, J.; Boisclair, Y.R.; et al. Circulating levels of IGF-1 directly regulate bone growth and density. J. Clin. Investig. 2002, 110, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Lui, J.C.; Finkielstain, G.P.; Barnes, K.M.; Baron, J. An imprinted gene network that controls mammalian somatic growth is down-regulated during postnatal growth deceleration in multiple organs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R189–R196. [Google Scholar] [CrossRef] [PubMed]
- Bann, D.; Holly, J.M.; Lashen, H.; Hardy, R.; Adams, J.; Kuh, D.; Ong, K.K.; Ben-Shlomo, Y. Changes in insulin-like growth factor-I and -II associated with fat but not lean mass in early old age. Obesity 2015, 23, 692–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blum, W.F.; Ranke, M.B. Insulin-like growth factor binding proteins (IGFBPs) with special reference to IGFBP-3. Acta Paediatr. Scand. Suppl. 1990, 367, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.C. Insulin-like growth factor (IGF)-binding proteins: Interactions with IGFs and intrinsic bioactivities. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E967–E976. [Google Scholar] [CrossRef] [PubMed]
- Ranke, M.B. Insulin-like growth factor binding-protein-3 (IGFBP-3). Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 701–711. [Google Scholar] [CrossRef]
- Probst-Hensch, N.M.; Steiner, J.H.; Schraml, P.; Varga, Z.; Zürrer-Härdi, U.; Storz, M.; Korol, D.; Fehr, M.K.; Fink, D.; Pestalozzi, B.C.; et al. IGFBP2 and IGFBP3 protein expressions in human breast cancer: Association with hormonal factors and obesity. Clin. Cancer Res. 2010, 16, 1025–1032. [Google Scholar] [CrossRef] [Green Version]
- Russo, V.C.; Azar, W.J.; Yau, S.W.; Sabin, M.A.; Werther, G.A. IGFBP-2: The dark horse in metabolism and cancer. Cytokine Growth Factor Rev. 2015, 26, 329–346. [Google Scholar] [CrossRef]
- Bach, L.A. IGF-binding proteins. J. Mol. Endocrinol. 2018, 61, T11–T28. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.H.; Michels, K.M.; Bondy, C.A. Localization of insulin-like growth factor binding protein-2 messenger RNA during postnatal brain development: Correlation with insulin-like growth factors I and II. Neuroscience 1993, 53, 251–265. [Google Scholar] [CrossRef]
- Boney, C.M.; Moats-Staats, B.M.; Stiles, A.D.; D’Ercole, A.J. Expression of insulin-like growth factor-I (IGF-I) and IGF-binding proteins during adipogenesis. Endocrinology 1994, 135, 1863–1868. [Google Scholar] [CrossRef] [PubMed]
- Gude, M.F.; Frystyk, J.; Flyvbjerg, A.; Bruun, J.M.; Richelsen, B.; Pedersen, S.B. The production and regulation of IGF and IGFBPs in human adipose tissue cultures. Growth Horm. IGF Res. 2012, 22, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Velloso, C.P. Regulation of muscle mass by growth hormone and IGF-I. Br. J. Pharmacol. 2008, 154, 557–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiess, W.; Yang, Y.; Kessler, U.; Hoeflich, A. Insulin-like growth factor II (IGF-II) and the IGF-II/mannose-6-phosphate receptor: The myth continues. Horm. Res. 1994, 41, 66–73. [Google Scholar] [CrossRef]
- Overgaard, M.T.; Boldt, H.B.; Laursen, L.S.; Sottrup-Jensen, L.; Conover, C.A.; Oxvig, C. Pregnancy-associated plasma protein-A2 (PAPP-A2), a novel insulin-like growth factor-binding protein-5 proteinase. J. Biol. Chem. 2001, 276, 21849–21853. [Google Scholar] [CrossRef] [Green Version]
- Kløverpris, S.; Mikkelsen, J.H.; Pedersen, J.H.; Jepsen, M.R.; Laursen, L.S.; Petersen, S.V.; Oxvig, C. Stanniocalcin-1 Potently Inhibits the Proteolytic Activity of the Metalloproteinase Pregnancy-associated Plasma Protein-A. J. Biol. Chem. 2015, 290, 21915–21924. [Google Scholar] [CrossRef] [Green Version]
- Jepsen, M.R.; Kløverpris, S.; Mikkelsen, J.H.; Pedersen, J.H.; Füchtbauer, E.M.; Laursen, L.S.; Oxvig, C. Stanniocalcin-2 inhibits mammalian growth by proteolytic inhibition of the insulin-like growth factor axis. J. Biol. Chem. 2015, 290, 3430–3439. [Google Scholar] [CrossRef] [Green Version]
- Hennebry, A.; Oldham, J.; Shavlakadze, T.; Grounds, M.D.; Sheard, P.; Fiorotto, M.L.; Falconer, S.; Smith, H.K.; Berry, C.; Jeanplong, F.; et al. IGF1 stimulates greater muscle hypertrophy in the absence of myostatin in male mice. J. Endocrinol. 2017, 234, 187–200. [Google Scholar] [CrossRef] [Green Version]
- Scavo, L.M.; Karas, M.; Murray, M.; Leroith, D. Insulin-like growth factor-I stimulates both cell growth and lipogenesis during differentiation of human mesenchymal stem cells into adipocytes. J. Clin. Endocrinol. Metab. 2004, 89, 3543–3553. [Google Scholar] [CrossRef]
- Argente, J.; Caballo, N.; Barrios, V.; Muñoz, M.T.; Pozo, J.; Chowen, J.A.; Hernández, M. Disturbances in the growth hormone-insulin-like growth factor axis in children and adolescents with different eating disorders. Horm. Res. 1997, 48, 16–18. [Google Scholar] [CrossRef]
- Reinhardt, R.R.; Bondy, C.A. Insulin-like growth factors cross the blood-brain barrier. Endocrinology 1994, 135, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Kastin, A.J. Interactions of IGF-1 with the blood-brain barrier in vivo and in situ. Neuroendocrinology 2000, 72, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Quesada, A.; Romeo, H.E.; Micevych, P. Distribution and localization Patterns of Estrogen Receptor-β and Insulin-Like Growth Factor-1 Receptors in Neurons and Glial Cells of the Female Rat Substantia Nigra. J. Comp. Neurol. 2007, 503, 198–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labandeira-Garcia, J.L.; Costa-Besada, M.A.; Labandeira, C.M.; Villar-Cheda, B.; Rodríguez-Perez, A.I. Insulin-Like Growth Factor-1 and Neuroinflammation. Front. Aging Neurosci. 2017, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- O’Kusky, J.R.; Ye, P.; D’Ercole, A.J. Insulin-like growth factor-I promotes neurogenesis and synaptogenesis in the hippocampal dentate gyrus during postnatal development. J. Neurosci. 2000, 20, 8435–8442. [Google Scholar] [CrossRef] [Green Version]
- Carro, E.; Torres-Aleman, I. The role of insulin and insulin-like growth factor I in the molecular and cellular mechanisms underlying the pathology of Alzheimer’s disease. Eur. J. Pharmacol. 2004, 490, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Genis, L.; Dávila, D.; Fernandez, S.; Pozo-Rodrigálvarez, A.; Martínez-Murillo, R.; Torres-Aleman, I. Astrocytes require insulin-like growth factor I to protect neurons against oxidative injury. F1000 Res. 2014, 3, 28. [Google Scholar] [CrossRef]
- Hernandez-Garzón, E.; Fernandez, A.M.; Perez-Alvarez, A.; Genis, L.; Bascuñana, P.; Fernandez de la Rosa, R.; Delgado, M.; Angel Pozo, M.; Moreno, E.; McCormick, P.J.; et al. The insulin-like growth factor I receptor regulates glucose transport by astrocytes. Glia 2016, 64, 1962–1971. [Google Scholar] [CrossRef]
- Chen, D.Y.; Stern, S.A.; Garcia-Osta, A.; Saunier-Rebori, B.; Pollonini, G.; Bambah-Mukku, D.; Blitzer, R.D.; Alberini, C.M. A critical role for IGF-II in memory consolidation and enhancement. Nature 2011, 469, 491–497. [Google Scholar] [CrossRef]
- Myer, D.J.; Gurkoff, G.G.; Lee, S.M.; Hovda, D.A.; Sofroniew, M.V. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain 2006, 129, 2761–2772. [Google Scholar] [CrossRef]
- Faulkner, J.R.; Herrmann, J.E.; Woo, M.J.; Tansey, K.E.; Doan, N.B.; Sofroniew, M.V. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 2004, 24, 2143–2155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liddelow, S.; Guttenplan, K.; Clarke, L.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Buckman, L.B.; Thompson, M.M.; Lippert, R.N.; Blackwell, T.S.; Yull, F.E.; Ellacott, K.L. Evidence for a novel functional role of astrocytes in the acute homeostatic response to high-fat diet intake in mice. Mol. Metab. 2014, 4, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Douglass, J.D.; Dorfman, M.D.; Fasnacht, R.; Shaffer, L.D.; Thaler, J.P. Astrocyte IKKβ/NF-κB signaling is required for diet-induced obesity and hypothalamic inflammation. Mol. Metab. 2017, 6, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, J.; Morales, L.; Barreto, G.E. Metabolic and Inflammatory Adaptation of Reactive Astrocytes: Role of PPARs. Mol. Neurobiol. 2017, 54, 2518–2538. [Google Scholar] [CrossRef] [PubMed]
- Komoly, S.; Hudson, L.D.; Webster, H.D.; Bondy, C.A. Insulin-like growth factor I gene expression is induced in astrocytes during experimental demyelination. Proc. Natl. Acad. Sci. USA 1992, 89, 1894–1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, A.M.; Garcia-Estrada, J.; Garcia-Segura, L.M.; Torres-Aleman, I. Insulin-like growth factor I modulates c-fos induction and astrocytosis in response to neurotoxic insult. Neuroscience 1996, 76, 117–122. [Google Scholar] [CrossRef] [Green Version]
- Ryu, B.R.; Ko, H.W.; Jou, I.; Noh, J.S.; Gwag, B.J. Phosphatidylinositol 3-kinase-mediated regulation of neuronal apoptosis and necrosis by insulin and IGF-I. J. Neurobiol. 1999, 39, 536–546. [Google Scholar] [CrossRef]
- Guerra-Cantera, S.; Frago, L.M.; Díaz, F.; Ros, P.; Jiménez-Hernaiz, M.; Freire-Regatillo, A.; Barrios, V.; Argente, J.; Chowen, J.A. Short-Term Diet Induced Changes in the Central and Circulating IGF Systems Are Sex Specific. Front. Endocrinol. 2020, 11, 513. [Google Scholar] [CrossRef]
- Hedbacker, K.; Birsoy, K.; Wysocki, R.W.; Asilmaz, E.; Ahima, R.S.; Farooqi, I.S.; Friedman, J.M. Antidiabetic effects of IGFBP2, a leptin-regulated gene. Cell Metab. 2010, 11, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Wheatcroft, S.B.; Kearney, M.T.; Shah, A.M.; Ezzat, V.A.; Miell, J.R.; Modo, M.; Williams, S.C.; Cawthorn, W.P.; Medina-Gomez, G.; Vidal-Puig, A.; et al. IGF-binding protein-2 protects against the development of obesity and insulin resistance. Diabetes 2007, 56, 285–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paruthiyil, S.; Hagiwara, S.I.; Kundassery, K.; Bhargava, A. Sexually dimorphic metabolic responses mediated by CRF2 receptor during nutritional stress in mice. Biol. Sex Differ. 2018, 9, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Wilde, J.; Smit, E.; Mohren, R.; Boekschoten, M.V.; de Groot, P.; van den Berg, S.A.; Bijland, S.; Voshol, P.J.; van Dijk, K.W.; de Wit, N.W.; et al. An 8-week high-fat diet induces obesity and insulin resistance with small changes in the muscle transcriptome of C57BL/6J mice. J. Nutrigenet. Nutr. 2009, 2, 280–291. [Google Scholar] [CrossRef]
- Ros, P.; Díaz, F.; Freire-Regatillo, A.; Argente-Arizón, P.; Barrios, V.; Argente, J.; Chowen, J.A. Sex Differences in Long-term Metabolic Effects of Maternal Resveratrol Intake in Adult Rat Offspring. Endocrinology 2020, 161, bqaa090. [Google Scholar] [CrossRef]
- Samuel, P.; Khan, M.A.; Nag, S.; Inagami, T.; Hussain, T. Angiotensin AT(2) receptor contributes towards gender bias in weight gain. PLoS ONE 2013, 8, e48425. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, U.S.; Waldén, T.B.; Carlsson, P.O.; Jansson, L.; Phillipson, M. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLoS ONE 2012, 7, e46057. [Google Scholar] [CrossRef] [PubMed]
- Pinos, H.; Carrillo, B.; Díaz, F.; Chowen, J.A.; Collado, P. Differential vulnerability to adverse nutritional conditions in male and female rats: Modulatory role of estradiol during development. Front. Neuroendocrinol. 2018, 48, 13–22. [Google Scholar] [CrossRef]
- Chowen, J.A.; Freire-Regatillo, A.; Argente, J. Neurobiological characteristics underlying metabolic differences between males and females. Prog. Neurobiol. 2019, 176, 18–32. [Google Scholar] [CrossRef]
- Peshti, V.; Obolensky, A.; Nahum, L.; Kanfi, Y.; Rathaus, M.; Avraham, M.; Tinman, S.; Alt, F.W.; Banin, E.; Cohen, H.Y. Characterization of physiological defects in adult SIRT6-/- mice. PLoS ONE 2017, 12, e0176371. [Google Scholar] [CrossRef] [Green Version]
- Frystyk, J.; Grønbaek, H.; Skjaerbaek, C.; Flyvbjerg, A.; Orskov, H.; Baxter, R.C. Developmental changes in serum levels of free and total insulin-like growth factor I (IGF-I), IGF-binding protein-1 and -3, and the acid-labile subunit in rats. Endocrinology 1998, 139, 4286–4292. [Google Scholar] [CrossRef]
- Argente, J.; Barrios, V.; Pozo, J.; Muñoz, M.T.; Hervás, F.; Stene, M.; Hernández, M. Normative data for insulin-like growth factors (IGFs), IGF-binding proteins, and growth hormone-binding protein in a healthy Spanish pediatric population: Age- and sex-related changes. J. Clin. Endocrinol. Metab. 1993, 77, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Waters, D.L.; Yau, C.L.; Montoya, G.D.; Baumgartner, R.N. Serum Sex Hormones, IGF-1, and IGFBP3 Exert a Sexually Dimorphic Effect on Lean Body Mass in Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, 648–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borski, R.J.; Tsai, W.; DeMott-Friberg, R.; Barkan, A.L. Regulation of somatic growth and the somatotropic axis by gonadal steroids: Primary effect on insulin-like growth factor I gene expression and secretion. Endocrinology 1996, 137, 3253–3259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, S.Y.; Lee, E.J.; Kim, K.R.; Cha, B.S.; Song, Y.D.; Lim, S.K.; Lee, H.C.; Huh, K.B. Effect of obesity on total and free insulin-like growth factor (IGF)-1, and their relationship to IGF-binding protein (BP)-1, IGFBP-2, IGFBP-3, insulin, and growth hormone. Int. J. Obes. Relat. Metab. Disord. 1997, 21, 355–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, S.L.; Donohoe, C.L.; Finn, S.P.; Howard, J.M.; Lithander, F.E.; Reynolds, J.V.; Pidgeon, G.P.; Lysaght, J. IGF-1 and its receptor in esophageal cancer: Association with adenocarcinoma and visceral obesity. Am. J. Gastroenterol. 2012, 107, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Xuan, L.; Ma, J.; Yu, M.; Yang, Z.; Huang, Y.; Guo, C.; Lu, Y.; Yan, L.; Shi, S. Insulin-like growth factor 2 promotes adipocyte proliferation, differentiation and lipid deposition in obese type 2 diabetes. J. Transl. Sci. 2019, 6, 1–7. [Google Scholar] [CrossRef]
- Buchanan, C.M.; Phillips, A.R.; Cooper, G.J. Preptin derived from proinsulin-like growth factor II (proIGF-II) is secreted from pancreatic islet beta cells and enhances insulin secretion. Biochem. J. 2001, 360, 431–439. [Google Scholar] [CrossRef]
- Argente, J.; Caballo, N.; Barrios, V.; Pozo, J.; Muñoz, M.T.; Chowen, J.A.; Hernández, M. Multiple endocrine abnormalities of the growth hormone and insulin-like growth factor axis in prepubertal children with exogenous obesity: Effect of short- and long-term weight reduction. J. Clin. Endocrinol. Metab. 1997, 82, 2076–2083. [Google Scholar] [CrossRef] [Green Version]
- Vahl, N.; Klausen, I.; Christiansen, J.S.; Jørgensen, J.O. Growth hormone (GH) status is an independent determinant of serum levels of cholesterol and triglycerides in healthy adults. Clin. Endocrinol. 1999, 51, 309–316. [Google Scholar] [CrossRef]
- Berryman, D.E.; Glad, C.A.; List, E.O.; Johannsson, G. The GH/IGF-1 axis in obesity: Pathophysiology and therapeutic considerations. Nat. Rev. Endocrinol. 2013, 9, 346–356. [Google Scholar] [CrossRef]
- Lewitt, M.S.; Dent, M.S.; Hall, K. The Insulin-Like Growth Factor System in Obesity, Insulin Resistance and Type 2 Diabetes Mellitus. J. Clin. Med. 2014, 3, 1561–1574. [Google Scholar] [CrossRef] [PubMed]
- Clemmons, D.R.; Snyder, D.K.; Busby, W.H., Jr. Variables controlling the secretion of insulin-like growth factor binding protein-2 in normal human subjects. J. Clin. Endocrinol. Metab. 1991, 73, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Yau, S.W.; Harcourt, B.E.; Kao, K.T.; Alexander, E.J.; Russo, V.C.; Werther, G.A.; Sabin, M.A. Serum IGFBP-2 levels are associated with reduced insulin sensitivity in obese children. Clin. Obes. 2018, 8, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Barrios, V.; Buño, M.; Pozo, J.; Muñoz, M.T.; Argente, J. Insulin-like growth factor-binding protein-2 levels in pediatric patients with growth hormone deficiency, eating disorders and acute lymphoblastic leukemia. Horm. Res. 2000, 53, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Hwang, L.L.; Wang, C.H.; Li, T.L.; Chang, S.D.; Lin, L.C.; Chen, C.P.; Chen, C.T.; Liang, K.C.; Ho, I.K.; Yang, W.S.; et al. Sex differences in high-fat diet-induced obesity, metabolic alterations and learning, and synaptic plasticity deficits in mice. Obesity 2010, 18, 463–469. [Google Scholar] [CrossRef]
- Wabitsch, M.; Hauner, H.; Heinze, E.; Teller, W.M. The role of growth hormone/insulin-like growth factors in adipocyte differentiation. Metabolism 1995, 44, 45–49. [Google Scholar] [CrossRef]
- Smith, P.J.; Wise, L.S.; Berkowitz, R.; Wan, C.; Rubin, C.S. Insulin-like growth factor-I is an essential regulator of the differentiation of 3T3-L1 adipocytes. J. Biol. Chem. 1988, 263, 9402–9408. [Google Scholar]
- Chang, H.R.; Kim, H.J.; Xu, X.; Ferrante, A.W., Jr. Macrophage and adipocyte IGF1 maintain adipose tissue homeostasis during metabolic stresses. Obesity 2016, 24, 172–183. [Google Scholar] [CrossRef] [Green Version]
- Morita, S.; Horii, T.; Kimura, M.; Arai, Y.; Kamei, Y.; Ogawa, Y.; Hatada, I. Paternal allele influences high fat diet-induced obesity. PLoS ONE 2014, 9, e85477. [Google Scholar] [CrossRef] [Green Version]
- Yau, S.W.; Russo, V.C.; Clarke, I.J.; Dunshea, F.R.; Werther, G.A.; Sabin, M.A. IGFBP-2 inhibits adipogenesis and lipogenesis in human visceral, but not subcutaneous, adipocytes. Int. J. Obes. 2015, 39, 770–781. [Google Scholar] [CrossRef]
- Li, Z.; Picard, F. Modulation of IGFBP2 mRNA expression in white adipose tissue upon aging and obesity. Horm. Metab. Res. 2010, 42, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Conover, C.A.; Harstad, S.L.; Tchkonia, T.; Kirkland, J.L. Preferential impact of pregnancy-associated plasma protein-A deficiency on visceral fat in mice on high-fat diet. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1145–E1153. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Rodriguez, R.; Lifshitz, L.M.; Bellve, K.D.; Min, S.Y.; Pires, J.; Leung, K.; Boeras, C.; Sert, A.; Draper, J.T.; Corvera, S.; et al. Human adipose tissue expansion in pregnancy is impaired in gestational diabetes mellitus. Diabetologia 2015, 58, 2106–2114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarapio, E.; De Souza, S.K.; Model, J.F.A.; Trapp, M.; Da Silva, R.S.M. Stanniocalcin-1 and -2 effects on glucose and lipid metabolism in white adipose tissue from fed and fasted rats. Can. J. Physiol. Pharmacol. 2019, 97, 916–923. [Google Scholar] [CrossRef]
- Baquedano, E.; Ruiz-Lopez, A.M.; Sustarsic, E.G.; Herpy, J.; List, E.O.; Chowen, J.A.; Frago, L.M.; Kopchick, J.J.; Argente, J. The absence of GH signaling affects the susceptibility to high-fat diet-induced hypothalamic inflammation in male mice. Endocrinology 2014, 155, 4856–4867. [Google Scholar] [CrossRef] [Green Version]
- Hong, H.; Cui, Z.Z.; Zhu, L.; Fu, S.P.; Rossi, M.; Cui, Y.H.; Zhu, B.M. Central IGF1 improves glucose tolerance and insulin sensitivity in mice. Nutr. Diabetes 2017, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Barrand, S.; Crowley, T.M.; Wood-Bradley, R.J.; De Jong, K.A.; Armitage, J.A. Impact of maternal high fat diet on hypothalamic transcriptome in neonatal Sprague Dawley rats. PLoS ONE 2017, 12, e0189492. [Google Scholar] [CrossRef] [Green Version]
- Iwamoto, T.; Ouchi, Y. Emerging evidence of insulin-like growth factor 2 as a memory enhancer: A unique animal model of cognitive dysfunction with impaired adult neurogenesis. Rev. Neurosci. 2014, 25, 559–574. [Google Scholar] [CrossRef]
- Uchimura, T.; Hollander, J.M.; Nakamura, D.S.; Liu, Z.; Rosen, C.J.; Georgakoudi, I.; Zeng, L. An essential role for IGF2 in cartilage development and glucose metabolism during postnatal long bone growth. Development 2017, 144, 3533–3546. [Google Scholar] [CrossRef] [Green Version]
- Gleason, C.E.; Ning, Y.; Cominski, T.P.; Gupta, R.; Kaestner, K.H.; Pintar, J.E.; Birnbaum, M.J. Role of insulin-like growth factor-binding protein 5 (IGFBP5) in organismal and pancreatic beta-cell growth. Mol. Endocrinol. 2010, 24, 178–192. [Google Scholar] [CrossRef] [Green Version]
- Thaler, J.P.; Yi, C.X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.O.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R.; et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdearcos, M.; Robblee, M.M.; Benjamin, D.I.; Nomura, D.K.; Xu, A.W.; Koliwad, S.K. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. 2014, 9, 2124–2138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemus, M.B.; Bayliss, J.A.; Lockie, S.H.; Santos, V.V.; Reichenbach, A.; Stark, R.; Andrews, Z.B. A stereological analysis of NPY, POMC, Orexin, GFAP astrocyte, and Iba1 microglia cell number and volume in diet-induced obese male mice. Endocrinology 2015, 156, 1701–1713. [Google Scholar] [CrossRef] [PubMed]
- Baufeld, C.; Osterloh, A.; Prokop, S.; Miller, K.R.; Heppner, F.L. High-fat diet-induced brain region-specific phenotypic spectrum of CNS resident microglia. Acta Neuropathol. 2016, 132, 361–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorfman, M.D.; Thaler, J.P. Hypothalamic inflammation and gliosis in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 325–330. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Pham, K.; Gammons, J.W.; Sutherland, D.; Liu, Y.; Smith, A.; Kaczorowski, C.C.; O’Connell, K.M. Diet composition, not calorie intake, rapidly alters intrinsic excitability of hypothalamic AgRP/NPY neurons in mice. Sci. Rep. 2015, 5, 16810. [Google Scholar] [CrossRef] [Green Version]
- Hassan, A.M.; Mancano, G.; Kashofer, K.; Fröhlich, E.E.; Matak, A.; Mayerhofer, R.; Reichmann, F.; Olivares, M.; Neyrinck, A.M.; Delzenne, N.M.; et al. High-fat diet induces depression-like behaviour in mice associated with changes in microbiome, neuropeptide Y, and brain metabolome. Nutr. Neurosci. 2019, 22, 877–893. [Google Scholar] [CrossRef] [Green Version]
- de Araujo, T.M.; Razolli, D.S.; Correa-da-Silva, F.; de Lima-Junior, J.C.; Gaspar, R.S.; Sidarta-Oliveira, D.; Victorio, S.C.; Donato, J., Jr.; Kim, Y.B.; Velloso, L.A. The partial inhibition of hypothalamic IRX3 exacerbates obesity. EBioMedicine 2019, 39, 448–460. [Google Scholar] [CrossRef] [Green Version]
- Mayer, C.M.; Belsham, D.D. Insulin directly regulates NPY and AgRP gene expression via the MAPK MEK/ERK signal transduction pathway in mHypoE-46 hypothalamic neurons. Mol. Cell Endocrinol. 2009, 307, 99–108. [Google Scholar] [CrossRef]
- Fujita, S.; Honda, K.; Yamaguchi, M.; Fukuzo, S.; Saneyasu, T.; Kamisoyama, H. Role of Insulin-like Growth Factor-1 in the Central Regulation of Feeding Behavior in Chicks. J. Poult. Sci. 2019, 56, 270–276. [Google Scholar] [CrossRef] [Green Version]
- Roepke, T.A.; Yasrebi, A.; Villalobos, A.; Krumm, E.A.; Yang, J.A.; Mamounis, K.J. Loss of ERα partially reverses the effects of maternal high-fat diet on energy homeostasis in female mice. Sci. Rep. 2017, 7, 6381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sclafani, A. Carbohydrate-induced hyperphagia and obesity in the rat: Effects of saccharide type, form, and taste. Neurosci. Biobehav. Rev. 1987, 11, 155–162. [Google Scholar] [CrossRef]
- Sclafani, A.; Xenakis, S. Influence of diet form on the hyperphagia-promoting effect of polysaccharide in rats. Life Sci. 1984, 34, 1253–1259. [Google Scholar] [CrossRef]
- Fuente-Martín, E.; García-Cáceres, C.; Granado, M.; Sánchez-Garrido, M.A.; Tena-Sempere, M.; Frago, L.M.; Argente, J.; Chowen, J.A. Early postnatal overnutrition increases adipose tissue accrual in response to a sucrose-enriched diet. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1586–E1598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuente-Martín, E.; Granado, M.; García-Cáceres, C.; Sanchez-Garrido, M.A.; Frago, L.M.; Tena-Sempere, M.; Argente, J.; Chowen, J.A. Early nutritional changes induce sexually dimorphic long-term effects on body weight gain and the response to sucrose intake in adult rats. Metabolism 2012, 61, 812–822. [Google Scholar] [CrossRef]
- Sandhu, M.S.; Gibson, J.M.; Heald, A.H.; Dunger, D.B.; Wareham, N.J. Low circulating IGF-II concentrations predict weight gain and obesity in humans. Diabetes 2003, 52, 1403–1408. [Google Scholar] [CrossRef] [Green Version]







| Chow Males | CCD Males | HFD Males | Chow Females | CCD Females | HFD Females | Significance | |
|---|---|---|---|---|---|---|---|
| Final body weight (g) | 24.6 ± 0.5 | 26.4 ± 0.3 | 35.2 ± 1.0 # | 19.6 ± 0.3 @ | 20.3 ± 0.3 @ | 24.2 ± 1.5 #,@ | a, p < 0.001 b, p < 0.001 c, p < 0.01 |
| Visceral adipose tissue (%) | 1.16 ± 0.07 | 1.22 ± 0.14 | 4.31 ± 0.28 # | 0.62 ± 0.07 @ | 0.99 ± 0.12 | 2.19 ± 0.46 #,@ | a, p < 0.001 b, p < 0.001 c, p < 0.001 |
| Subcutaneous adipose tissue (%) | 0.52 ± 0.04 | 0.57 ± 0.05 | 1.86 ± 0.16 # | 0.67 ± 0.04 @ | 0.96 ± 0.09 @ | 1.52 ± 0.21 #,@ | b, p < 0.001 c, p < 0.001 |
| Glycemia (mg/dl) | 67.8 ± 2.0 | 71.8 ± 3.7 | 80.6 ± 3.2 | 63.6 ± 3.9 | 68.7 ± 4.3 | 79.6 ± 4.9 | b, p < 0.01 |
| Kcal/mouse/ day | 11.7 ± 0.1 | 10.9 ± 0.8 | 13.8 ± 0.5 | 10.0 ± 0.2 @ | 9.9 ± 0.2 | 24.9 ± 0.4 #,@ | a, p < 0.001 b, p < 0.001 c, p < 0.001 |
| Kcal/mouse/ day/100 g | 46.2 ± 0.7 | 42.3 ± 2.0 | 45.8 ± 0.7 | 48.7 ± 1.2 | 47.6 ± 0.9 | 114.5 ± 3.3 #,@ | a, p < 0.001 b, p < 0.001 c, p < 0.001 |
| Energy efficiency (%) | 0.68 ± 0.02 | 0.96 ± 0.11 | 1.84 ± 0.06 # | 0.56 ± 0.02 @ | 0.50 ± 0.05 @ | 0.45 ± 0.07 @ | a, p < 0.001 b, p < 0.001 c, p < 0.001 |
| Leptin (ng/mL) | 0.77 ± 0.28 | 0.65 ± 0.21 | 10.05 ± 2.10 # | 1.06 ± 0.34 | 1.22 ± 0.49 | 4.52 ± 1.43 # | b, p < 0.01 c, p < 0.05 |
| Plasma IGF2 | Plasma IGFBP2 | VAT IGF2 mRNA | VAT IGFBP2 mRNA | HPT IGF2 mRNA | HPT IGFBP2 mRNA | |
|---|---|---|---|---|---|---|
| Body weight (males) | 0.661 ** | −0.470 * | −0.523 * | −0.306 | 0.088 | 0.090 |
| Body weight (females) | 0.286 | −0.225 | −0.107 | −0.132 | 0.579 ** | 0.598 ** |
| Glycemia (males) | 0.159 | −0.102 | −0.218 | 0.075 | −0.092 | 0.138 |
| Glycemia (females) | 0.268 | −0.016 | −0.157 | −0.293 | 0.570 ** | 0.623 ** |
| Chow Males | CCD Males | HFD Males | Chow Females | CCD Females | HFD Females | Significance | |
|---|---|---|---|---|---|---|---|
| GFAP | 100.0 ± 2.1 | 115.6 ± 6.8 | 107.4 ± 9.5 | 103.9 ± 7.6 | 97.9 ± 3.0 | 103.3 ± 4.5 | ns |
| Iba1 | 100.0 ± 3.2 | 117.1 ± 3.6 | 110.4 ± 12.5 | 114.8 ± 7.4 | 101.5 ± 4.5 | 97.7 ± 6.9 | ns |
| pJNK | 100.0 ± 10.8 | 84.0 ± 11.5 | 71.6 ± 5.2 | 107.5 ± 18.3 | 86.7 ± 13.9 | 95.3 ± 14.1 | ns |
| Antibody | Class | Dilution | Host | Commercial Source | Reference |
|---|---|---|---|---|---|
| pJNK | Polyclonal | 1:3000 | Rabbit | Promega | V7932 |
| GAPDH | Polyclonal | 1:10,000 | Rabbit | Sigma-Aldrich | G9545 |
| GFAP | Polyclonal | 1:5000 | Guinea pig | Synaptic Systems | 173004 |
| Iba1 | Polyclonal | 1:1000 | Rabbit | Synaptic Systems | 234003 |
| α-guinea pig HRP-conjugated | Polyclonal | 1:2000 | Goat | AbD Serotec | AHP861P |
| α-rabbit HRP-conjugated | Polyclonal | 1:20,000 | Goat | Invitrogen | 31460 |
| Name | Gene | Reference | Commercial Source |
|---|---|---|---|
| Agouti-related protein | Agrp | Mm00475829_g1 | Applied Biosystems |
| Cyclophilin A (Peptidylprolyl isomerase A) | Ppia | Mm02342430_g1 | Applied Biosystems |
| Cocaine and amphetamine regulated transcript prepropeptide | Cartpt | Mm04210469_m1 | Applied Biosystems |
| Insulin-like growth factor 1 | Igf1 | Mm00439560_m1 | Applied Biosystems |
| Insulin-like growth factor 1 receptor | Igf1r | Mm00802831_m1 | Applied Biosystems |
| Insulin-like growth factor 2 | Igf2 | Mm00439564_m1 | Applied Biosystems |
| Insulin-like growth factor 2 receptor | Igf2r | Mm00439576_m1 | Applied Biosystems |
| Insulin-like growth factor-binding protein 2 | Igfbp2 | Mm00492632_m1 | Applied Biosystems |
| Insulin-like growth factor-binding protein 3 | Igfbp3 | Mm01187817_m1 | Applied Biosystems |
| Insulin-like growth factor-binding protein 4 | Igfbp4 | Mm00494922_m1 | Applied Biosystems |
| Insulin-like growth factor-binding protein 5 | Igfbp5 | Mm00516037_m1 | Applied Biosystems |
| Neuropeptide Y | Npy | Mm03048253_m1 | Applied Biosystems |
| Pregnancy-associated plasma protein A | Pappa | Mm01259244_m1 | Applied Biosystems |
| Pregnancy-associated plasma protein A-2 | Pappa2 | Mm01284029_m1 | Applied Biosystems |
| Pro-opiomelanocortin | Pomc | Mm00435874_m1 | Applied Biosystems |
| Stanniocalcin-1 | Stc1 | Mm01322191_m1 | Applied Biosystems |
| Stanniocalcin-2 | Stc2 | Mm00441560_m1 | Applied Biosystems |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerra-Cantera, S.; Frago, L.M.; Jiménez-Hernaiz, M.; Ros, P.; Freire-Regatillo, A.; Barrios, V.; Argente, J.; Chowen, J.A. Impact of Long-Term HFD Intake on the Peripheral and Central IGF System in Male and Female Mice. Metabolites 2020, 10, 462. https://doi.org/10.3390/metabo10110462
Guerra-Cantera S, Frago LM, Jiménez-Hernaiz M, Ros P, Freire-Regatillo A, Barrios V, Argente J, Chowen JA. Impact of Long-Term HFD Intake on the Peripheral and Central IGF System in Male and Female Mice. Metabolites. 2020; 10(11):462. https://doi.org/10.3390/metabo10110462
Chicago/Turabian StyleGuerra-Cantera, Santiago, Laura M. Frago, María Jiménez-Hernaiz, Purificación Ros, Alejandra Freire-Regatillo, Vicente Barrios, Jesús Argente, and Julie A. Chowen. 2020. "Impact of Long-Term HFD Intake on the Peripheral and Central IGF System in Male and Female Mice" Metabolites 10, no. 11: 462. https://doi.org/10.3390/metabo10110462
APA StyleGuerra-Cantera, S., Frago, L. M., Jiménez-Hernaiz, M., Ros, P., Freire-Regatillo, A., Barrios, V., Argente, J., & Chowen, J. A. (2020). Impact of Long-Term HFD Intake on the Peripheral and Central IGF System in Male and Female Mice. Metabolites, 10(11), 462. https://doi.org/10.3390/metabo10110462