Prediction of Autoimmune Diseases by Targeted Metabolomic Assay of Urinary Organic Acids
Abstract
:1. Introduction
2. Results
2.1. Distinct Levels of Organic Acids in ADs
2.2. Pathways Analysis
2.3. Development of Predictive Models
3. Discussion
4. Methods
4.1. Study Design
- RA: ACR/EULAR 2010 Rheumatoid Arthritis Classification Criteria [38]
- IBD: the Lennard-Jones diagnostic criteria for Ulcerative colitis and Crohn’s disease [39]
- PSO: The presence of chronic psoriasis plaque and the (Psoriasis Area and Severity Index) PASI score was used to assess the severity of the disease.
- THY: Diagnosis and assessment of disease severity were performed by evaluating the levels of the thyroid gland hormones T3 T4 and TSH, and images of the thyroid gland ultrasound.
- MS: Revised McDonald 2010 diagnostic criteria [40]
4.2. Chemicals
4.3. Sample Preparation
4.4. Statistical Analysis
4.5. Matching Analysis
4.6. Enrichment and Pathway Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Data Availability Statement
References
- Wang, L.; Wang, F.-S.; Gershwin, M.E. Human autoimmune diseases: A comprehensive update. J. Internet Med. 2015, 278, 369–395. [Google Scholar] [CrossRef] [PubMed]
- American Autoimmune Related Disease Association Autoimmune Diseases Statistics. Available online: https://www.aarda.org/news-information/statistics/#1488234345468-3bf2d325-1052 (accessed on 29 November 2020).
- ENVI European Parliament Autoimmune Diseases-Modern Diseases; Directorate for Internal Policies (Workshop): Brussels, Belgium, 2017; Available online: https://www.europarl.europa.eu/committees/en/autoimmune-diseases-modern-diseases/product-details/20170822WKS00741 (accessed on 29 November 2020).
- Smith, E.; Hoy, D.G.; Cross, M.; Vos, T.; Naghavi, M.; Buchbinder, R.; Woolf, A.D.; March, L. The global burden of other musculoskeletal disorders: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.A.; Kawabata, H.; Ray, N.; Baheti, A.; Suissa, S.; Esdaile, J.M. Prevalence of Co-existing Autoimmune Disease in Rheumatoid Arthritis: A Cross-Sectional Study. Adv. Ther. 2017, 34, 2481–2490. [Google Scholar] [CrossRef] [Green Version]
- Boelaert, K.; Newby, P.R.; Simmonds, M.J.; Holder, R.L.; Carr-Smith, J.D.; Heward, J.M.; Manji, N.; Allahabadia, A.; Armitage, M.; Chatterjee, K.V.; et al. Prevalence and Relative Risk of Other Autoimmune Diseases in Subjects with Autoimmune Thyroid Disease. Am. J. Med. 2010, 123, 183.e1–183.e9. [Google Scholar] [CrossRef]
- Ziade, N.; El Khoury, B.; Zoghbi, M.; Merheb, G.; Abi Karam, G.; Mroue’, K.; Messaykeh, J. Prevalence and pattern of comorbidities in chronic rheumatic and musculoskeletal diseases: The COMORD study. Sci. Rep. 2020, 10, 7683. [Google Scholar] [CrossRef] [PubMed]
- Elkon, K.; Casali, P. Nature and functions of autoantibodies. Nat. Clin. Pr. Rheumatol. 2008, 4, 491–498. [Google Scholar] [CrossRef] [Green Version]
- Docea, A.O.; Gofita, E.; Calina, D.; Ioan, Z.S.; Valcea, D.I.; Mitrut, P. Autoimmune disorders due to double antiviral therapy with peginterferon and ribavirin in patients with hepatitis C virus infection. Farmacia 2016, 64, 605–611. [Google Scholar]
- Hollifield, R.D.; Harbige, L.S.; Pham-Dinh, D.; Sharief, M.K. Evidence for Cytokine Dysregulation in Multiple Sclerosis: Peripheral Blood Mononuclear Cell Production of Pro-inflammatory and Anti-inflammatory Cytokines During Relapse and Remission. Autoimmunity 2003, 36, 133–141. [Google Scholar] [CrossRef]
- Mititelu, R.R.; Pădureanu, R.; Băcănoiu, M.; Pădureanu, V.; Docea, A.O.; Calina, D.; Barbulescu, A.L.; Buga, A.M. Inflammatory and Oxidative Stress Markers—Mirror Tools in Rheumatoid Arthritis. Biomedicines 2020, 8, 125. [Google Scholar] [CrossRef]
- Tsoukalas, D.; Sarandi, E.; Thanasoula, M.; Docea, A.O.; Tsilimidos, G.; Calina, D.; Tsatsakis, A. Metabolic Fingerprint of Chronic Obstructive Lung Diseases: A New Diagnostic Perspective. Metabolites 2019, 9, 290. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, D.K.; Hollywood, K.A.; Goodacre, R. Metabolomics for the masses: The future of metabolomics in a personalized world. New Horiz. Transl. Med. 2017, 3, 294–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef] [Green Version]
- Tsoukalas, D.; Alegakis, A.; Fragkiadaki, P.; Papakonstantinou, E.; Nikitovic, D.; Karataraki, A.; Nosyrev, A.E.; Papadakis, E.G.; Spandidos, D.A.; Drakoulis, N.; et al. Application of metabolomics: Focus on the quantification of organic acids in healthy adults. Int. J. Mol. Med. 2017, 40, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Sarandi, E.; Thanasoula, M.; Anamaterou, C.; Papakonstantinou, E.; Geraci, F.; Papamichael, M.M.; Itsiopoulos, C.; Tsoukalas, D. Metabolic Profiling of Organic and Fatty Acids in Chronic and Autoimmune Diseases, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Lubin, J.H.; Colt, J.S.; Camann, D.; Davis, S.; Cerhan, J.R.; Severson, R.K.; Bernstein, L.; Hartge, P. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ. Health Perspect. 2004, 112, 1691–1696. [Google Scholar] [CrossRef] [PubMed]
- Tsoukalas, D.; Fragoulakis, V.; Sarandi, E.; Docea, A.O.; Papakonstantinou, E.; Tsilimidos, G.; Anamaterou, C.; Fragkiadaki, P.; Aschner, M.; Tsatsakis, A.; et al. Targeted Metabolomic Analysis of Serum Fatty Acids for the Prediction of Autoimmune Diseases. Front. Mol. Biosci. 2019, 6, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lord, R.S. Long-term patterns of urinary pyroglutamic acid in healthy humans. Physiol. Rep. 2016, 4, e12706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, J.; Liu, R.; Jiaying, Y.; Liu, X.; Zhao, X.; Li, Y.; Liu, L.; Sun, C. Fasting serum α-hydroxybutyrate and pyroglutamic acid as important metabolites for detecting isolated post-challenge diabetes based on organic acid profiles. J. Chromatogr. B 2018, 1100–1101, 6–16. [Google Scholar] [CrossRef]
- Yoshinari, O.; Igarashi, K. Anti-diabetic effect of pyroglutamic acid in type 2 diabetic Goto-Kakizaki rats and KK-A y mice. Br. J. Nutr. 2011, 106, 995–1004. [Google Scholar] [CrossRef] [Green Version]
- Hannibal, L.; Lysne, V.; Behringer, S. Biomarkers and Algorithms for the Diagnosis of Vitamin B 12 Deficiency. Front. Mol. Biosci. 2016, 3, 27. [Google Scholar] [CrossRef] [Green Version]
- Choi, C.; Ganji, S.K.; DeBerardinis, R.J.; Hatanpaa, K.J.; Rakheja, D.; Kovacs, Z.; Yang, X.-L.; Mashimo, T.; Raisanen, J.M.; Marin-Valencia, I.; et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat. Med. 2012, 18, 624–629. [Google Scholar] [CrossRef] [Green Version]
- Seijo-Martínez, M.; Navarro, C.; Castro del Río, M.; Vila, O.; Puig, M.; Ribes, A.; Butron, M. L-2-Hydroxyglutaric Aciduria: Clinical, Neuroimaging, and Neuropathological Findings. Arch. Neurol. 2005, 62, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, H.; Deng, P.; Liu, C.; Li, D.; Jie, H.; Zhang, H.; Zhou, Z.; Zhao, Y.-L. Tissue metabolic profiling of human gastric cancer assessed by 1H NMR. BMC Cancer 2016, 16, 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mutsaers, H.A.M.; Engelke, U.F.H.; Wilmer, M.J.G.; Wetzels, J.F.M.; Wevers, R.A.; van den Heuvel, L.P.; Hoenderop, J.G.; Masereeuw, R. Optimized Metabolomic Approach to Identify Uremic Solutes in Plasma of Stage 3–4 Chronic Kidney Disease Patients. PLoS ONE 2013, 8, e71199. [Google Scholar] [CrossRef] [Green Version]
- Irwin, C.; Mienie, L.J.; Wevers, R.A.; Mason, S.; Westerhuis, J.A.; Van Reenen, M.; Reinecke, C.J. GC-MS-based urinary organic acid profiling reveals multiple dysregulated metabolic pathways following experimental acute alcohol consumption. Sci. Rep. 2018, 8, 5775. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.B.; Petersen, K.F.; Shulman, G.I. Mechanisms of insulin resistance in humans and possible links with inflammation. Hypertension 2005, 45, 828–833. [Google Scholar] [CrossRef]
- Herebian, D.; Seibt, A.; Smits, S.H.J.; Rodenburg, R.J.; Mayatepek, E.; Distelmaier, F. 4-Hydroxybenzoic acid restores CoQ10 biosynthesis in human COQ2 deficiency. Ann. Clin. Transl. Neurol. 2017, 4, 902–908. [Google Scholar] [CrossRef]
- Lindblad, B.; Lindstedt, G.; Lindstedt, S. Mechanism of enzymic formation of homogentisate from p-hydroxyphenylpyruvate. J. Am. Chem. Soc. 1970, 92, 7446–7449. [Google Scholar] [CrossRef]
- Yousri, N.A.; Mook-Kanamori, D.O.; Selim, M.M.E.D.; Takiddin, A.H.; Al-Homsi, H.; Al-Mahmoud, K.A.S.; Karoly, E.D.; Krumsiek, J.; Do, K.T.; Neumaier, U.; et al. A systems view of type 2 diabetes-associated metabolic perturbations in saliva, blood and urine at different timescales of glycaemic control. Diabetologia 2015, 58, 1855–1867. [Google Scholar] [CrossRef] [Green Version]
- Blackmore, D.; Li, L.; Wang, N.; Maksymowych, W.; Yacyshyn, E.; Siddiqi, Z.A. Metabolomic profile overlap in prototypical autoimmune humoral disease: A comparison of myasthenia gravis and rheumatoid arthritis. Metabolomics 2020, 16, 10. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rodrigues, C.F.; Sharopov, F.; Docea, A.O.; Karaca, A.C.; Sharifi-Rad, M.; Karincaoglu, D.K.; Gülseren, G.; Şenol, E.; Demircan, E.; et al. Diet, lifestyle and cardiovascular diseases: Linking pathophysiology to cardioprotective effects of natural bioactive compounds. Int. J. Environ. Res. Public Health 2020, 17, 2326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnald, A.; Julià, A.; Vinaixa, M.; Domènech, E.; Fernández-Nebro, A.; Cañete, J.D.; Ferrándiz, C.; Tornero, J.; Gisbert, J.P.; Nos, P.; et al. Urine metabolome profiling of immune-mediated inflammatory diseases. BMC Med. 2016, 14, 133. [Google Scholar]
- Nyamundanda, G.; Gormley, I.C.; Fan, Y.; Gallagher, W.M.; Brennan, L. MetSizeR: Selecting the optimal sample size for metabolomic studies using an analysis based approach. BMC Bioinform. 2013, 14, 338. [Google Scholar] [CrossRef] [Green Version]
- Schulz, K.F.; Grimes, D.A. Case-control studies: Research in reverse. Lancet 2002, 359, 431–434. [Google Scholar] [CrossRef]
- Kay, J.; Upchurch, K.S. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology (UK) 2012, 51, 5–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherlock, M.E.; Benchimol, E.I. Classification of inflammatory bowel disease in children. In Pediatric Inflammatory Bowel Disease, 3rd ed.; Mamula, P., Grossman, A.B., Eds.; Springer: Cham, Switzerland, 2017; pp. 181–191. [Google Scholar]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [Green Version]
- IBM SPSS Software. Available online: https://www.ibm.com/analytics/spss-statistics-software (accessed on 29 November 2020).
- Foundation, T.R. The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 29 November 2020).
- Hart, A. Mann-Whitney test is not just a test of medians: Differences in spread can be important. BMJ 2001, 323, 391–393. [Google Scholar] [CrossRef] [Green Version]
- Vinaixa, M.; Samino, S.; Saez, I.; Duran, J.; Guinovart, J.J.; Yanes, O. A guideline to univariate statistical analysis for LC/MS-based untargeted metabolomics-derived data. Metabolites 2012, 2, 775–795. [Google Scholar] [CrossRef]
- Heinze, G.; Wallisch, C.; Dunkler, D. Variable selection—A review and recommendations for the practicing statistician. Biom. J. 2018, 60, 431–449. [Google Scholar] [CrossRef] [Green Version]
- Sordo, M. Introduction to neural networks in healthcare. Open Clin. 2002. Available online: https://www.academia.edu/20719514/Introduction_to_neural_networks_in_healthcare (accessed on 29 November 2020).
- Yu, K.-H.; Beam, A.L.; Kohane, I.S. Artificial intelligence in healthcare. Nat. Biomed. Eng. 2018, 2, 719–731. [Google Scholar] [CrossRef]
- Hajian-Tilaki, K. Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation. Casp. J. Internet Med. 2013, 4, 627. [Google Scholar]
- Rose, S.; van der Laan, M.J. Why Match? Investigating Matched Case-Control Study Designs with Causal Effect Estimation. Int. J. Biostat. 2009, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faresjö, T.; Faresjö, Å. To match or not to match in epidemiological studies—Same outcome but less power. Int. J. Environ. Res. Public Health 2010, 7, 325–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinf. 2019, 68, e86. [Google Scholar] [CrossRef]


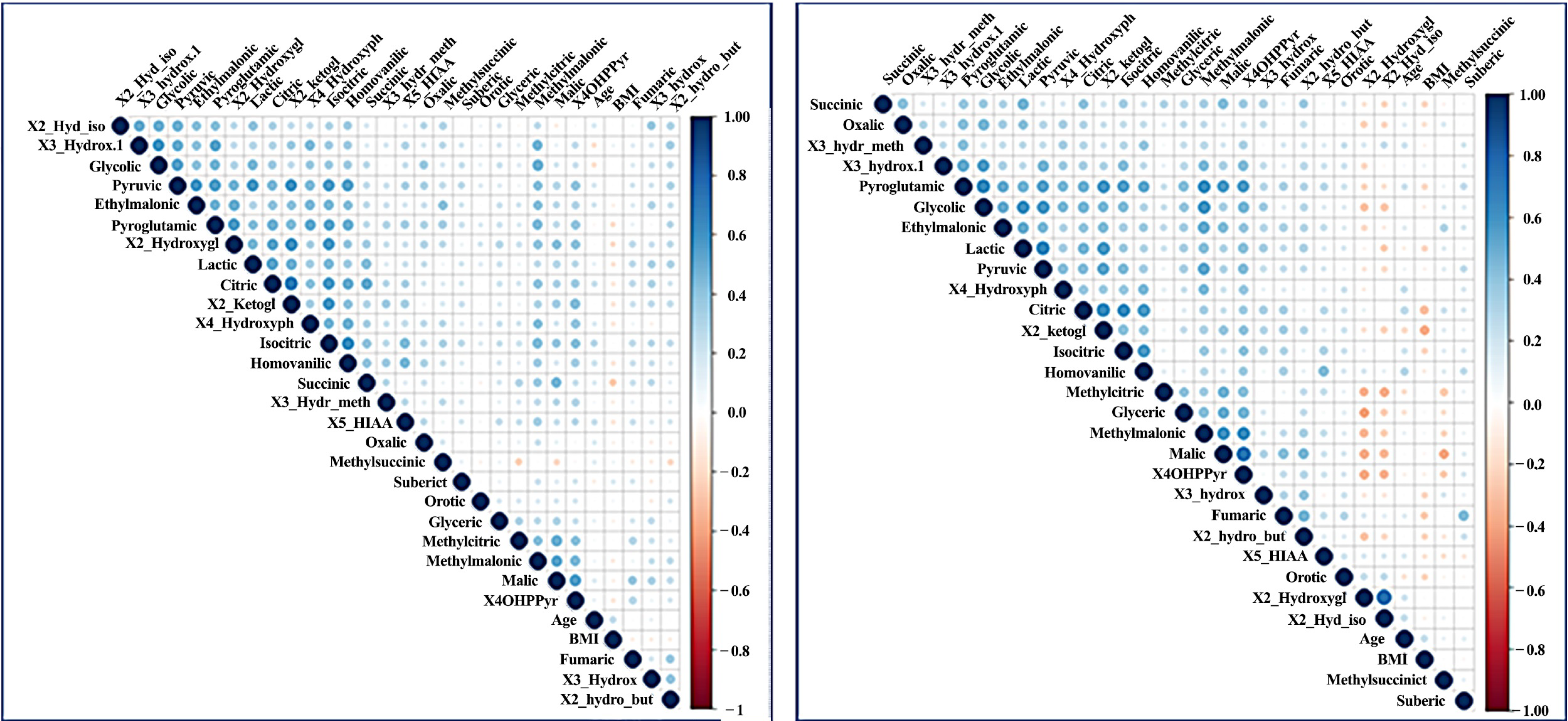



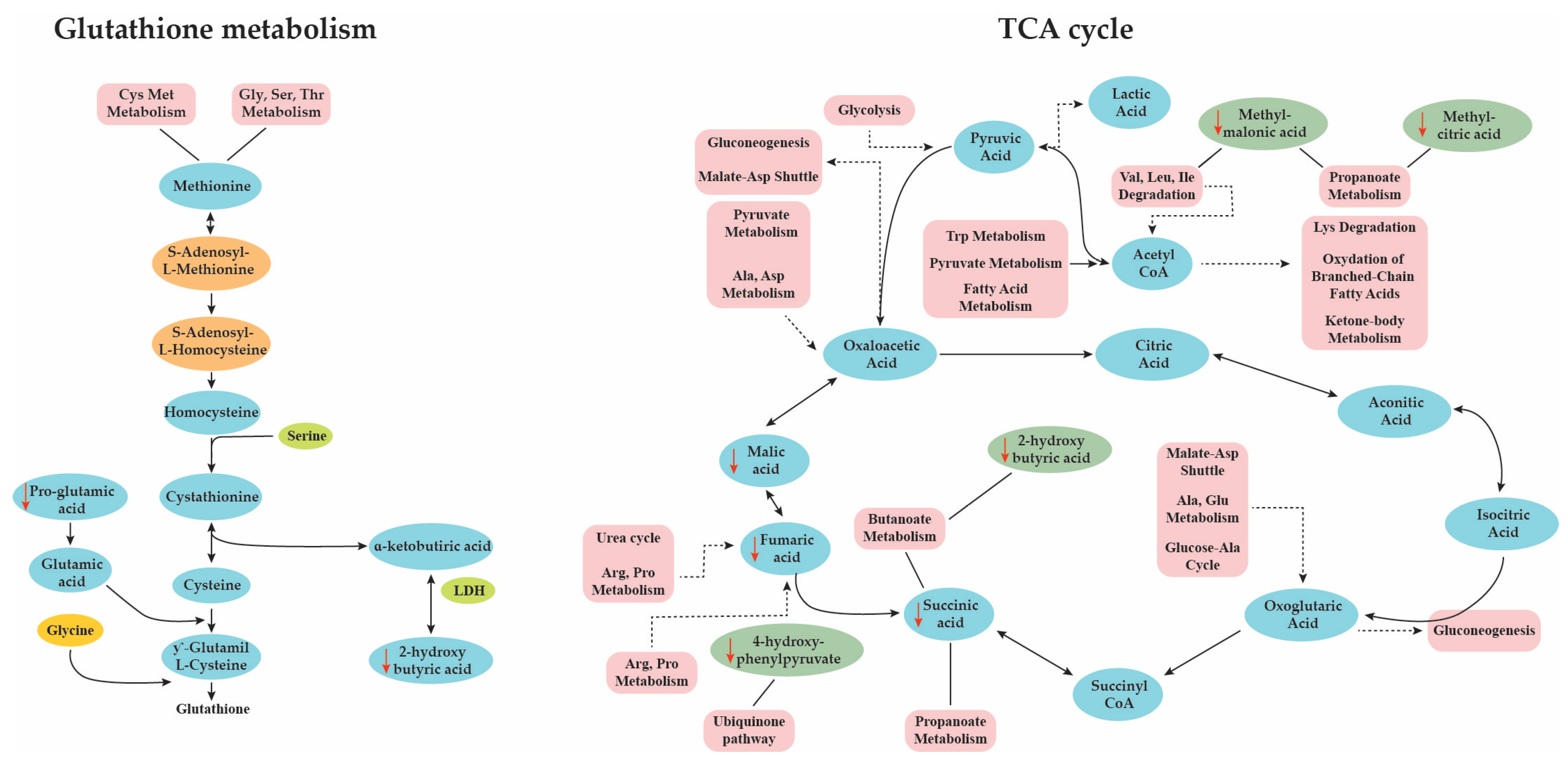
| AD | Control | ||||
|---|---|---|---|---|---|
| Mean ± SD | Median | Mean ± SD | Median | p-Value | |
| Citric acid | 88.45 ± 66.17 | 72.70 | 96.2 ± 75.7 | 75.70 | >0.90 |
| Isocitric acid | 5.04 ± 4.99 | 4.00 | 5.21 ± 3.76 | 4.30 | >0.90 |
| 2-ketoglutaric acid | 11.99 ± 11.54 | 8.90 | 15.86 ± 16.57 | 11.20 | 0.145 |
| Succinic acid | 3.07 ± 7.27 | 1.40 | 4.91 ± 13.82 | 2.00 | <0.001 |
| Fumaric acid | 0.04 ± 0.27 | 0.00 | 0.07 ± 0.31 | 0.00 | >0.90 |
| Malic acid | 0.40 ± 0.86 | 0.00 | 0.66 ± 0.63 | 1.00 | <0.001 |
| 3-hydroxy3-methylglutaric acid | 2.17 ± 1.75 | 1.70 | 2.19 ± 2.13 | 1.80 | >0.90 |
| Lactic acid | 7.88 ± 9.63 | 5.60 | 16.81 ± 75.43 | 7.00 | 0.232 |
| Pyruvic acid | 7.76 ± 6.04 | 6.60 | 8.61 ± 6.4 | 6.80 | >0.90 |
| 3-hydroxybutyric acid | 9.14 ± 54.47 | 0.00 | 5.44 ± 16.3 | 1.00 | 0.638 |
| Pyroglutamic acid | 19.04 ± 16.90 | 16.70 | 23.97 ± 16.29 | 21.10 | <0.001 |
| 3-hydroxyisovaleric acid | 10.25 ± 10.52 | 7.80 | 13.98 ± 15.29 | 9.90 | 0.087 |
| Methylmalonic acid | 0.63 ± 0.97 | 0.00 | 0.95 ± 0.87 | 1.00 | <0.001 |
| Homovanillic acid | 2.12 ± 1.63 | 1.70 | 2.57 ± 2.4 | 2.10 | 0.29 |
| 5-HIAA | 2.69 ± 3.01 | 2.10 | 3.51 ± 5.52 | 2.50 | 0.725 |
| 4 Hydroxyphenylacetic acid | 11.40 ± 13.41 | 7.50 | 10.96 ± 8.86 | 8.00 | >0.90 |
| Orotic acid | 0.01 ± 0.16 | 0.00 | 0.01 ± 0.11 | 0.00 | >0.90 |
| 2-Hydroxyglutaric acid | 2.53 ± 1.71 | 2.20 | 1.95 ± 4.23 | 1.30 | <0.001 |
| Glycolic acid | 22.68 ± 17.91 | 18.70 | 26.86 ± 23.11 | 22.30 | >0.90 |
| Oxalic acid | 4.66 ± 3.55 | 4.00 | 5.95 ± 4.54 | 5.00 | >0.90 |
| Glyceric acid | 2.04 ± 7.56 | 0.00 | 1.52 ± 4.08 | 1.30 | >0.90 |
| 2-hydroxy isobutyric acid | 4.75 ± 2.81 | 4.20 | 2.79 ± 3.94 | 0.00 | <0.001 |
| 2-hydroxy butyric acid | 0.16 ± 0.77 | 0.00 | 0.39 ± 0.96 | 0.00 | <0.001 |
| Ethylmalonic acid | 1.64 ± 2.26 | 1.20 | 1.9 ± 1.9 | 1.40 | 0.812 |
| Methylsuccinic acid | 0.34 ± 0.86 | 0.00 | 0.17 ± 0.54 | 0.00 | >0.90 |
| Suberic acid | 0.08 ± 0.55 | 0.00 | 0.1 ± 0.39 | 0.00 | >0.90 |
| Methylcitric acid | 0.11 ± 0.31 | 0.00 | 0.27 ± 0.45 | 0.00 | <0.001 |
| 4HPPA | 0.55 ± 0.88 | 0.00 | 0.79 ± 0.76 | 1.00 | <0.001 |
| B | St Error | Exp (B) | 95% LCI | 95% UCI | p-Value | |
|---|---|---|---|---|---|---|
| Succinic acid | 0.018 | 0.012 | 1.018 | 0.994 | 1.044 | 0.147 |
| Malic acid | 0.059 | 0.181 | 1.060 | 0.744 | 1.512 | 0.747 |
| Pyroglutamic acid | 0.015 | 0.010 | 1.015 | 0.995 | 1.036 | 0.151 |
| Methylmalonic acid | 0.005 | 0.180 | 1.005 | 0.706 | 1.431 | 0.976 |
| 2-Hydroxy-glutaric acid | −0.069 | 0.048 | 0.933 | 0.850 | 1.024 | 0.145 |
| 2-hydroxy isobutyric acid | −0.180 | 0.044 | 0.835 | 0.766 | 0.910 | 0.000 |
| 2-hydroxy butyric acid | 0.420 | 0.172 | 1.521 | 1.086 | 2.131 | 0.015 |
| Methylcitric acid | 0.389 | 0.332 | 1.476 | 0.769 | 2.831 | 0.241 |
| 4HPPA | 0.188 | 0.175 | 1.207 | 0.857 | 1.700 | 0.281 |
| Female | −0.450 | 0.271 | 0.638 | 0.375 | 1.085 | 0.097 |
| Age | −0.009 | 0.011 | 0.991 | 0.969 | 1.014 | 0.440 |
| No Exercise | −0.985 | 0.262 | 0.373 | 0.223 | 0.624 | 0.000 |
| No Alcohol | 0.830 | 0.264 | 2.294 | 1.367 | 3.849 | 0.002 |
| BMI | −0.009 | 0.027 | 0.991 | 0.939 | 1.045 | 0.735 |
| Constant | 0.352 | 0.837 | 1.422 | 0.674 |
| B | St Error | Exp (B) | 95% LCI | 95% UCI | p-Value | |
|---|---|---|---|---|---|---|
| Factor 1 | 0.076 | 0.129 | 1.079 | 0.838 | 1.388 | 0.557 |
| Factor 2 | 0.137 | 0.124 | 1.146 | 0.899 | 1.461 | 0.270 |
| Factor 3 | 0.454 | 0.203 | 1.575 | 1.059 | 2.344 | 0.025 |
| Factor 4 | −0.611 | 0.126 | 0.543 | 0.424 | 0.695 | 0.000 |
| Factor 5 | 0.297 | 0.129 | 1.346 | 1.045 | 1.735 | 0.022 |
| Factor 6 | 0.061 | 0.112 | 1.063 | 0.853 | 1.324 | 0.586 |
| Factor 7 | −0.031 | 0.125 | 0.969 | 0.759 | 1.239 | 0.804 |
| Factor 8 | −0.185 | 0.166 | 0.831 | 0.600 | 1.151 | 0.265 |
| Factor 9 | 0.047 | 0.116 | 1.049 | 0.836 | 1.316 | 0.682 |
| Factor 10 | −0.300 | 0.168 | 0.741 | 0.533 | 1.030 | 0.074 |
| Male | −0.095 | 0.251 | 0.909 | 0.556 | 1.487 | 0.705 |
| Age | −0.014 | 0.012 | 0.986 | 0.964 | 1.009 | 0.231 |
| BMI | −0.002 | 0.027 | 0.998 | 0.947 | 1.052 | 0.952 |
| Exercise | 0.993 | 0.261 | 2.698 | 1.617 | 4.502 | 0.000 |
| Alcohol | −0.850 | 0.261 | 0.427 | 0.256 | 0.713 | 0.001 |
| Constant | −0.202 | 0.779 | 0.817 | 0.795 |
| Predicted | ||||
|---|---|---|---|---|
| Case | Control | % Correct | ||
| Training | Case | 164 | 7 | 95.9% |
| Control | 49 | 51 | 51.0% | |
| Overall Percent | 78.6% | 21.4% | 79.3% | |
| Testing | Case | 42 | 3 | 93.3% |
| Control | 12 | 15 | 55.6% | |
| Overall Percent | 75.0% | 25.0% | 79.2% | |
| Holdout | Case | 25 | 2 | 92.6% |
| Control | 15 | 9 | 37.5% | |
| Overall Percent | 78.4% | 21.6% | 66.7% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsoukalas, D.; Fragoulakis, V.; Papakonstantinou, E.; Antonaki, M.; Vozikis, A.; Tsatsakis, A.; Buga, A.M.; Mitroi, M.; Calina, D. Prediction of Autoimmune Diseases by Targeted Metabolomic Assay of Urinary Organic Acids. Metabolites 2020, 10, 502. https://doi.org/10.3390/metabo10120502
Tsoukalas D, Fragoulakis V, Papakonstantinou E, Antonaki M, Vozikis A, Tsatsakis A, Buga AM, Mitroi M, Calina D. Prediction of Autoimmune Diseases by Targeted Metabolomic Assay of Urinary Organic Acids. Metabolites. 2020; 10(12):502. https://doi.org/10.3390/metabo10120502
Chicago/Turabian StyleTsoukalas, Dimitris, Vassileios Fragoulakis, Evangelos Papakonstantinou, Maria Antonaki, Athanassios Vozikis, Aristidis Tsatsakis, Ana Maria Buga, Mihaela Mitroi, and Daniela Calina. 2020. "Prediction of Autoimmune Diseases by Targeted Metabolomic Assay of Urinary Organic Acids" Metabolites 10, no. 12: 502. https://doi.org/10.3390/metabo10120502
APA StyleTsoukalas, D., Fragoulakis, V., Papakonstantinou, E., Antonaki, M., Vozikis, A., Tsatsakis, A., Buga, A. M., Mitroi, M., & Calina, D. (2020). Prediction of Autoimmune Diseases by Targeted Metabolomic Assay of Urinary Organic Acids. Metabolites, 10(12), 502. https://doi.org/10.3390/metabo10120502










