Exercise Promotes the Osteoinduction of HA/β-TCP Biomaterials via the Wnt Signaling Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
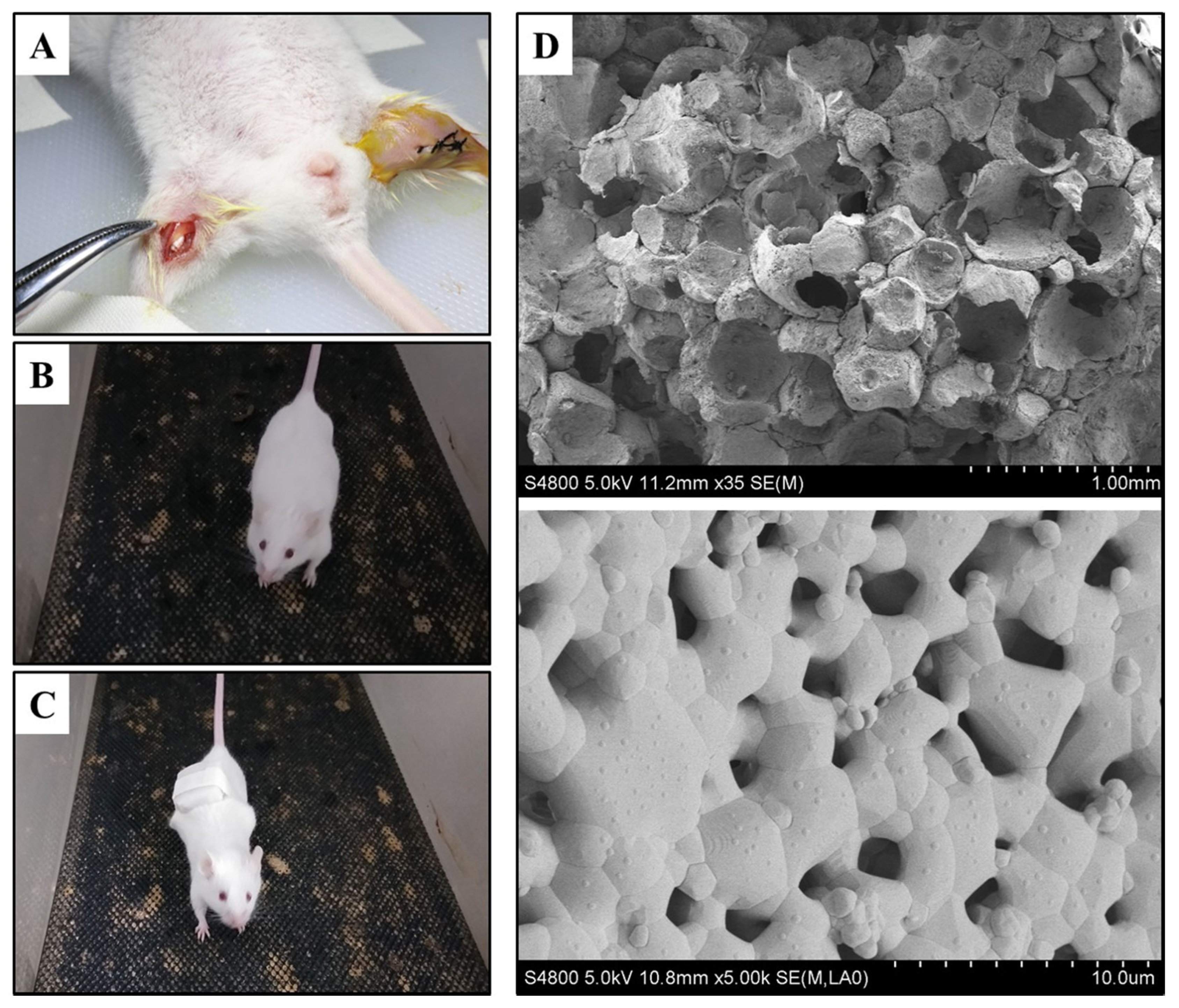
2.2. Animal Surgery
2.3. Animal Grouping
2.4. Sample Harvesting
2.5. Enzyme-Linked Immunosorbent Assay
2.6. Histological Staining
2.7. The Area Percentage of New Bone Tissues
2.8. Western Blot
2.9. Statistical Analysis
3. Results
3.1. The Characteristic of Biomaterials
3.2. Inflammation after Implantation
3.3. Immune Regulation through Exercise
3.4. New Bone and Bone Marrow Formation
3.5. The Osteoinductive Efficiency
3.6. Wnt/β-catenin Signaling Pathway
3.7. The Expression of Osteogenesis Related Proteins
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burge, R.; Dawson-Hughes, B.; Solomon, D.H.; Wong, J.B.; King, A.; Tosteson, A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J. Bone Miner. Res. 2007, 22, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Liu, Z.; Yan, S.; Chen, Z.; Zou, L.; Shi, Z. The role of osteoclasts in osteoinduction triggered by calcium phosphate biomaterials in mice. Biomed. Mater. Eng. 2019, 30, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Boccaccio, A.; Uva, A.E.; Fiorentino, M.; Monno, G.; Ballini, A.; Desiate, A. Optimal load for bone tissue scaffolds with an assigned geometry. Int. J. Med. Sci. 2018, 15, 16–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballini, A.; Cantore, S.; Scacco, S.; Coletti, D.; Tatullo, M. Mesenchymal stem cells as promoters, enhancers, and playmakers of the translational regenerative medicine 2018. Stem Cells Int. 2018, 2018, 6927401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekegren, C.L.; Beck, B.; Climie, R.E.; Owen, N.; Dunstan, D.W.; Gabbe, B.J. Physical activity and sedentary behavior subsequent to serious orthopedic injury: A systematic review. Arch. Phys. Med. Rehabil. 2018, 99, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Tveit, M.; Rosengren, B.E.; Nilsson, J.Å.; Karlsson, M.K. Exercise in youth: High bone mass, large bone size, and low fracture risk in old age. Scand. J. Med. Sci. Sports 2015, 25, 453–461. [Google Scholar] [CrossRef]
- Lombardi, G.; Ziemann, E.; Banfi, G. Physical activity and bone health: What is the role of immune system? A narrative review of the third way. Front. Endocrinol. 2019, 7, 60. [Google Scholar] [CrossRef] [Green Version]
- Ross, M.; Lithgow, H.; Hayes, L.; Florida-James, G. Potential cellular and biochemical mechanisms of exercise and physical activity on the ageing process. Subcell. Biochem. 2019, 91, 311–338. [Google Scholar]
- Cheng, L.; Yan, S.; Zhu, J.; Cai, P.; Wang, T.; Shi, Z. Exercise enhance the ectopic bone formation of calcium phosphate biomaterials in muscles of mice. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 1, 136–141. [Google Scholar] [CrossRef]
- Nusse, R.; Clevers, H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef]
- McEnerney, L.; Duncan, K.; Bang, B.R.; Elmasry, S.; Li, M.; Miki, T.; Ramakrishnan, S.K.; Shah, Y.M.; Saito, T. Dual modulation of human hepatic zonation via canonical and non-canonical Wnt pathways. Exp. Mol. Med. 2017, 49, e413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, Y.; Sims, C.; Murray, M.J.; Kuhlmann, P.K.; Fuentes-Antrás, J.; Weatherbee, S.D.; Krumlauf, R. Multiple modes of Lrp4 function in modulation of Wnt/β-catenin signaling during tooth development. Development 2017, 144, 2824–2836. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Li, Y.; Wang, X.; Zou, S.; Hu, J.; Luo, E. The effect of uniaxial mechanical stretch on wnt/β-catenin pathway in bone mesenchymal stem cells. J. Craniofac. Surg. 2017, 28, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in chronic wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef] [PubMed]
- Sung, P.S.; Huang, T.F.; Hsieh, S.L. Extracellular vesicles from clec2-activated platelets enhance dengue virus-induced lethality via clec5a/tlr2. Nat. Commun. 2019, 10, 2402. [Google Scholar] [CrossRef] [Green Version]
- Fendl, B.; Eichhorn, T.; Weiss, R.; Tripisciano, C.; Spittler, A.; Fischer, M.B.; Weber, V. Differential Interaction of platelet-derived extracellular vesicles with circulating immune cells: Roles of tam receptors, CD11b, and phosphatidylserine. Front. Immunol. 2018, 9, 2797. [Google Scholar] [CrossRef]
- Zuchtriegel, G.; Uhl, B.; Hessenauer, M.E.; Kurz, A.R.; Rehberg, M.; Lauber, K.; Krombach, F.; Reichel, C.A. Spatiotemporal expression dynamics of selectins govern the sequential extravasation of neutrophils and monocytes in the acute inflammatory response. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 899–910. [Google Scholar] [CrossRef] [Green Version]
- Kotwal, G.J.; Chien, S. Macrophage differentiation in normal and accelerated wound healing. Results Probl. Cell Differ. 2017, 62, 353–364. [Google Scholar]
- Castro, S.A.; Collighan, R.; Lambert, P.A.; Dias, I.H.; Chauhan, P.; Bland, C.E.; Milic, I.; Milward, M.R.; Cooper, P.R.; Devitt, A. Porphyromonas gingivalis gingipains cause defective macrophage migration towards apoptotic cells and inhibit phagocytosis of primary apoptotic neutrophils. Cell Death Dis. 2017, 8, e2644. [Google Scholar] [CrossRef]
- Yang, H.L.; Tsai, Y.C.; Korivi, M.; Chang, C.T.; Hseu, Y.C. Lucidone promotes the cutaneous wound healing process via activation of the PI3K/AKT, Wnt/β-catenin and NF-κB signaling pathways. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 151–168. [Google Scholar] [CrossRef]
- Loi, F.; Córdova, L.A.; Pajarinen, J.; Lin, T.H.; Yao, Z.; Goodman, S.B. Inflammation, fracture and bone repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, L.; Ye, F.; Yang, R.; Lu, X.; Shi, Y.; Li, L.; Fan, H.; Bu, H. Osteoinduction of hydroxyapatite/beta-tricalcium phosphate bioceramics in mice with a fractured fibula. Acta Biomater. 2010, 6, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Shi, L.; Chen, L.; Liu, X.; Qu, X.; Wang, K.; Wei, F. Endothelial cells modified by adenovirus vector containing nine copies hypoxia response elements and human vascular endothelial growth factor as the novel seed cells for bone tissue engineering. Acta Biochim. Biophys. Sin. 2017, 49, 973–978. [Google Scholar] [CrossRef] [Green Version]
- Hausherr, T.C.; Nuss, K.; Thein, E.; Krähenbühl, S.; Applegate, L.A.; Pioletti, D.P. Effect of temporal onsets of mechanical loading on bone formation inside a tissue engineering scaffold combined with cell therapy. Bone Rep. 2018, 8, 173–179. [Google Scholar] [CrossRef]
- Hojman, P. Exercise protects from cancer through regulation of immune function and inflammation. Biochem. Soc. Trans. 2017, 45, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Idorn, M.; Hojman, P. Exercise-dependent regulation of NK cells in cancer protection. Trends Mol. Med. 2016, 22, 565–577. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Feng, X.; Desierto, M.J.; Keyvanfar, K.; Young, N.S. IFN-γ-mediated hematopoietic cell destruction in murine models of immune-mediated bone marrow failure. Blood 2015, 126, 2621–2631. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Honda, Y.; Arima, Y.; Yasui, K.; Inami, K.; Nishiura, A.; Hashimoto, Y.; Matsumoto, N. Interferon-γ enhances the efficacy of autogenous bone grafts by inhibiting postoperative bone resorption in rat calvarial defects. J. Prosthodont. Res. 2016, 60, 167–176. [Google Scholar] [CrossRef]
- Li, H.; Nie, B.; Du, Z.; Zhang, S.; Long, T.; Yue, B. Bacitracin promotes osteogenic differentiation of human bone marrow mesenchymal stem cells by stimulating the bone morphogenetic protein-2/Smad axis. Biomed. Pharmacother. 2018, 103, 588–597. [Google Scholar] [CrossRef]
- Duan, P.; Bonewald, L.F. The role of the wnt/β-catenin signaling pathway in formation and maintenance of bone and teeth. Int. J. Biochem. Cell Biol. 2016, 77, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Ziouti, F.; Ebert, R.; Rummler, M.; Krug, M.; Müller-Deubert, S.; Lüdemann, M.; Jakob, F.; Willie, B.M.; Jundt, F. Notch signaling is activated through mechanical strain in human bone marrow-derived mesenchymal stromal cells. Stem Cells Int. 2019, 2019, 5150634. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, B.; Zhao, Q. Hedgehog signaling regulates osteoblast differentiation in zebrafish larvae through modulation of autophagy. Biol. Open 2019, 8, bio040840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, L.; Yang, L.; Niu, B.; Yan, S.; Shi, Z.; Liu, R.; Liu, Z.; Zou, L. Identify the source of osteoblasts and osteogenic signaling pathway during bone induction in mice. J. Biomater. Tissue Eng. 2019, 9, 1–7. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, L.; Khalaf, A.T.; Lin, T.; Ran, L.; Shi, Z.; Wan, J.; Zhou, X.; Zou, L. Exercise Promotes the Osteoinduction of HA/β-TCP Biomaterials via the Wnt Signaling Pathway. Metabolites 2020, 10, 90. https://doi.org/10.3390/metabo10030090
Cheng L, Khalaf AT, Lin T, Ran L, Shi Z, Wan J, Zhou X, Zou L. Exercise Promotes the Osteoinduction of HA/β-TCP Biomaterials via the Wnt Signaling Pathway. Metabolites. 2020; 10(3):90. https://doi.org/10.3390/metabo10030090
Chicago/Turabian StyleCheng, Lijia, Ahmad Taha Khalaf, Tianchang Lin, Ling Ran, Zheng Shi, Jun Wan, Xin Zhou, and Liang Zou. 2020. "Exercise Promotes the Osteoinduction of HA/β-TCP Biomaterials via the Wnt Signaling Pathway" Metabolites 10, no. 3: 90. https://doi.org/10.3390/metabo10030090
APA StyleCheng, L., Khalaf, A. T., Lin, T., Ran, L., Shi, Z., Wan, J., Zhou, X., & Zou, L. (2020). Exercise Promotes the Osteoinduction of HA/β-TCP Biomaterials via the Wnt Signaling Pathway. Metabolites, 10(3), 90. https://doi.org/10.3390/metabo10030090







