Synthesis and Biological Evaluation of Imidazo[2,1-b]Thiazole based Sulfonyl Piperazines as Novel Carbonic Anhydrase II Inhibitors
Abstract
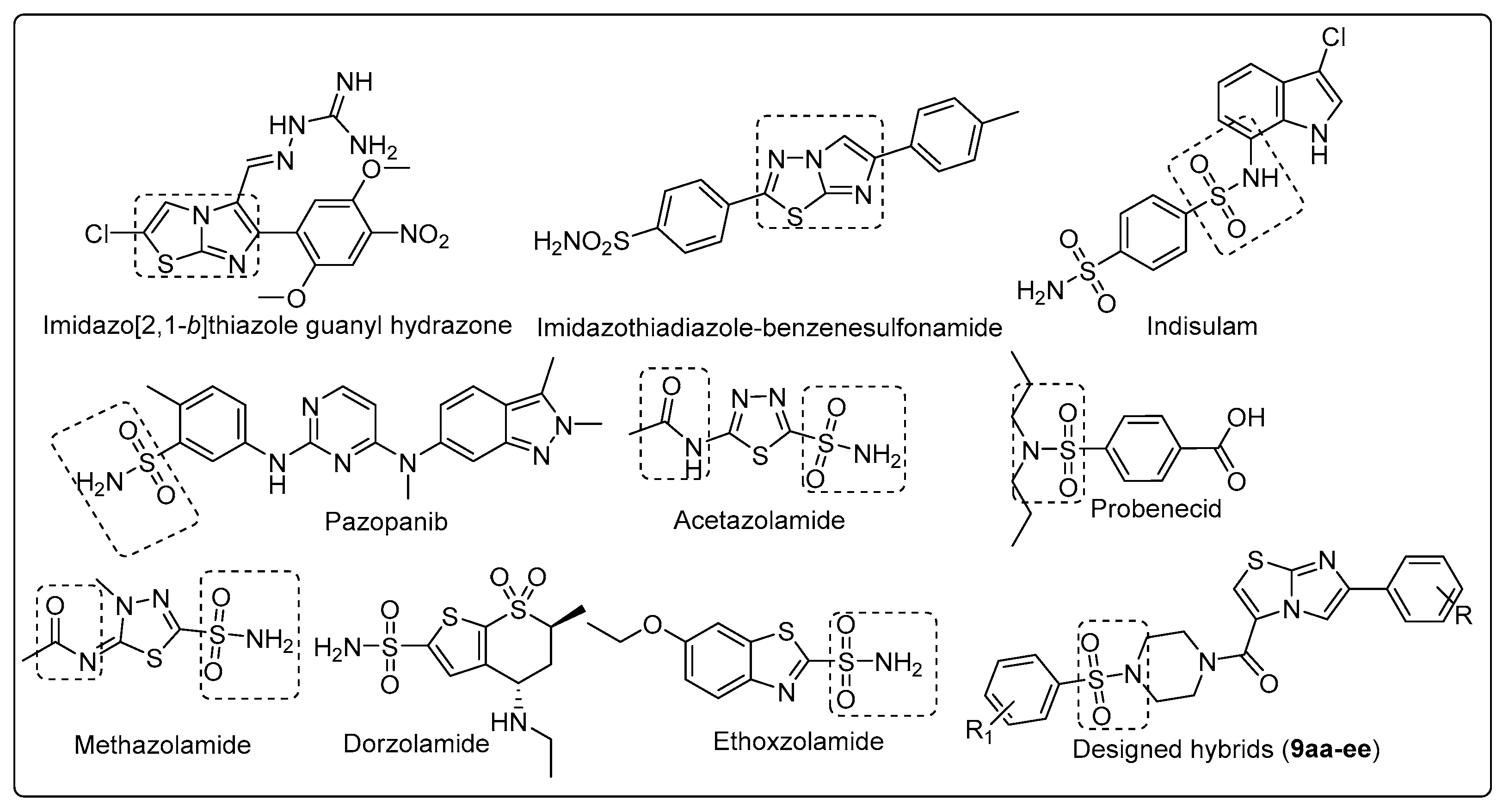
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Carbonic Anhydrase Inhibition
- i.
- The cytosolic isoforms hCA I and tumor associated isoform hCA IX, hCAXII were not inhibited by the compounds 9aa-ee (Ki > 100 µM).
- ii.
- The compounds 9aa-ee showed varied inhibitory profiles against cytosolic isoform hCA II. Compound 9ae, 9bb, 9ca, 9cc-ce and 9da elicited inhibitory potencies (Ki) of 57.7–67.9 µM. Potent compounds (9ca, 9cc-ce) having chlorine (R) at aromatic ring of imidazo thiazole whereas other compounds are ineffective. It is assumed that these compounds, i.e., 9ae (Ki = 57.7 µM), 9bb (Ki = 76.4 µM), 9ca (Ki =79.9 µM), 9cc (Ki = 57.8 µM), 9cd (Ki = 71.2 µM), 9ce (Ki = 62.1 µM), 9da (Ki = 67.9 µM) were showing inhibition probably due to the presence of 4-OCH3, 4-CH3, 4-Cl and 4-F as R group and 4-t-butyl, 4-CH3, 4-OCH3, 4-Cl and H as R1 group.
- iii.
- It is evident from the Ki values (Table 1), compound 9ae bearing that R-group (imidazothiazole part) substituted with 4-methoxy and R1 substituted with 4-t-butyl (sulfonyl piperazine part) showed promising inhibitory activity.
- iv.
- Among all the congeners, some of the potent compounds exhibited selective inhibition of hCA II as compared to hCA IX and hCAXII in micromolar range.
3. Conclusions
4. Experimental Section
4.1. Materials and Methods
4.1.1. General Synthetic Procedure for the Preparation of Compound 3
4.1.2. General Synthetic Procedure for the Preparation of Compound 5a-e
4.1.3. General Synthetic Procedure for the Preparation of Compound 6a-e
4.1.4. General Synthetic Procedure for the Preparation of Compound 8a-e
4.1.5. General Synthetic Procedure for the Preparation of (5-(aryl)imidazo[2,1-b]thiazol-2-yl)(4-((aryl)sulfonyl)piperazin-1-yl)methanone (9aa-ee)
(5-(4-Methoxyphenyl)imidazo[2,1-b]thiazol-2-yl)(4-((4-methoxyphenyl)sulfonyl)piperazin-1-yl)methanone (9aa)
(5-(4-Methoxyphenyl)imidazo[2,1-b]thiazol-2-yl)(4-tosylpiperazin-1-yl)methanone (9ab)
(1-(4-Methoxyphenyl)-9H-pyrido[3,4-b]indol-3-yl)(4-tosylpiperazin-1-yl)methanone (9ac)
(5-(4-Methoxyphenyl)imidazo[2,1-b]thiazol-2-yl)(4-(phenylsulfonyl)piperazin-1-yl)methanone (9ad)
(4-((4-(Tert-butyl)phenyl)sulfonyl)piperazin-1-yl)(5-(4-methoxyphenyl)imidazo[2,1-b]thiazol-2-yl)methanone (9ae)
(4-((4-Methoxyphenyl)sulfonyl)piperazin-1-yl)(5-(p-tolyl)imidazo[2,1-b]thiazol-2-yl)methanone (9ba)
(5-(p-Tolyl)imidazo[2,1-b]thiazol-2-yl)(4-tosylpiperazin-1-yl)methanone (9bb)
(4-((4-Chlorophenyl)sulfonyl)piperazin-1-yl)(5-(p-tolyl)imidazo[2,1-b]thiazol-2-yl)methanone (9bc)
(4-((4-(Tert-butyl)phenyl)sulfonyl)piperazin-1-yl)(5-(p-tolyl)imidazo[2,1-b]thiazol-2-yl)methanone (9bd)
(5-(4-Chlorophenyl)imidazo[2,1-b]thiazol-2-yl)(4-((4-methoxyphenyl)sulfonyl)piperazin-1-yl)methanone (9ca)
(5-(4-Chlorophenyl)imidazo[2,1-b]thiazol-2-yl)(4-tosylpiperazin-1-yl)methanone (9cb)
(5-(4-Chlorophenyl)imidazo[2,1-b]thiazol-2-yl)(4-((4-chlorophenyl)sulfonyl)piperazin-1-yl)methanone (9cc)
(5-(4-Chlorophenyl)imidazo[2,1-b]thiazol-2-yl)(4-(phenylsulfonyl)piperazin-1-yl)methanone (9cd)
(4-((4-(Tert-butyl)phenyl)sulfonyl)piperazin-1-yl)(5-(4-chlorophenyl)imidazo[2,1-b]thiazol-2-yl)methanone (9ce)
(5-(4-Fluorophenyl)imidazo[2,1-b]thiazol-2-yl)(4-((4-methoxyphenyl)sulfonyl)piperazin-1-yl)methanone (9da)
(5-(4-Fluorophenyl)imidazo[2,1-b]thiazol-2-yl)(4-tosylpiperazin-1-yl)methanone (9db)
(4-((4-Chlorophenyl)sulfonyl)piperazin-1-yl)(5-(4-fluorophenyl)imidazo[2,1-b]thiazol-2-yl)methanone (9dc)
(5-(4-Fluorophenyl)imidazo[2,1-b]thiazol-2-yl)(4-(phenylsulfonyl)piperazin-1-yl)methanone (9dd)
(4-((4-(Tert-butyl)phenyl)sulfonyl)piperazin-1-yl)(5-(4-fluorophenyl)imidazo[2,1-b]thiazol-2-yl)methanone (9de)
(4-((4-Methoxyphenyl)sulfonyl)piperazin-1-yl)(5-phenylimidazo[2,1-b]thiazol-2-yl)methanone (9ea)
(5-Phenylimidazo[2,1-b]thiazol-2-yl)(4-tosylpiperazin-1-yl)methanone (9eb)
(4-((4-Chlorophenyl)sulfonyl)piperazin-1-yl)(5-phenylimidazo[2,1-b]thiazol-2-yl)methanone (9ec)
(5-Phenylimidazo[2,1-b]thiazol-2-yl)(4-(phenylsulfonyl)piperazin-1-yl)methanone (9ed)
(4-((4-(Tert-butyl)phenyl)sulfonyl)piperazin-1-yl)(5-phenylimidazo[2,1-b]thiazol-2-yl)methanone (9ee)
4.2. CA Inhibition
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Supuran, C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008, 7, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Structure and function of carbonic anhydrases. Biochem. J. 2016, 473, 2023–2032. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic anhydrases and metabolism. Metabolites 2018, 8, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karki, S.S.; Panjamurthy, K.; Kumar, S.; Nambiar, M.; Ramareddy, S.A.; Chiruvella, K.K.; Raghavan, S.C. Synthesis and biological evaluation of novel 2-aralkyl-5-substituted-6-(4′-fluorophenyl)-imidazo [2, 1-b][1, 3, 4] thiadiazole derivatives as potent anticancer agents. Eur. J. Med. Chem. 2011, 46, 2109–2116. [Google Scholar] [CrossRef] [PubMed]
- Andreani, A.; Granaiola, M.; Leoni, A.; Locatelli, A.; Morigi, R.; Rambaldi, M.; Lenaz, G.; Fato, R.; Bergamini, C.; Farruggia, G. Potential Antitumor Agents. 37. Synthesis and Antitumor Activity of Guanylhydrazones from Imidazo[2,1-b]thiazoles and from the New Heterocyclic System Thiazolo[2′,3′:2,3]imidazo[4,5-c]quinoline. J. Med. Chem. 2005, 48, 3085–3089. [Google Scholar] [CrossRef] [PubMed]
- Demirayak, S.; Zitouni, G.; Chevallet, P.; Erol, K.; Kilic, F.S. Synthesis and vasodilatory activity of some thiazolo-triazole derivative. II Farm. 1993, 48, 707–712. [Google Scholar]
- Ibrahim, D.A. Synthesis and biological evaluation of 3,6-disubstituted [1,2,4]triazolo[3,4-b][1,3,4]thiadiazole derivatives as a novel class of potential anti-tumor agents. Eur. J. Med. Chem. 2009, 44, 2776–2781. [Google Scholar] [CrossRef]
- Kumar, R.; Bua, S.; Ram, S.; Prete, S.D.; Capasso, C.; Supuran, C.T.; Sharma, P.K. Benzenesulfonamide bearing imidazothiadiazole and thiazolotriazole scaffolds as potent tumor associated human carbonic anhydrase IX and XII inhibitors. Bioorg. Med. Chem. 2017, 25, 1286–1293. [Google Scholar] [CrossRef]
- Nishimori, I.; Vullo, D.; Innocenti, A.; Scozzafava, A.; Mastrolorenzo, A.; Supuran, C.T. Carbonic Anhydrase Inhibitors. The Mitochondrial Isozyme VB as a New Target for Sulfonamide and Sulfamate Inhibitors. J. Med. Chem. 2005, 48, 7860–7866. [Google Scholar] [CrossRef]
- Scozzafava, A.; Menabuoni, L.; Mincione, F.; Supuran, C.T. Carbonic Anhydrase Inhibitors. A General Approach for the Preparation of Water-Soluble Sulfonamides Incorporating Polyamino–Polycarboxylate Tails and of Their Metal Complexes Possessing Long-Lasting, Topical Intraocular Pressure-Lowering Properties. J. Med. Chem. 2002, 45, 1466–1476. [Google Scholar] [CrossRef]
- Tanpure, R.P.; Ren, B.; Peat, T.S.; Bornaghi, L.F.; Vullo, D.; Supuran, C.T.; Poulsen, S.-A. Carbonic Anhydrase Inhibitors with Dual-Tail Moieties to Match the Hydrophobic and Hydrophilic Halves of the Carbonic Anhydrase Active Site. J. Med. Chem. 2015, 58, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Angapelly, S.; Ramya, P.V.S.; Angeli, A.; Prete, S.D.; Capasso, C.; Arifuddin, M.; Supuran, C.T. Development of sulfonamides incorporating phenylacrylamido functionalities as carbonic anhydrase isoforms I, II, IX and XII inhibitors. Bioorg. Med. Chem. 2017, 25, 5726–5732. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin. Drug. Discov. 2017, 12, 61–88. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T.; Vullo, D.; Manole, G.; Casini, A.; Scozzafava, A. Designing of Novel Carbonic Anhydrase Inhibitors and Activators. Curr. Med. Chem. 2004, 2, 49–68. [Google Scholar] [CrossRef]
- Supuran, C.T. Exploring the multiple binding modes of inhibitors to carbonic anhydrases for novel drug discovery. Expert Opin. Drug Discov. 2020, 25, 1–16, in press. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic anhydrases: From biomedical applications of the inhibitors and activators to biotechnological use for CO2 capture. J. Enzym. Inhib. Med. Chem. 2013, 28, 229–230. [Google Scholar] [CrossRef] [Green Version]
- Angeli, A.; Chiaramonte, N.; Manetti, D.; Romanelli, M.N.; Supuran, C.T. Investigation of piperazines as human carbonic anhydrase I, II, IV and VII activators. J. Enzym. Inhib. Med. Chem. 2017, 33, 303–308. [Google Scholar] [CrossRef] [Green Version]
- Shaik, S.P.; Nayak, V.L.; Sultana, F.; Rao, A.V.S.; Shaik, A.B.; Babu, K.S.; Kamal, A. Design and synthesis of imidazo [2,1-b] thiazole linked triazole conjugates: Microtubule-destabilizing agents. Eur. J. Med. Chem. 2017, 126, 36–51. [Google Scholar] [CrossRef]
- Jadala, C.; Sathish, M.; Anchi, P.; Tokala, R.; Lakshmi, U.J.; Reddy, V.G.; Shankaraiah, N.; Godugu, C.; Kamal, A. Synthesis of Combretastatin-A4 Carboxamidest that Mimic Sulfonyl Piperazines by a Molecular Hybridization Approach: In vitro Cytotoxicity Evaluation and Inhibition of Tubulin Polymerization. Chemmedchem 2019, 14, 2052–2060. [Google Scholar] [CrossRef] [Green Version]
- Khalifah, R.J. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J. Biol. Chem. 1971, 246, 2561–2573. [Google Scholar]
- Maresca, A.; Temperini, C.; Vu, H.; Pham, N.B.; Poulsen, S.A.; Scozzatava, A.; Quinn, P.J.; Supuran, C.T. Non-zinc mediated inhibition of carbonic anhydrases: Coumarins are a new class of suicide inhibitors. J. Am. Chem. Soc. 2009, 131, 3057–3062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Supuran, C.T. Carbonic anhydrase inhibitors and their potential in a range of therapeutic areas. Expert Opin Ther Pat. 2018, 28, 709–712. [Google Scholar] [CrossRef] [Green Version]
- Korkmaz, N.; Obaidi, O.A.; Senturk, M.; Astley, D.; Ekinci, D.; Supuran, C.T. Synthesis and biological activity of novel thiourea derivatives as carbonic anhydrase inhibitors. J. Enzym. Inhib. Med. Chem. 2015, 30, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.; Nicolae, A.; Popescu, A. Carbonic anhydrase inhibitors. Part 35. Synthesis of Schiff bases derived from sulfanilamide and aromatic aldehydes: The first inhibitors with equally high affinity towards cytosolic and membrane-bound isozymes. Eur. J. Med. Chem. 1996, 31, 431–438. [Google Scholar] [CrossRef]
- Carta, F.; Aggarwal, M.; Maresca, A.; Scozzafava, A.; McKenna, R.; Masini, E.; Supuran, C.T. Dithiocarbamates strongly inhibit carbonic anhydrases and show antiglaucoma action in vivo. J. Med. Chem. 2012, 55, 1721–1730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceruso, M.; Bragagni, M.; AlOthman, Z.; Osman, S.M.; Supuran, C.T. New series of sulfonamides containing amino acid moiety act as effective and selective inhibitors of tumor-associated carbonic anhydrase XII. J. Enzym. Inhib. Med. Chem. 2015, 30, 430–434. [Google Scholar] [CrossRef]
- Alafeefy, A.M.; Abdel-Aziz, H.A.; Carta, F.; Supuran, C.T.; Pathak, S.K.; Prasad, O.; Sinha, L. Exploring QSARs of some benzenesulfonamides incorporating cyanoacrylamide moieties as a carbonic anhydrase inhibitors (specifically against tumorassociated isoforms IX and XII). J. Enzym. Inhib. Med. Chem. 2015, 30, 519–523. [Google Scholar] [CrossRef] [Green Version]
- Nocentini, A.; Supuran, C.T. Advances in the structural annotation of human carbonic anhydrases and impact on future drug discovery. Expert Opin. Drug. Discov. 2019, 14, 1175–1197. [Google Scholar] [CrossRef]
- Supuran, C.T. How many carbonic anhydrase inhibition mechanisms exist? J. Enzym. Inhib. Med. Chem. 2016, 31, 345–360. [Google Scholar] [CrossRef]

| Compound | Structure | hCA I | hCA II | hCA IX | hCA XII |
|---|---|---|---|---|---|
| 9aa |  | >100 | >100 | >100 | >100 |
| 9ab |  | >100 | >100 | >100 | >100 |
| 9ac |  | >100 | >100 | >100 | >100 |
| 9ad |  | >100 | >100 | >100 | >100 |
| 9ae |  | >100 | 57.7 | >100 | >100 |
| 9ba |  | >100 | >100 | >100 | >100 |
| 9bb |  | >100 | 76.4 | >100 | >100 |
| 9bc |  | >100 | >100 | >100 | >100 |
| 9bd |  | nd | nd | nd | nd |
| 9ca |  | >100 | 79.9 | >100 | >100 |
| 9cb |  | >100 | >100 | >100 | >100 |
| 9cc |  | >100 | 57.8 | >100 | >100 |
| 9cd |  | >100 | 71.2 | >100 | >100 |
| 9ce |  | >100 | 62.1 | >100 | >100 |
| 9da |  | >100 | 67.9 | >100 | >100 |
| 9db |  | >100 | >100 | >100 | >100 |
| 9dc |  | >100 | 98.2 | >100 | >100 |
| 9dd |  | >100 | >100 | >100 | >100 |
| 9de |  | >100 | >100 | >100 | >100 |
| 9ea |  | >100 | >100 | >100 | >100 |
| 9eb |  | >100 | >100 | >100 | >100 |
| 9ec |  | >100 | >100 | >100 | >100 |
| 9ed |  | >100 | >100 | >100 | >100 |
| 9ee |  | >100 | >100 | >100 | >100 |
| AAZ |  | 0.25 | 0.012 | 0.026 | 0.006 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manasa, K.L.; Pujitha, S.; Sethi, A.; Arifuddin, M.; Alvala, M.; Angeli, A.; Supuran, C.T. Synthesis and Biological Evaluation of Imidazo[2,1-b]Thiazole based Sulfonyl Piperazines as Novel Carbonic Anhydrase II Inhibitors. Metabolites 2020, 10, 136. https://doi.org/10.3390/metabo10040136
Manasa KL, Pujitha S, Sethi A, Arifuddin M, Alvala M, Angeli A, Supuran CT. Synthesis and Biological Evaluation of Imidazo[2,1-b]Thiazole based Sulfonyl Piperazines as Novel Carbonic Anhydrase II Inhibitors. Metabolites. 2020; 10(4):136. https://doi.org/10.3390/metabo10040136
Chicago/Turabian StyleManasa, Kesari Lakshmi, Sravya Pujitha, Aaftaab Sethi, Mohammed Arifuddin, Mallika Alvala, Andrea Angeli, and Claudiu T. Supuran. 2020. "Synthesis and Biological Evaluation of Imidazo[2,1-b]Thiazole based Sulfonyl Piperazines as Novel Carbonic Anhydrase II Inhibitors" Metabolites 10, no. 4: 136. https://doi.org/10.3390/metabo10040136
APA StyleManasa, K. L., Pujitha, S., Sethi, A., Arifuddin, M., Alvala, M., Angeli, A., & Supuran, C. T. (2020). Synthesis and Biological Evaluation of Imidazo[2,1-b]Thiazole based Sulfonyl Piperazines as Novel Carbonic Anhydrase II Inhibitors. Metabolites, 10(4), 136. https://doi.org/10.3390/metabo10040136







