Chronic Influence of Inspiratory Muscle Training at Different Intensities on the Serum Metabolome
Abstract
1. Introduction
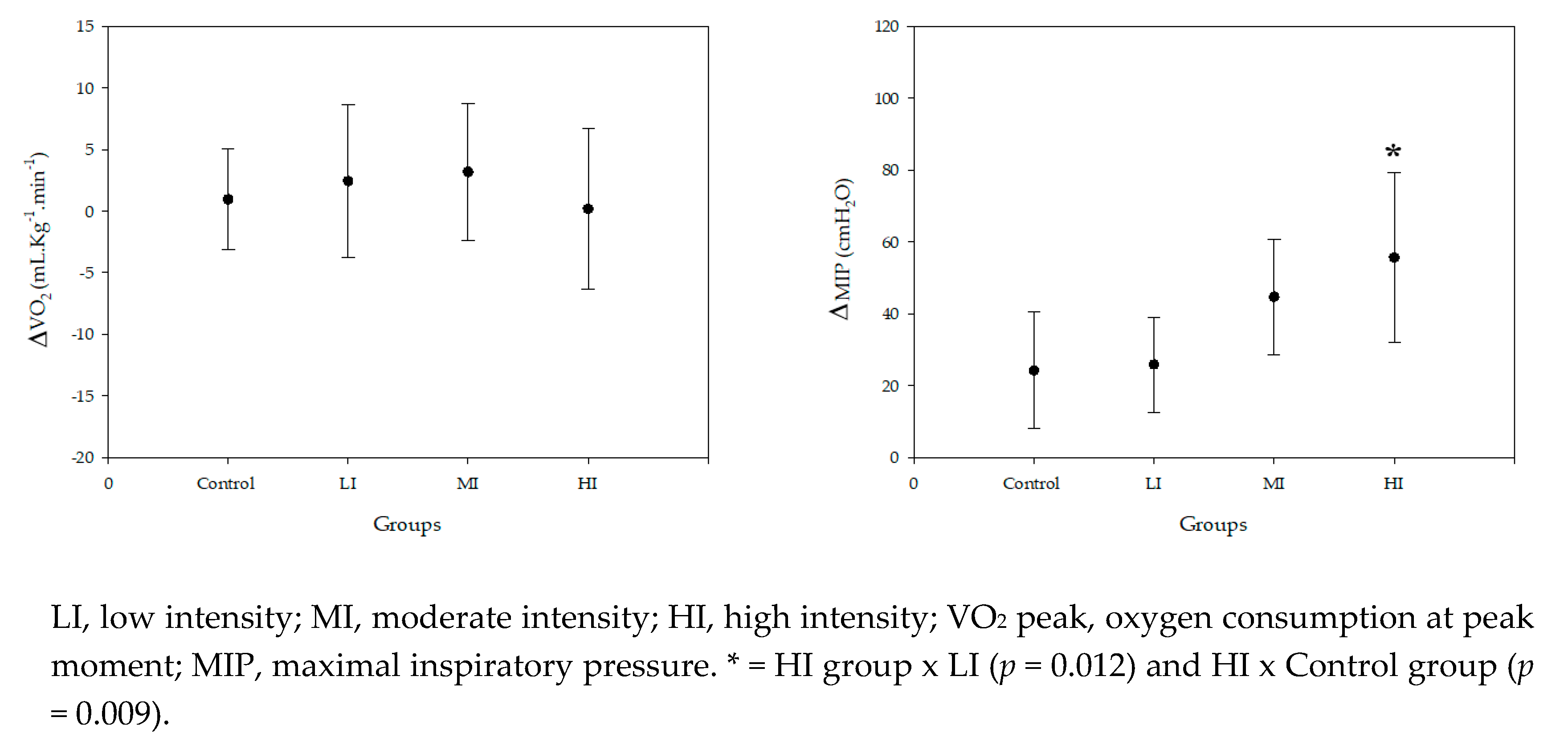
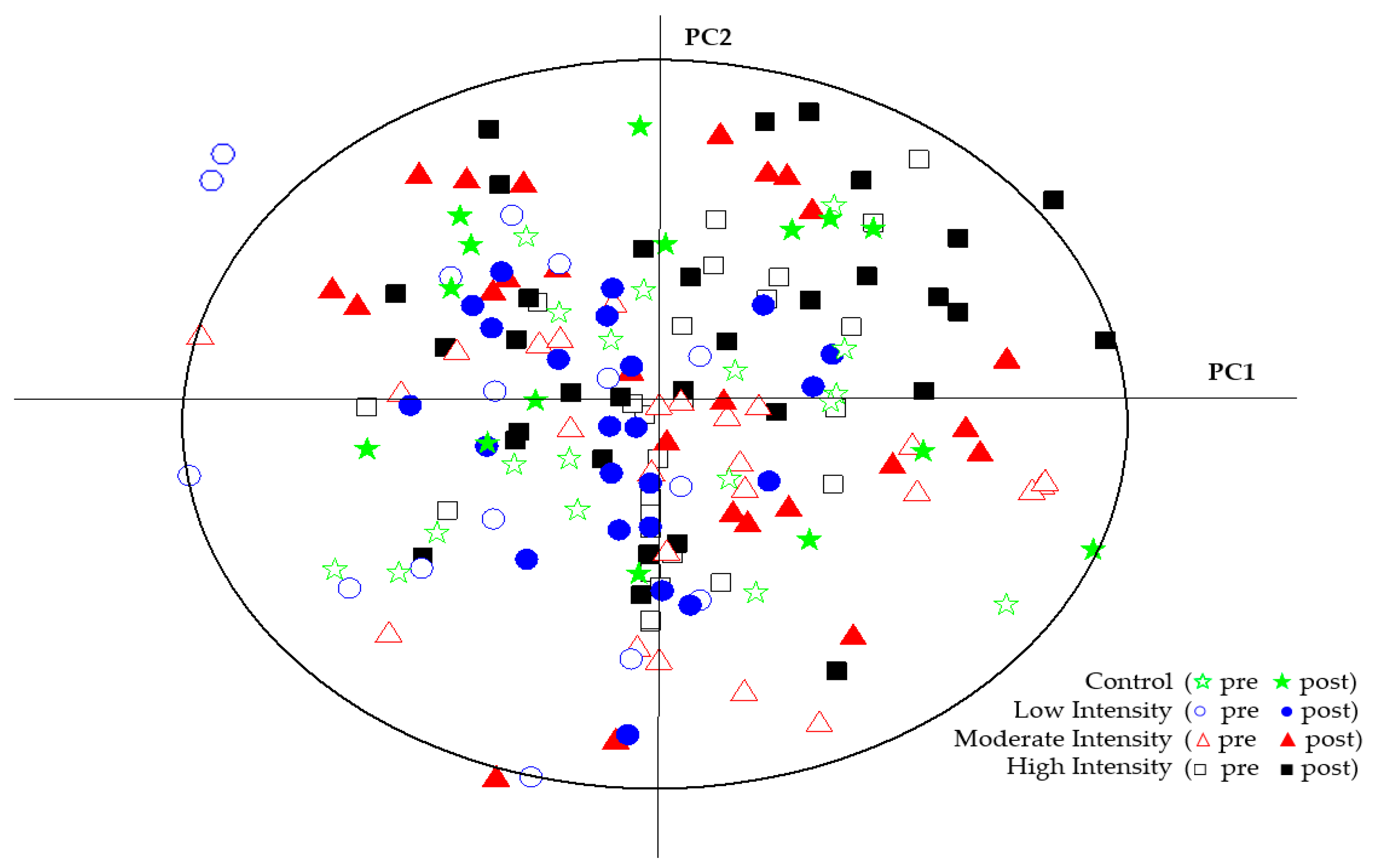
2. Results
3. Discussion
4. Materials and Methods
4.1. Participants
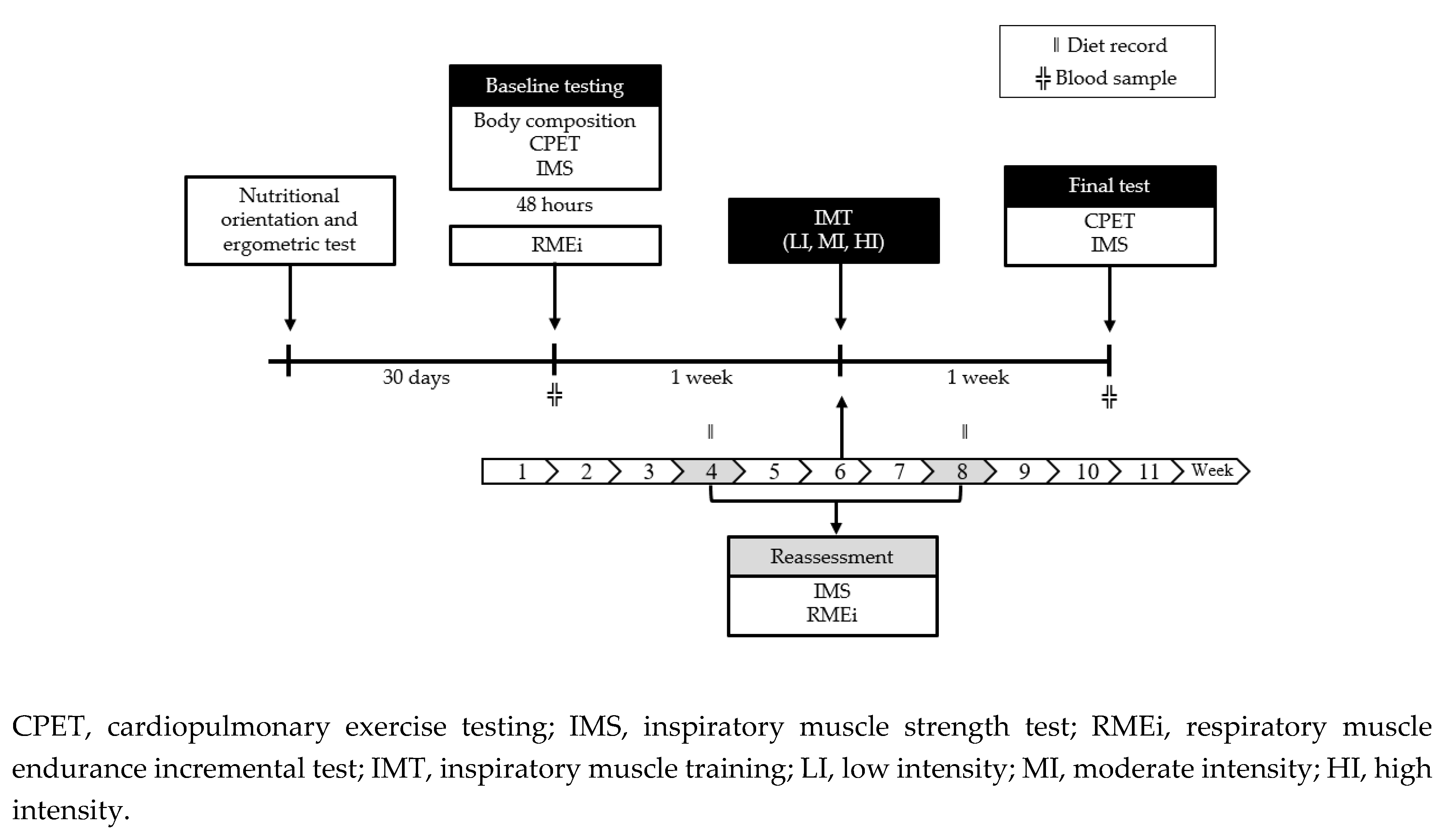
4.2. Research Design
4.3. Experimental Section
4.3.1. Baseline Testing
4.3.2. Inspiratory Muscle Training (IMT)
4.4. Sample Preparation for NMR Analysis
4.5. NMR Data Acquisition and Metabolite Identification
4.6. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Sakaguchi, C.A.; Nieman, D.C.; Signini, E.F.; Abreu, R.M.; Catai, A.M. Metabolomics-Based Studies Assessing Exercise-Induced Alterations of the Human Metabolome: A Systematic Review. Metabolites 2019, 9, 164. [Google Scholar] [CrossRef]
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef]
- San-Millán, I. Blood Biomarkers in Sports Medicine and Performance and the Future of Metabolomics. Methods Mol. Biol. 2019, 1978, 431–446. [Google Scholar]
- HajGhanbari, B.; Yamabayashi, C.; Buna, T.R.; Coelho, J.D.; Freedman, K.D.; Morton, T.A.; Palmer, S.A.; Toy, M.A.; Walsh, C.; Sheel, A.W.; et al. Effects of respiratory muscle training on performance in athletes: a systematic review with meta-analyses. J. Strength Cond. Res. 2013, 27, 1643–1663. [Google Scholar] [CrossRef]
- Illi, S.K.; Held, U.; Frank, I.; Spengler, C.M. Effect of respiratory muscle training on exercise performance in healthy individuals: a systematic review and meta-analysis. Sports Med. 2012, 42, 707–724. [Google Scholar] [CrossRef]
- Sheel, A.W. Respiratory muscle training in healthy individuals: physiological rationale and implications for exercise performance. Sports Med. 2002, 32, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante Silva, R.L.; Hall, E.; Maior, A.S. Inspiratory muscle training improves performance of a repeated sprints ability test in professional soccer players. J. Bodyw. Mov. Ther. 2019, 23, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Spengler, C.M.; Roos, M.; Laube, S.M.; Boutellier, U. Decreased exercise blood lactate concentrations after respiratory endurance training in humans. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 79, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Geary, C.M.; Welch, J.F.; McDonald, M.R.; Peters, C.M.; Leahy, M.G.; Reinhard, P.A.; Sheel, A.W. Diaphragm fatigue and inspiratory muscle metaboreflex in men and women matched for absolute diaphragmatic work during pressure-threshold loading. J. Physiol. 2019, 597, 4797–4808. [Google Scholar] [CrossRef] [PubMed]
- Martins de Abreu, R.; Porta, A.; Rehder-Santos, P.; Cairo, B.; Donisete da Silva, C.; De Favari Signini, É.; Sakaguchi, C.A.; Catai, A.M. Effects of inspiratory muscle-training intensity on cardiovascular control in amateur cyclists. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R891–R902. [Google Scholar] [CrossRef] [PubMed]
- Archiza, B.; Andaku, D.K.; Caruso, F.C.R.; Bonjorno, J.C.; de Oliveira, C.R.; Ricci, P.A.; do Amaral, A.C.; Mattiello, S.M.; Libardi, C.A.; Phillips, S.A.; et al. Effects of inspiratory muscle training in professional women football players: a randomized sham-controlled trial. J. Sports Sci. 2018, 36, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Grapov, D.; Fiehn, O.; Campbell, C.; Chandler, C.J.; Burnett, D.J.; Souza, E.C.; Casazza, G.A.; Keim, N.L.; Newman, J.W.; Hunter, G.R.; et al. Exercise plasma metabolomics and xenometabolomics in obese, sedentary, insulin-resistant women: impact of a fitness and weight loss intervention. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E999–E1014. [Google Scholar] [CrossRef] [PubMed]
- Siopi, A.; Mougios, V. Metabolomics in human acute-exercise trials: study design and preparation. Methods Mol. Biol. 2018, 1738, 279–287. [Google Scholar] [PubMed]
- Neal, C.M.; Hunter, A.M.; Brennan, L.; O’Sullivan, A.; Hamilton, D.L.; De Vito, G.; Galloway, S.D.R. Six weeks of a polarized training-intensity distribution leads to greater physiological and performance adaptations than a threshold model in trained cyclists. J. Appl. Physiol. 2013, 114, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Pechlivanis, A.; Kostidis, S.; Saraslanidis, P.; Petridou, A.; Tsalis, G.; Veselkov, K.; Mikros, E.; Mougios, V.; Theodoridis, G.A. 1H NMR study on the short- and long-term impact of two training programs of sprint running on the metabolic fingerprint of human serum. J. Proteome Res. 2013, 12, 470–480. [Google Scholar] [CrossRef]
- Castro, A.; Duft, R.G.; Ferreira, M.L.V.; de Andrade, A.L.L.; Gáspari, A.F.; de Marchi Silva, L.; de Oliveira-Nunes, S.G.; Cavaglieri, C.R.; Ghosh, S.; Bouchard, C.; et al. Association of skeletal muscle and serum metabolites with maximum power output gains in response to continuous endurance or high-intensity interval training programs: The TIMES study - A randomized controlled trial. PLoS ONE 2019, 14, e0212115. [Google Scholar] [CrossRef]
- Moghetti, P.; Bacchi, E.; Brangani, C.; Donà, S.; Negri, C. Metabolic Effects of Exercise. Front. Horm. Res. 2016, 47, 44–57. [Google Scholar]
- Karsten, M.; Ribeiro, G.S.; Esquivel, M.S.; Matte, D.L. The effects of inspiratory muscle training with linear workload devices on the sports performance and cardiopulmonary function of athletes: A systematic review and meta-analysis. Phys. Ther. Sport 2018, 34, 92–104. [Google Scholar] [CrossRef]
- Brennan, A.M.; Benson, M.; Morningstar, J.; Herzig, M.; Robbins, J.; Gerszten, R.E.; Ross, R. Plasma Metabolite Profiles in Response to Chronic Exercise. Med. Sci. Sports Exerc. 2018, 50, 1480–1486. [Google Scholar] [CrossRef]
- Dellweg, D.; Reissig, K.; Hoehn, E.; Siemon, K.; Haidl, P. Inspiratory muscle training during rehabilitation in successfully weaned hypercapnic patients with COPD. Respir. Med. 2017, 123, 116–123. [Google Scholar] [CrossRef]
- Neves, L.M.T.; Karsten, M.; Neves, V.R.; Beltrame, T.; Borghi-Silva, A.; Catai, A.M. Relationship between inspiratory muscle capacity and peak exercise tolerance in patients post-myocardial infarction. Heart Lung 2012, 41, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Gosselink, R.; De Vos, J.; van den Heuvel, S.P.; Segers, J.; Decramer, M.; Kwakkel, G. Impact of inspiratory muscle training in patients with COPD: what is the evidence? Eur. Respir. J. 2011, 37, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.M.; Toledo-Arruda, A.C.; Lima, J.S.; Duarte, C.S.; Villacorta, H.; Nóbrega, A.C.L. Inspiratory Muscle Training Improves Intercostal and Forearm Muscle Oxygenation in Patients With Chronic Heart Failure: Evidence of the Origin of the Respiratory Metaboreflex. J. Card. Fail. 2017, 23, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Hossein Pour, A.H.; Gholami, M.; Saki, M.; Birjandi, M. The effect of inspiratory muscle training on fatigue and dyspnea in patients with heart failure: A randomized, controlled trial. Jpn. J. Nurs. Sci. 2019, e12290. [Google Scholar] [CrossRef]
- de Abreu, R.M.; Rehder-Santos, P.; Minatel, V.; Dos Santos, G.L.; Catai, A.M. Effects of inspiratory muscle training on cardiovascular autonomic control: A systematic review. Auton. Neurosci. 2017, 208, 29–35. [Google Scholar] [CrossRef]
- Wang, M.-H.; Yeh, M.-L. Respiratory training interventions improve health status of heart failure patients: A systematic review and network meta-analysis of randomized controlled trials. World J. Clin. Cases 2019, 7, 2760–2775. [Google Scholar] [CrossRef]
- Figueiredo, P.H.S.; Lima, M.M.O.; Costa, H.S.; Martins, J.B.; Flecha, O.D.; Gonçalves, P.F.; Alves, F.L.; Rodrigues, V.G.B.; Maciel, E.H.B.; Mendonça, V.A.; et al. Effects of the inspiratory muscle training and aerobic training on respiratory and functional parameters, inflammatory biomarkers, redox status and quality of life in hemodialysis patients: A randomized clinical trial. PLoS ONE 2018, 13, e0200727. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT Group CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Int. J. Surg. 2011, 9, 672–677. [Google Scholar] [CrossRef]
- Rehder-Santos, P.; Minatel, V.; Milan-Mattos, J.C.; Signini, É.D.F.; de Abreu, R.M.; Dato, C.C.; Catai, A.M. Critical inspiratory pressure - a new methodology for evaluating and training the inspiratory musculature for recreational cyclists: study protocol for a randomized controlled trial. Trials 2019, 20, 258. [Google Scholar] [CrossRef]
- Wasserman, K.W.M.; MD, J.E.H.; MD, D.Y.S.; MD, W.W.S.; MD, K.E.S.; MD, X.-G.S.; DSc, B.J.W.P. Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Applications, 5th ed.; LWW: Philadelphia, PA, USA, 2011. [Google Scholar]
- Beckonert, O.; Keun, H.C.; Ebbels, T.M.D.; Bundy, J.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2007, 2, 2692–2703. [Google Scholar] [CrossRef]

| Variable | Control (n = 6) | LI (n = 7) | MI (n = 10) | HI (n = 11) | P-Value |
|---|---|---|---|---|---|
| Age (years) | 30.8 ± 5.6 | 29.6 ± 4.9 | 31.9 ± 7.1 | 30.3 ± 7.2 | 0.896 |
| Height (m) | 1.74 ± 0.12 | 1.79 ± 0.03 | 1.75 ± 0.05 | 1.77 ± 0.06 | 0.580 |
| Body mass (kg) | 75.9 ± 18.0 | 75.9 ± 12.4 | 73.8 ± 8.2 | 78.5 ± 10.2 | 0.838 |
| BMI (kg/m2) | 24.9 ± 4.3 | 23.8 ± 4.4 | 23.9 ± 1.9 | 25.1 ± 3.4 | 0.804 |
| Body fat (%) | 22.0 ± 3.27 | 21.6 ± 6.31 | 21.1 ± 3.4 | 22.1 ± 4.72 | 0.958 |
| Lean mass (%) | 75.1 ± 3.52 | 74.9 ± 5.79 | 75.8 ± 3.25 | 74.8 ± 4.27 | 0.946 |
| VO2 peak (ml.kg−1.min−1) | 52.6 ± 15.2 | 40.9 ± 8.77 | 48.9 ± 9.60 | 48.8 ± 12.7 | 0.321 |
| MIP (cmH2O) | 151 ± 11.4 | 150 ± 10.4 | 159 ± 24.8 | 145 ± 13.7 | 0.303 |
| Metabolites | Chemical Shift (ppm) | Control | Low Intensity | Moderate Intensity | High Intensity | P-Values: Interaction | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Training | Post-Training | Pre-Training | Post-Training | Pre-Training | Post-Training | Pre-Training | Post-Training | |||
| Leucine | 0.9 | 402 ± 41.8 | 425 ± 80.8 | 405 ± 52.1 | 400 ± 48.8 | 444 ± 73.0 | 388 ± 47.8 | 414 ± 46.1 | 428 ± 70.7 | 0.142 |
| (CH2)n FA | 1.26 | 1536 ± 180 | 1621 ± 316 | 1549 ± 192 | 1545 ± 195 | 1743 ± 290 | 1522 ± 202 | 1613 ± 168 | 1682 ± 296 | 0.144 |
| Lactate | 1.33 | 572 ± 104 | 598 ± 99.8 | 631 ± 110 | 642 ± 115 | 617 ± 120 | 574 ± 110 | 595 ± 111 | 595 ± 115 | 0.817 |
| Alanine | 1.47 | 47.4 ± 9.40 | 48.4 ± 4.88 | 53.8 ± 4.52 | 48.2 ± 16.7 | 48.0 ± 9.86 | 50.4 ± 10.05 | 52.1 ± 12.2 | 54.4 ± 10.7 | 0.540 |
| CH2=CH2-CO lipids | 1.6 | 251 ± 31.0 | 262 ± 46.8 | 249 ± 29.2 | 244 ± 34.5 | 267 ± 45.0 | 235 ± 31.5 | 254 ± 25.1 | 263 ± 41.7 | 0.216 |
| Acetate | 1.9 | 3.40 ± 1.09 | 4.44 ± 1.59 | 3.14 ± 1.04 | 3.08 ± 0.98 | 3.50 ± 1.20 | 3.13 ± 0.72 | 3.46 ± 1.66 | 2.79 ± 1.27 | 0.118 |
| Acetone | 2.2 | 8.97 ± 3.40 | 9.80 ± 2.97 | 9.14 ± 4.71 | 7.51 ± 1.81 | 28.1 ± 55.5 | 17.3 ± 31.5 | 16.6 ± 15.2 | 8.73 ± 5.07 | 0.924 |
| Creatine | 3.02 | 1.92 ± 0.90 | 1.97 ± 0.61 | 1.99 ± 0.69 | 1.91 ± 0.83 | 2.02 ± 0.50 | 2.18 ± 1.24 | 2.12 ± 0.89 | 2.68 ± 1.11 | 0.516 |
| Creatinine | 3.05 | 7.58 ± 2.06 | 7.27 ± 1.12 | 9.45 ± 1.93 | 8.05 ± 2.47 | 8.17 ± 1.31 | 7.76 ± 1.60 | 7.97 ± 0.99 | 8.22 ± 1.56 | 0.404 |
| Arginine | 3.19 | 64.4 ± 7.53 | 72.5 ± 11.8 | 66.4 ± 13.1 | 61.5 ± 11.6 | 64.6 ± 11.8 | 63.5 ± 14.5 | 68.0 ± 12.8 | 71.7 ± 13.3 | 0.264 |
| Betaine | 3.25 | 119 ± 20.8 | 137 ± 18.8 | 122 ± 24.3 | 120 ± 16.7 | 134 ± 23.3 | 121 ± 16.2 | 128 ± 22.9 | 129 ± 33.8 | 0.137 |
| Glycine | 3.7 | 63.2 ± 6.82 | 71.0 ± 11. 7 | 67.8 ± 10.8 | 64.4 ± 11.8 | 64.7 ± 10.3 | 64.4 ± 13.6 | 68.4 ± 10.1 | 70.1 ± 12.0 | 0.378 |
| Proline | 4.2 | 50.3 ± 8.10 | 54.5 ± 10.1 | 51.2 ± 5.79 | 51.0 ± 5.53 | 55.3 ± 9.81 | 51.1 ± 6.56 | 52.8 ± 5.96 | 55.2 ± 11.0 | 0.342 |
| Glucose | 4.5 | 82.5 ± 11.8 | 95.7 ± 16.4 | 88.3 ± 16.0 | 83.9 ± 15.8 | 83.7 ± 13.4 | 83.2 ± 17.8 | 91.0 ± 13.7 | 91.8 ± 16.0 | 0.270 |
| Glucose | 5.2 | 62.0 ± 8.02 | 70.1 ± 11.4 | 67.2 ± 10.6 | 63.7 ± 12.5 | 64.1 ± 10.3 | 63.6 ± 13.1 | 68.1 ± 10.2 | 70.0 ± 11.9 | 0.352 |
| FA=CH | 5.3 | 342 ± 36.3 | 366 ± 74.0 | 349 ± 52.6 | 346 ± 49.1 | 387 ± 63.9 | 349 ± 49.5 | 369 ± 36.5 | 377 ± 66.4 | 0.315 |
| Urea | 5.7 | 141 ± 31.1 | 162 ± 50.6 | 152 ± 34.4 | 136 ± 45.1 | 143 ± 40.4 | 126 ± 19.8 | 154 ± 36.7 | 145 ± 53.0 | 0.375 |
| Tyrosine | 6.8 | 4.46 ± 0.84 | 4.85 ± 0.62 | 4.67 ± 0.58 | 4.23 ± 1.09 | 4.46 ± 0.80 | 4.41 ± 0.88 | 4.56 ± 0.69 | 4.89 ± 0.95 | 0.417 |
| Methyl histidine | 7.0 | 2.42 ± 0.25 | 2.54 ± 0.39 | 2.60 ± 0.22 | 2.02 ± 0.95 | 2.52 ± 0.35 | 2.43 ± 0.40 | 2.54 ± 0.36 | 2.51 ± 0.35 | 0.155 |
| Phenyalanine | 7.4 | 2.09 ± 0.38 | 2.32 ± 0.24 | 2.24 ± 0.24 | 2.01 ± 0.62 | 2.21 ± 0.35 | 2.11 ± 0.35 | 2.06 ± 0.31 | 2.24 ± 0.36 | 0.105 |
| Histidine | 7.7 | 2.55 ± 0.19 | 2.73 ± 0.42 | 2.77 ± 0.20 | 2.18 ± 1.06 | 2.70 ± 0.39 | 2.63 ± 0.44 | 2.72 ± 0.39 | 2.69 ± 0.34 | 0.156 |
| Tryptophan | 7.72 | 2.10 ± 0.47 | 2.41 ± 0.28 | 2.37 ± 0.25 | 2.13 ± 0.50 | 2.13 ± 0.35 | 2.16 ± 0.31 | 2.13 ± 0.35 | 2.34 ± 0.34 | 0.056 |
| Formate | 8.5 | 0.31 ± 0.07 | 0.30 ± 0.04 | 0.27 ± 0.06 | 0.29 ± 0.04 | 0.30 ± 0.06 | 0.30 ± 0.08 | 0.30 ± 0.07 | 0.26 ± 0.06 | 0.512 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakaguchi, C.A.; Nieman, D.C.; F. Signini, E.; M. de Abreu, R.; Silva, C.D.; Rehder-Santos, P.; Carosio, M.G.A.; M. Maria, R.; Dato, C.C.; de Araújo, H.S.S.; et al. Chronic Influence of Inspiratory Muscle Training at Different Intensities on the Serum Metabolome. Metabolites 2020, 10, 78. https://doi.org/10.3390/metabo10020078
Sakaguchi CA, Nieman DC, F. Signini E, M. de Abreu R, Silva CD, Rehder-Santos P, Carosio MGA, M. Maria R, Dato CC, de Araújo HSS, et al. Chronic Influence of Inspiratory Muscle Training at Different Intensities on the Serum Metabolome. Metabolites. 2020; 10(2):78. https://doi.org/10.3390/metabo10020078
Chicago/Turabian StyleSakaguchi, Camila A., David C. Nieman, Etore F. Signini, Raphael M. de Abreu, Claudio D. Silva, Patrícia Rehder-Santos, Maria G. A. Carosio, Roberta M. Maria, Carla C. Dato, Heloisa S. S. de Araújo, and et al. 2020. "Chronic Influence of Inspiratory Muscle Training at Different Intensities on the Serum Metabolome" Metabolites 10, no. 2: 78. https://doi.org/10.3390/metabo10020078
APA StyleSakaguchi, C. A., Nieman, D. C., F. Signini, E., M. de Abreu, R., Silva, C. D., Rehder-Santos, P., Carosio, M. G. A., M. Maria, R., Dato, C. C., de Araújo, H. S. S., Venâncio, T., Ferreira, A. G., & Catai, A. M. (2020). Chronic Influence of Inspiratory Muscle Training at Different Intensities on the Serum Metabolome. Metabolites, 10(2), 78. https://doi.org/10.3390/metabo10020078






