Age-Dependent Metabolic Profiles Unravel the Metabolic Relationships within and between Flax Leaves (Linum usitatissimum)
Abstract
1. Introduction
2. Results
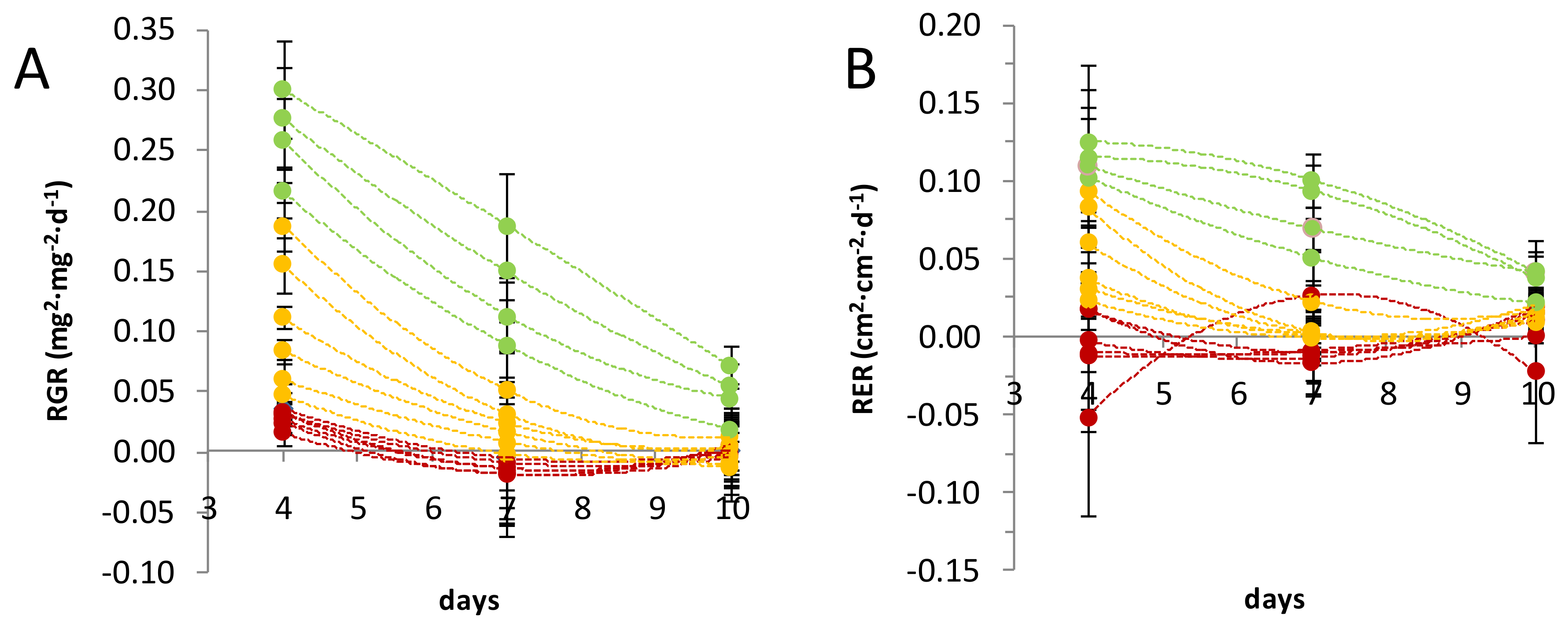
2.1. Growth Parameters of Three Flax Leaf Populations at Different Stages of Development
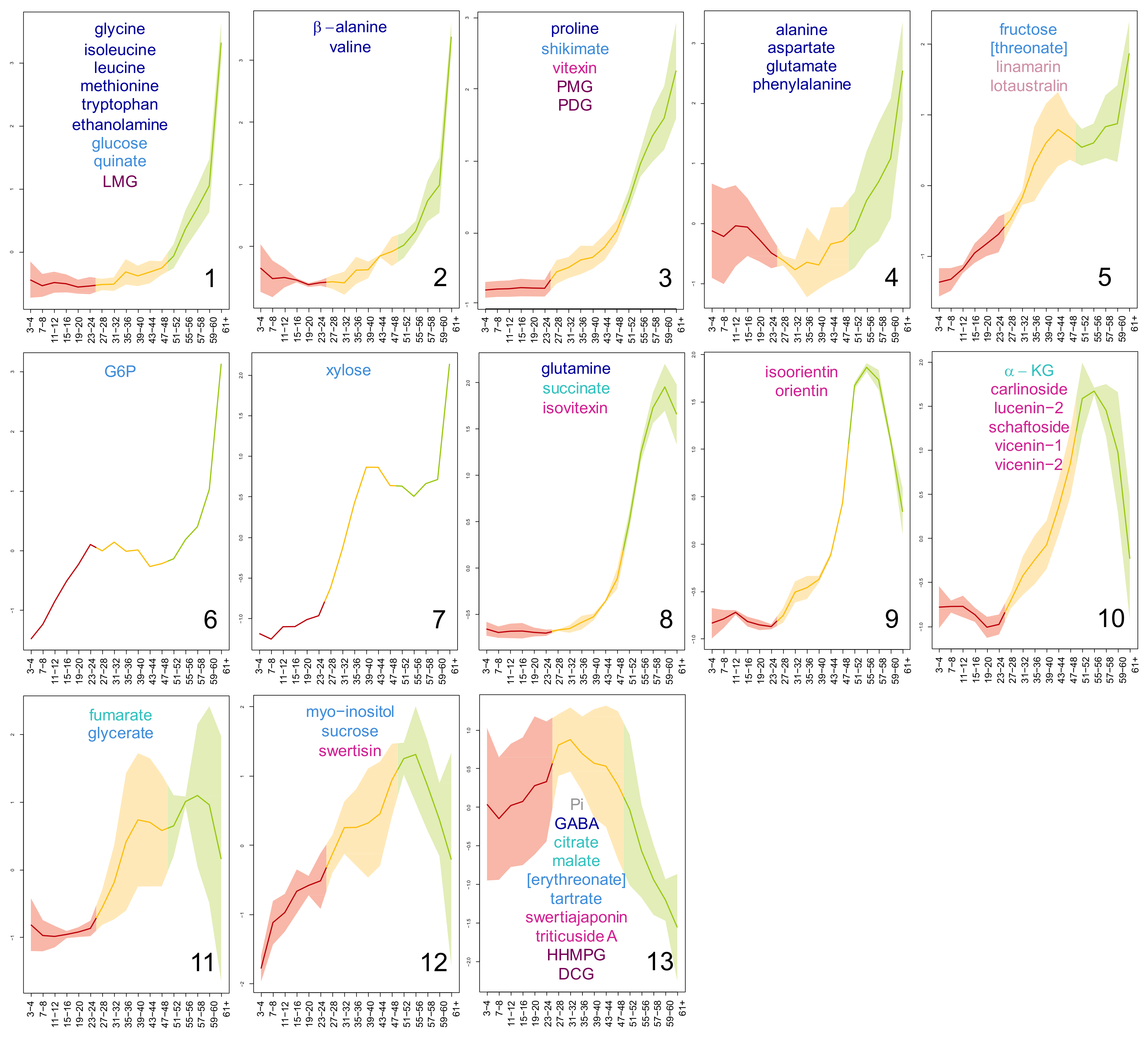
2.2. Age-Dependent Distribution of Flax Leaf Metabolites
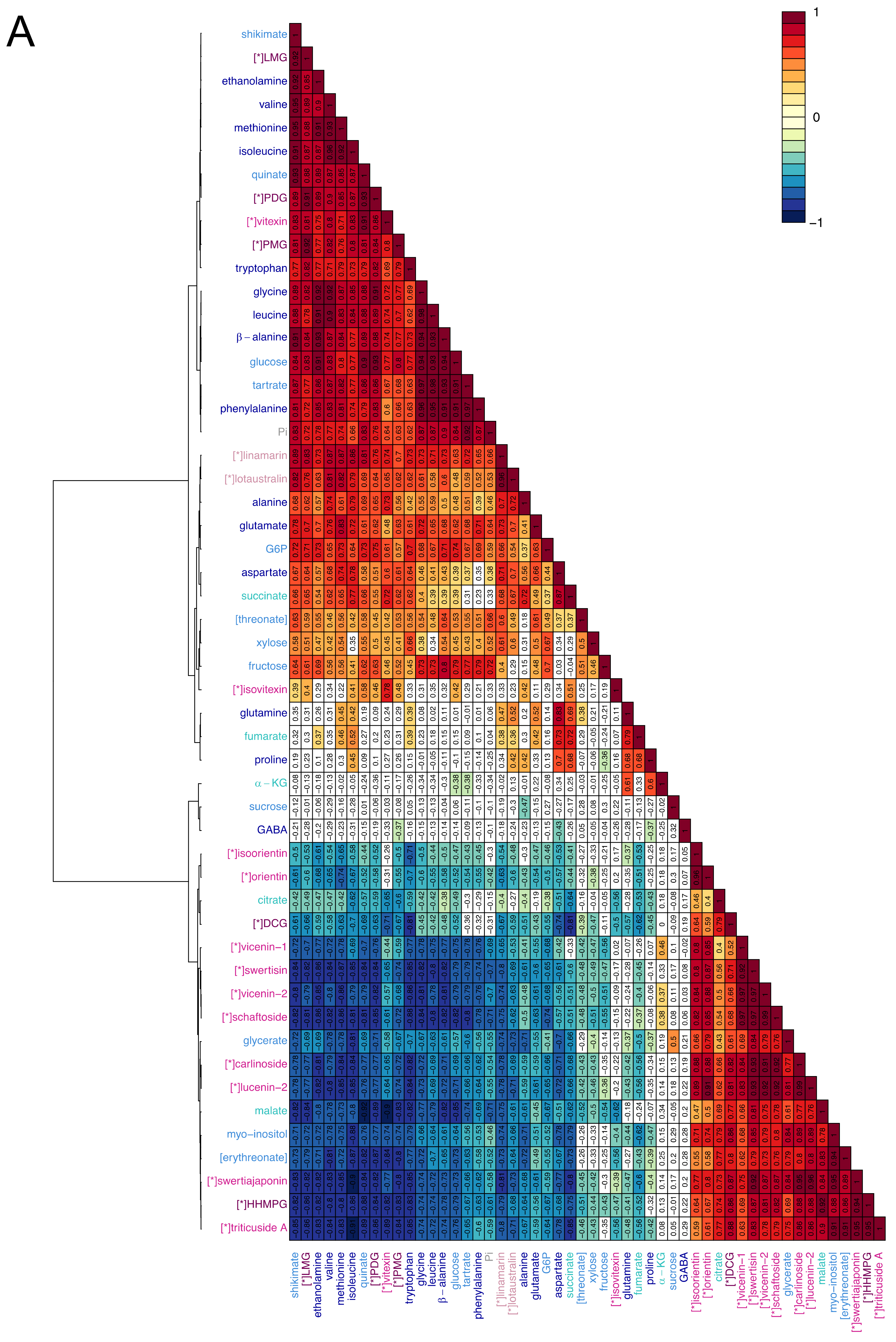
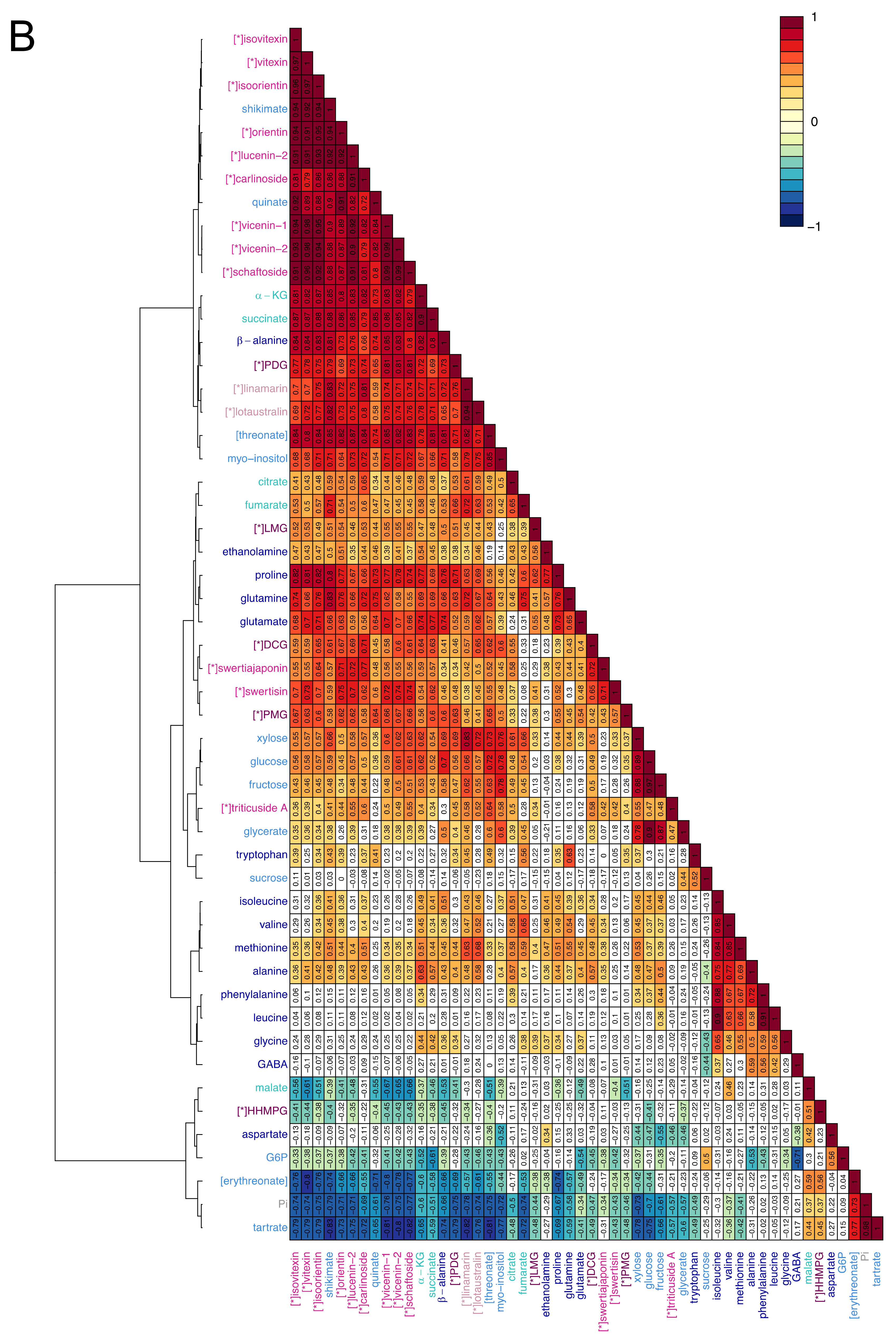
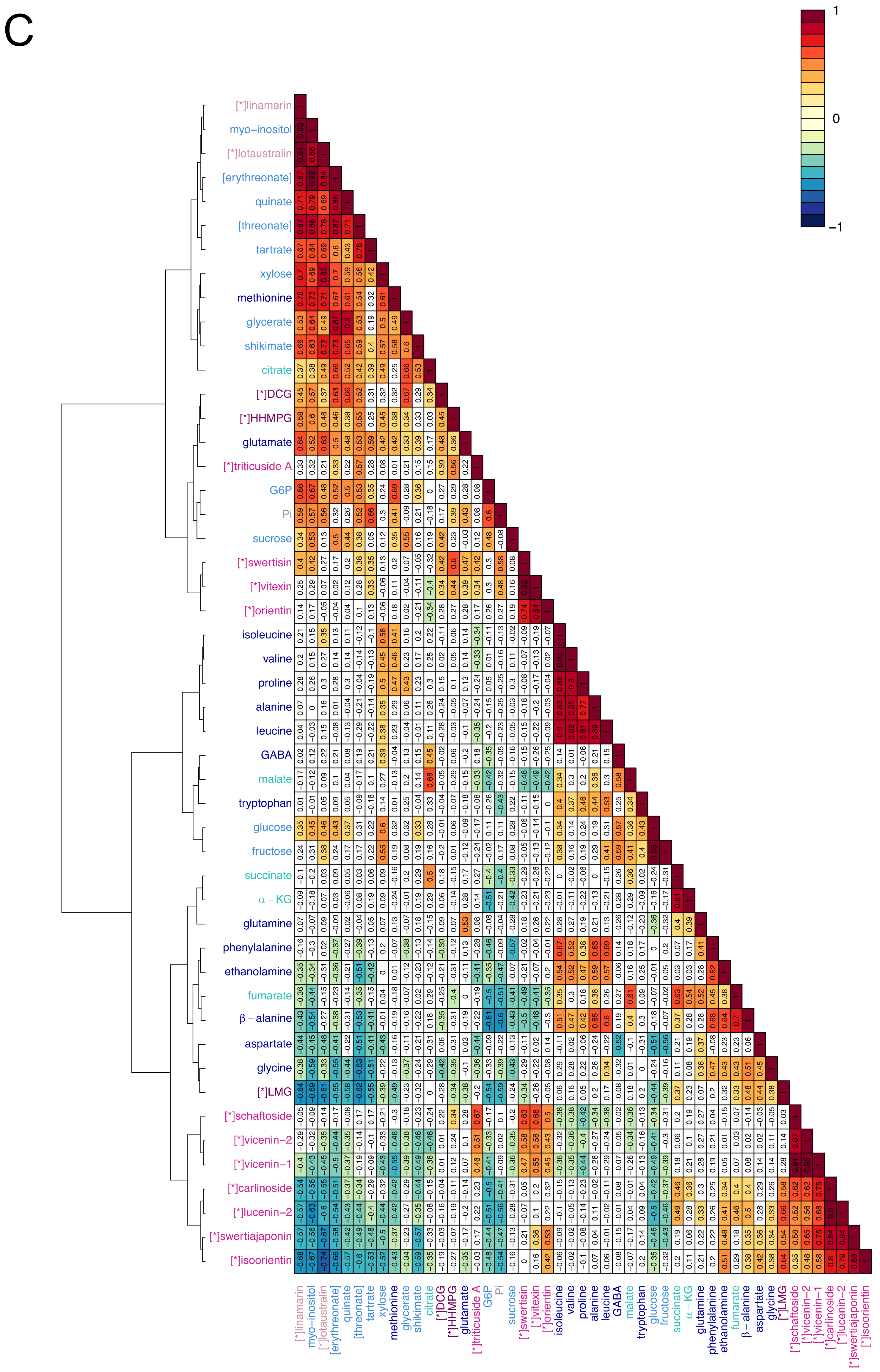
2.3. Visualisation of the Metabolic Correlations within Leaf Populations
3. Discussion
3.1. Flax Leaves Show Age-Dependent Distributions of Metabolites
3.2. Correlations between Primary Metabolites Are Indicative of the Metabolic Fluxes Between Differently Developed Leaves
3.3. C-glycosyl Flavonoids Distribution Sheds Light on Their Biosynthetic Pathways in Flax
4. Material and Methods
4.1. Plant Material
4.2. Leaf Dry Mass Weight and Relative Growth Rate (RGR)
4.3. Leaf Surface and Relative Expansion Rate (RER)
4.4. Metabolic Profiling
4.5. Data Treatment
4.5.1. Statistical Analysis
4.5.2. Multivariate Analysis
4.5.3. Kohonen Self-Organising Map (SOM)
4.5.4. Hierarchical Cluster Analysis (HCA)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sumner, L.W.; Mendes, P.; Dixon, R.A. Plant metabolomics: Large-scale phytochemistry in the functional genomics era. Phytochemistry 2003, 62, 817–836. [Google Scholar] [CrossRef]
- Kehr, J. High resolution spatial analysis of plant systems. Curr. Opin. Plant Boil. 2001, 4, 197–201. [Google Scholar] [CrossRef]
- Turgeon, R. The sink-source transition in leaves. Annu. Rev. Plant Physiol. Plant Mol Biol. 1989, 40, 119–138. [Google Scholar] [CrossRef]
- Fatichi, S.; Leuzinger, S.; Körner, C. Moving beyond photosynthesis: From carbon source to sink-driven vegetation modeling. New Phytol. 2014, 201, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.E. Phenotypic plasticity for plant development, function and life history. Trends Plant Sci. 2000, 5, 537–542. [Google Scholar] [CrossRef]
- Yu, S.-M.; Lo, S.-F.; Ho, T.-H.D. Source-sink communication: Regulated by hormone, nutrient, and stress cross-signaling. Trends Plant Sci. 2015, 20, 844–857. [Google Scholar] [CrossRef]
- Wink, M. Plant breeding: Importance of plant secondary metabolites for protection against pathogens and herbivores. Theoret. Appl. Genetics 1988, 75, 225–233. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, S.-Y.; Lim, S.-H.; Yeo, Y.; Cho, H.S.; Ha, S.-H. Comparative metabolic profiling of pigmented rice (Oryza sativa L.) cultivars reveals primary metabolites are correlated with secondary metabolites. J. Cereal Sci. 2013, 57, 14–20. [Google Scholar] [CrossRef]
- Koricheva, J. Meta-analysis of sources of variation in fitness costs of plant antiherbivore defenses. Ecology 2002, 83, 176–190. [Google Scholar] [CrossRef]
- Goyal, A.; Sharma, V.; Upadhyay, N.; Gill, S.; Sihag, M. Flax and flaxseed oil: An ancient medicine & modern functional food. J. Food Sci. Technol. 2014, 51, 1633–1653. [Google Scholar] [PubMed]
- Tchoumtchoua, J.; Mathiron, D.; Pontarin, N.; Gagneul, D.; van Bohemen, A.-I.; Otogo N’nang, E.; Mesnard, F.; Petit, E.; Fontaine, J.-X.; Molinié, R.; et al. Phenolic profiling of flax highlights contrasting patterns in winter and spring varieties. Molecules 2019, 24, 4303. [Google Scholar] [CrossRef] [PubMed]
- Schwachtje, J.; Baldwin, I.T. Why does herbivore attack reconfigure primary metabolism? Plant Physiol. 2008, 146, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Aharoni, A.; Galili, G. Metabolic engineering of the plant primary-secondary metabolism interface. Curr. Opin. Biotechnol. 2011, 22, 239–244. [Google Scholar] [CrossRef]
- Kim, H.K.; Choi, Y.H.; Verpoorte, R. Metabolomic analysis of Catharanthus roseus using NMR and principal component analysis. In Plant Metabolomics; Saito, K., Dixon, R.A., Willmitzer, L., Eds.; Springer: Berlin, Heidelberg, 2006; pp. 261–276. [Google Scholar]
- Weiner, J. Allocation, plasticity and allometry in plants. Perspect. Plant Ecol. Evol. Syst. 2004, 6, 207–215. [Google Scholar] [CrossRef]
- Steuer, R. Review: On the analysis and interpretation of correlations in metabolomic data. Brief Bioinform. 2006, 7, 151–158. [Google Scholar] [CrossRef]
- Erickson, R.O.; Michelini, F.J. The plastochron index. Am. J. Bot. 1957, 44, 297–305. [Google Scholar] [CrossRef]
- Pantin, F.; Simonneau, T.; Muller, B. Coming of leaf age: Control of growth by hydraulics and metabolics during leaf ontogeny. New Phytol. 2012, 196, 349–366. [Google Scholar] [CrossRef]
- Freitas, J.R.L.; Vendramini, P.H.; Melo, J.O.F.; Eberlin, M.N.; Augusti, R. An appraisal on the source-to-sink relationship in plants: An application of desorption electrospray ionization mass spectrometry imaging. J. Braz. Chem. Soc. 2018, 29, 17–23. [Google Scholar] [CrossRef]
- Bénard, C.; Bernillon, S.; Biais, B.; Osorio, S.; Maucourt, M.; Ballias, P.; Deborde, C.; Colombié, S.; Cabasson, C.; Jacob, D.; et al. Metabolomic profiling in tomato reveals diel compositional changes in fruit affected by source-sink relationships. J. Exp. Bot. 2015, 66, 3391–3404. [Google Scholar] [CrossRef]
- Masclaux, C.; Valadier, M.H.; Brugière, N.; Morot-Gaudry, J.F.; Hirel, B. Characterization of the sink/source transition in tobacco (Nicotiana tabacum L.) shoots in relation to nitrogen management and leaf senescence. Planta 2000, 211, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.L.; Jiang, H.; Chen, H.-S.; Tsai, C.-J.; Harding, S.A. Metabolic profiling of the sink-to-source transition in developing leaves of quaking aspen. Plant Physiol. 2004, 136, 3364–3375. [Google Scholar] [CrossRef] [PubMed]
- Desbrosses, G.G.; Kopka, J.; Udvardi, M.K. Lotus japonicus metabolic profiling. Development of gas chromatography-mass spectrometry resources for the study of plant-microbe interactions. Plant Physiol. 2005, 137, 1302–1318. [Google Scholar] [CrossRef] [PubMed]
- Kusano, M.; Jonsson, P.; Fukushima, A.; Gullberg, J.; Sjöström, M.; Trygg, J.; Moritz, T. Metabolite signature during short-day induced growth cessation in Populus. Front. Plant Sci. 2011, 2. [Google Scholar] [CrossRef] [PubMed]
- Fester, T.; Fetzer, I.; Härtig, C. A core set of metabolite sink/source ratios indicative for plant organ productivity in Lotus japonicus. Planta 2013, 237, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Balazadeh, S.; Tohge, T.; Erban, A.; Giavalisco, P.; Kopka, J.; Mueller-Roeber, B.; Fernie, A.R.; Hoefgen, R. Comprehensive dissection of spatiotemporal metabolic shifts in primary, secondary, and lipid metabolism during developmental senescence in Arabidopsis. Plant Physiol. 2013, 162, 1290–1310. [Google Scholar] [CrossRef]
- Kleiner, K.W.; Raffa, K.F.; Dickson, R.E. Partitioning of 14C-labeled photosynthate to allelochemicals and primary metabolites in source and sink leaves of aspen: Evidence for secondary metabolite turnover. Oecologia 1999, 119, 408–418. [Google Scholar] [CrossRef]
- Matsuki, S.; Sano, Y.; Koike, T. Chemical and physical defence in early and late leaves in three heterophyllous birch species native to northern Japan. Ann. bot. 2004, 93, 141–147. [Google Scholar] [CrossRef]
- Matsuda, F.; Hirai, M.Y.; Sasaki, E.; Akiyama, K.; Yonekura-Sakakibara, K.; Provart, N.J.; Sakurai, T.; Shimada, Y.; Saito, K. AtMetExpress development: A phytochemical atlas of Arabidopsis development. Plant Physiol. 2010, 152, 566–578. [Google Scholar] [CrossRef]
- McCall, A.C.; Fordyce, J.A. Can optimal defence theory be used to predict the distribution of plant chemical defences? J. Ecol. 2010, 98, 985–992. [Google Scholar] [CrossRef]
- Legrand, G.; Delporte, M.; Khelifi, C.; Harant, A.; Vuylsteker, C.; Mörchen, M.; Hance, P.; Hilbert, J.-L.; Gagneul, D. Identification and characterization of five BAHD acyltransferases involved in hydroxycinnamoyl ester metabolism in chicory. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Yamawo, A.; Suzuki, N.; Tagawa, J.; Hada, Y. Leaf ageing promotes the shift in defence tactics in Mallotus japonicus from direct to indirect defence. J. Ecol. 2012, 100, 802–809. [Google Scholar] [CrossRef]
- Kaur, H.; Heinzel, N.; Schöttner, M.; Baldwin, I.T.; Gális, I. R2R3-NaMYB8 Regulates the accumulation of phenylpropanoid-polyamine conjugates, which are essential for local and systemic defense against insect herbivores in Nicotiana attenuata. Plant Physiology 2010, 152, 1731–1747. [Google Scholar] [CrossRef] [PubMed]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [PubMed]
- Ncube, B.; Van Staden, J. Tilting plant metabolism for improved metabolite biosynthesis and enhanced human benefit. Molecules 2015, 20, 12698–12731. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.; Eagles, J.E. Translocation of 14carbon within and between leaves. Ann. bot. 1962, 26, 505–510. [Google Scholar] [CrossRef]
- Fellows, R.J.; Geiger, D.R. Structural and physiological changes in sugar beet leaves during sink to source conversion. Plant Physiol. 1974, 54, 877–885. [Google Scholar] [CrossRef]
- Roberts, A.G.; Cruz, S.S.; Roberts, I.M.; Prior, D.; Turgeon, R.; Oparka, K.J. Phloem unloading in sink leaves of Nicotiana benthamiana: Comparison of a fluorescent solute with a fluorescent virus. Plant Cell 1997, 9, 1381–1396. [Google Scholar] [CrossRef]
- Imlau, A.; Truernit, E.; Sauer, N. Cell-to-cell and long-distance trafficking of the green fluorescent protein in the phloem and symplastic unloading of the protein into sink tissues. Plant Cell 1999, 11, 309–322. [Google Scholar] [CrossRef]
- Meng, Q.; Siebke, K.; Lippert, P.; Baur, B.; Mukherjee, U.; Weis, E. Sink–source transition in tobacco leaves visualized using chlorophyll fluorescence imaging. New Phytol. 2001, 151, 585–595. [Google Scholar] [CrossRef]
- Merry, A.M.; Evans, K.J.; Corkrey, R.; Wilson, S.J. Coincidence of maximum severity of powdery mildew on grape leaves and the carbohydrate sink-to-source transition. Plant Pathol. 2013, 62, 842–850. [Google Scholar] [CrossRef]
- Camacho, D.; de la Fuente, A.; Mendes, P. The origin of correlations in metabolomics data. Metabolomics 2005, 1, 53–63. [Google Scholar] [CrossRef]
- Weckwerth, W.; Loureiro, M.E.; Wenzel, K.; Fiehn, O. Differential metabolic networks unravel the effects of silent plant phenotypes. Proc. Natl. Acad. Sci. USA 2004, 101, 7809–7814. [Google Scholar] [CrossRef] [PubMed]
- Guschina, I.A.; Everard, J.D.; Kinney, A.J.; Quant, P.A.; Harwood, J.L. Studies on the regulation of lipid biosynthesis in plants: Application of control analysis to soybean. Biochim. Biophys. Acta 2014, 1838, 1488–1500. [Google Scholar] [CrossRef]
- Voet, D.; Voet, J.G.; Pratt, C.W. Fundamentals of biochemistry: Life at the molecular level, 5th ed.; John Wiley and Sons: Hoboken, New Jersey, USA, 2016; ISBN 978-1-118-91840-1. [Google Scholar]
- Ibrahim, R.K. Chromatographic and spectrophotometric evidence for the occurrence of mixed O- and C-glycoflavones in flax (Linum usitatissimum) cotyledons. Biochim. Biophys. Acta 1969, 192, 549–552. [Google Scholar] [CrossRef]
- Dubois, J.; Mabry, T.J. The C-glycosylflavonoids of flax, Linum usitatissimum. Phytochemistry 1971, 10, 2839–2840. [Google Scholar] [CrossRef]
- Czemplik, M.; Mierziak, J.; Szopa, J.; Kulma, A. Flavonoid C-glucosides derived from flax straw extracts reduce human breast cancer cell growth in vitro and induce apoptosis. Front. Pharmacol. 2016, 7. [Google Scholar] [CrossRef]
- Ito, T.; Fujimoto, S.; Suito, F.; Shimosaka, M.; Taguchi, G. C-Glycosyltransferases catalyzing the formation of di-C-glucosyl flavonoids in citrus plants. Plant J. 2017, 91, 187–198. [Google Scholar] [CrossRef]
- Talhi, O.; Silva, A.M.S. Advances in C-glycosylflavonoid research. Curr. Org. Chem. 2012, 16, 859–896. [Google Scholar]
- Xiao, J.; Capanoglu, E.; Jassbi, A.R.; Miron, A. Advance on the flavonoid C-glycosides and health benefits. Crit Rev Food Sci Nutr 2016, 56(Suppl. 1), S29–S45. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- McNally, D.J.; Wurms, K.V.; Labbé, C.; Quideau, S.; Bélanger, R.R. Complex C-glycosyl flavonoid phytoalexins from Cucumis sativus. J. Nat. Prod. 2003, 66, 1280–1283. [Google Scholar] [CrossRef] [PubMed]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.L.; Kuate, S.P.; Brazier-Hicks, M.; Caulfield, J.C.; Rose, R.; Edwards, R.; Torto, B.; Pickett, J.A.; Hooper, A.M. Elucidation of the biosynthesis of the di-C-glycosylflavone isoschaftoside, an allelopathic component from Desmodium spp. that inhibits Striga spp. development. Phytochemistry 2012, 84, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Schwab, W. Metabolome diversity: Too few genes, too many metabolites? Phytochemistry 2003, 62, 837–849. [Google Scholar] [CrossRef]
- Quéro, A.; Molinié, R.; Elboutachfaiti, R.; Petit, E.; Pau-Roblot, C.; Guillot, X.; Mesnard, F.; Courtois, J. Osmotic stress alters the balance between organic and inorganic solutes in flax (Linum usitatissimum). J. Plant Physiol. 2014, 171, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Quéro, A.; Fliniaux, O.; Elboutachfaiti, R.; Petit, E.; Guillot, X.; Hawkins, S.; Courtois, J.; Mesnard, F. β-Aminobutyric acid increases drought tolerance and reorganizes solute content and water homeostasis in flax (Linum usitatissimum). Metabolomics 2015, 11, 1363–1375. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pontarin, N.; Molinié, R.; Mathiron, D.; Tchoumtchoua, J.; Bassard, S.; Gagneul, D.; Thiombiano, B.; Demailly, H.; Fontaine, J.-X.; Guillot, X.; et al. Age-Dependent Metabolic Profiles Unravel the Metabolic Relationships within and between Flax Leaves (Linum usitatissimum). Metabolites 2020, 10, 218. https://doi.org/10.3390/metabo10060218
Pontarin N, Molinié R, Mathiron D, Tchoumtchoua J, Bassard S, Gagneul D, Thiombiano B, Demailly H, Fontaine J-X, Guillot X, et al. Age-Dependent Metabolic Profiles Unravel the Metabolic Relationships within and between Flax Leaves (Linum usitatissimum). Metabolites. 2020; 10(6):218. https://doi.org/10.3390/metabo10060218
Chicago/Turabian StylePontarin, Nicole, Roland Molinié, David Mathiron, Job Tchoumtchoua, Solène Bassard, David Gagneul, Benjamin Thiombiano, Hervé Demailly, Jean-Xavier Fontaine, Xavier Guillot, and et al. 2020. "Age-Dependent Metabolic Profiles Unravel the Metabolic Relationships within and between Flax Leaves (Linum usitatissimum)" Metabolites 10, no. 6: 218. https://doi.org/10.3390/metabo10060218
APA StylePontarin, N., Molinié, R., Mathiron, D., Tchoumtchoua, J., Bassard, S., Gagneul, D., Thiombiano, B., Demailly, H., Fontaine, J.-X., Guillot, X., Sarazin, V., Quéro, A., & Mesnard, F. (2020). Age-Dependent Metabolic Profiles Unravel the Metabolic Relationships within and between Flax Leaves (Linum usitatissimum). Metabolites, 10(6), 218. https://doi.org/10.3390/metabo10060218





