Serum Metabolomic Alterations Associated with Cesium-137 Internal Emitter Delivered in Various Dose Rates
Abstract
:1. Introduction
2. Results
2.1. 137Cs Dose and DosE-Rate Calculation
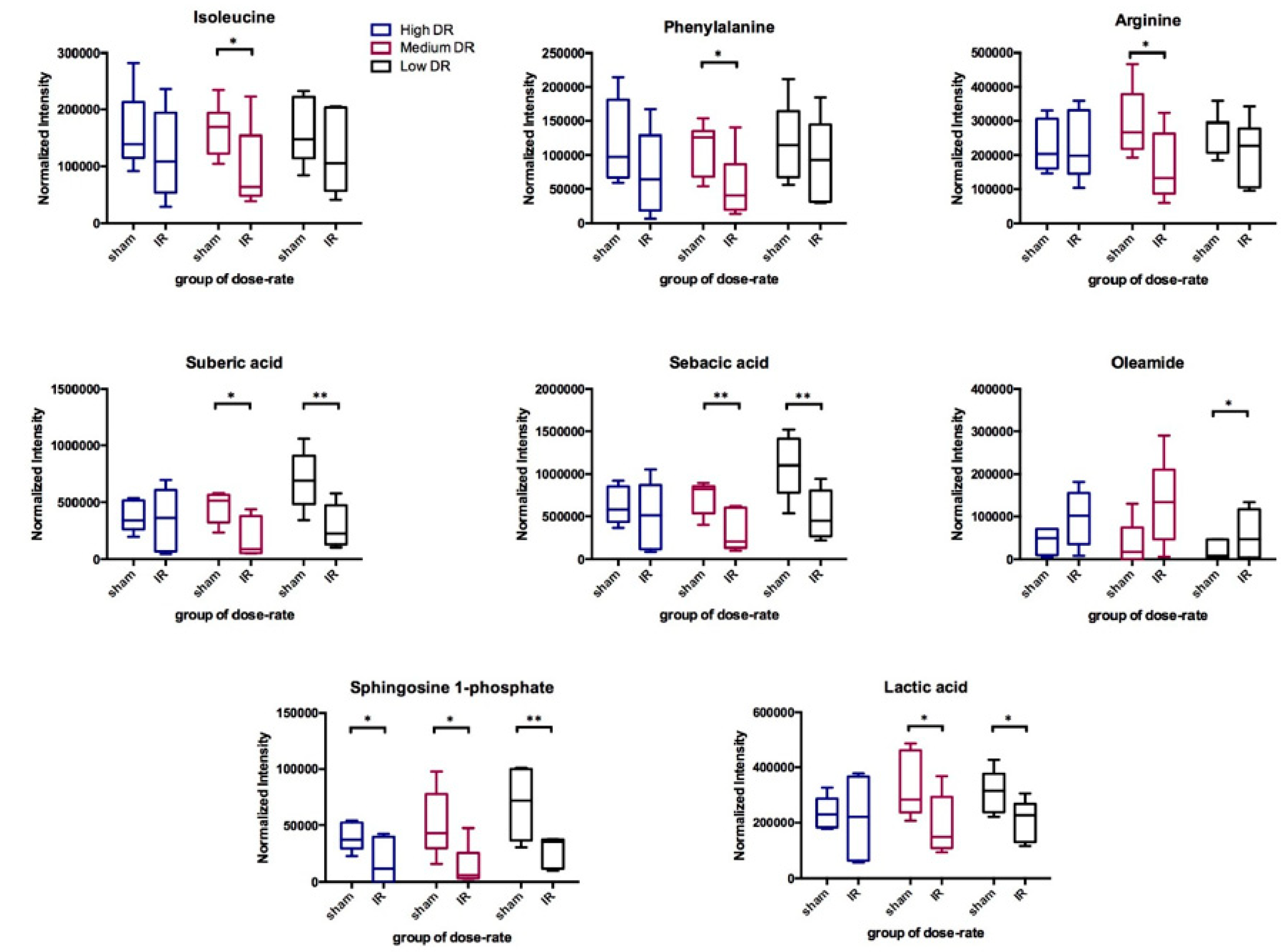
2.2. Determine Differential Ions after Exposure to Internal Emitter
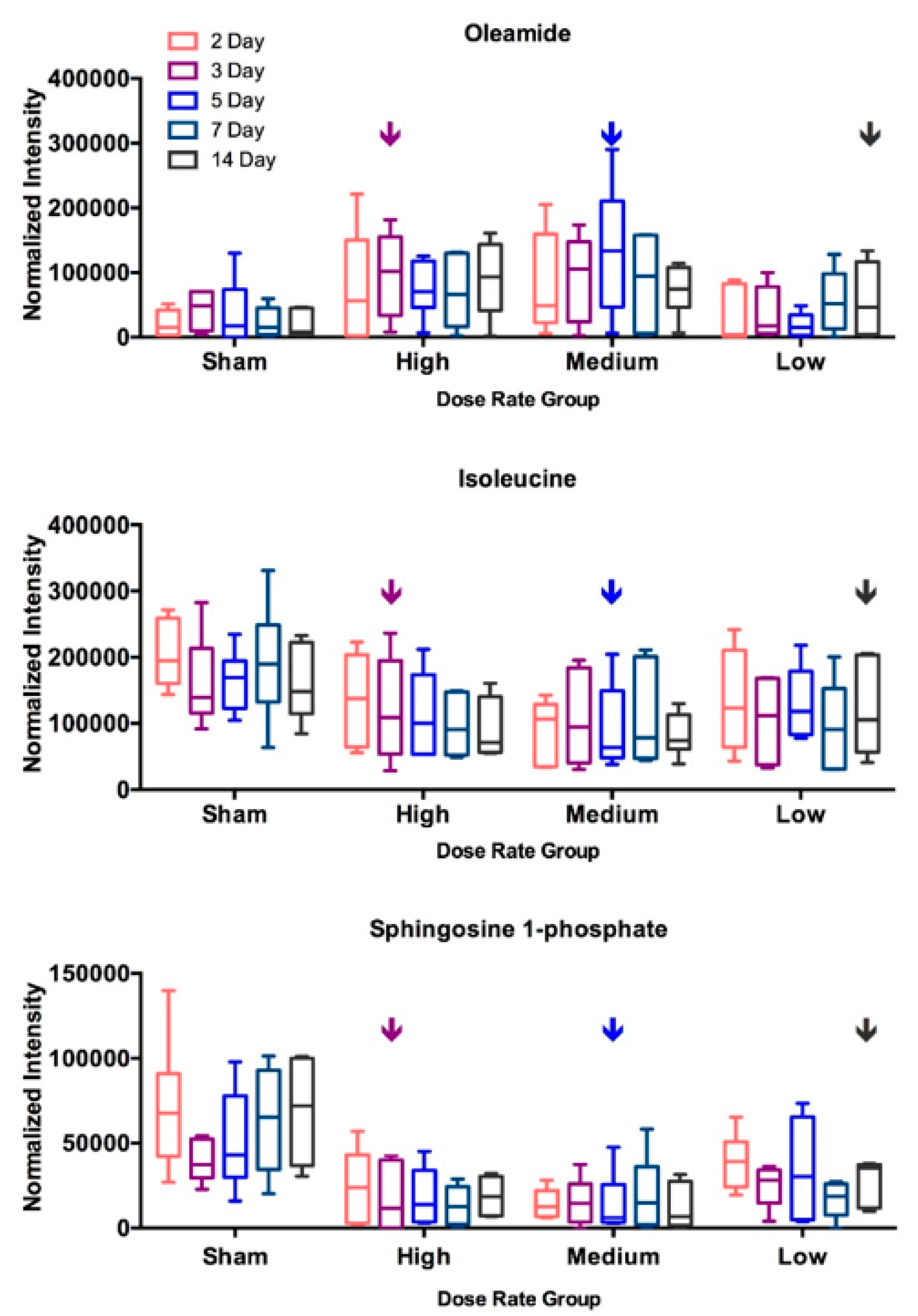
2.3. DosE-Rate Effects on Metabolite Changes
3. Discussion
4. Materials and Methods
4.1. Animal Irradiation and Sample Collection
4.2. Sample Preparation for Mass Spectrometry Analysis
4.3. Mass Spectrometry Analysis
4.4. Data Processing and Multivariate Data Analysis
4.5. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kimura, Y.; Okubo, Y.; Hayashida, N.; Takahashi, J.; Gutevich, A.; Chorniy, S.; Kudo, T.; Takamura, N. Evaluation of the relationship between current internal 137cs exposure in residents and soil contamination west of chernobyl in northern ukraine. PLoS ONE 2015, 10, e0139007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akahane, K. Reconstruction of internal and external exposures of fukushima residents. Radiat. Prot. Dosim. 2016, 171, 3–6. [Google Scholar] [CrossRef]
- Sakamoto-Hojo, E.T. Lessons from the accident with 137cesium in goiania, brazil: Contributions to biological dosimetry in case of human exposure to ionizing radiation. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018, 836, 72–77. [Google Scholar] [CrossRef]
- Jorgensen, T.J. Predicting the public health consequences of a nuclear terrorism attack: Drawing on the experiences of hiroshima and fukushima. Health Phys. 2018, 115, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Pujol-Canadell, M.; Taveras, M.; Garty, G.; Perrier, J.; Bueno-Beti, C.; Shuryak, I.; Brenner, D.J.; Turner, H.C. DNA damage response in peripheral mouse blood leukocytes in vivo after variable, low-dose rate exposure. Radiat. Environ. Biophys. 2020, 59, 89–98. [Google Scholar] [CrossRef]
- Bertho, J.M.; Bô, R.; Magneron, V.; Legendre, A.; Cochard, M.; Broggio, D.; Tack, K. Co-exposure to internal and external radiation alters cesium biokinetics and retention in the mouse. J. Radiol. Prot. 2020, 40, 504. [Google Scholar] [CrossRef] [PubMed]
- Melo, D.R.; Lipsztein, J.L.; de Oliveira, C.A.; Bertelli, L. 137cs internal contamination involving a brazilian accident, and the efficacy of prussian blue treatment. Health Phys. 1994, 66, 245–252. [Google Scholar] [CrossRef]
- Coleman, C.N.; Adams, S.; Adrianopoli, C.; Ansari, A.; Bader, J.L.; Buddemeier, B.; Caro, J.J.; Casagrande, R.; Case, C.; Caspary, K.; et al. Medical planning and response for a nuclear detonation: A practical guide. Biosecur. Bioterror. 2012, 10, 346–371. [Google Scholar] [CrossRef]
- Herate, C.; Sabatier, L. Retrospective biodosimetry techniques: Focus on cytogenetics assays for individuals exposed to ionizing radiation. Mutat. Res. 2020, 783, 108287. [Google Scholar] [CrossRef]
- Paul, S.; Ghandhi, S.A.; Weber, W.; Doyle-Eisele, M.; Melo, D.; Guilmette, R.; Amundson, S.A. Gene expression response of mice after a single dose of 137cs as an internal emitter. Radiat. Res. 2014, 182, 380–389. [Google Scholar] [CrossRef]
- Turner, H.C.; Shuryak, I.; Weber, W.; Doyle-Eisele, M.; Melo, D.; Guilmette, R.; Amundson, S.A.; Brenner, D.J. Γ-h2ax kinetic profile in mouse lymphocytes exposed to the internal emitters cesium-137 and strontium-90. PLoS ONE 2015, 10, e0143815. [Google Scholar] [CrossRef] [PubMed]
- Turner, H.C.; Lee, Y.; Weber, W.; Melo, D.; Kowell, A.; Ghandhi, S.A.; Amundson, S.A.; Brenner, D.J.; Shuryak, I. Effect of dose and dose rate on temporal γ-h2ax kinetics in mouse blood and spleen mononuclear cells in vivo following cesium-137 administration. BMC Mol. Cell Biol. 2019, 20, 13. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.S.; Uppal, M.; Randhawa, S.; Cheema, M.S.; Aghdam, N.; Usala, R.L.; Ghosh, S.P.; Cheema, A.K.; Dritschilo, A. Radiation metabolomics: Current status and future directions. Front. Oncol. 2016, 6, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laiakis, E.C.; Strassburg, K.; Bogumil, R.; Lai, S.; Vreeken, R.J.; Hankemeier, T.; Langridge, J.; Plumb, R.S.; Fornace, A.J.; Astarita, G. Metabolic phenotyping reveals a lipid mediator response to ionizing radiation. J. Proteome Res. 2014, 13, 4143–4154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golla, S.; Golla, J.P.; Krausz, K.W.; Manna, S.K.; Simillion, C.; Beyoğlu, D.; Idle, J.R.; Gonzalez, F.J. Metabolomic analysis of mice exposed to gamma radiation reveals a systemic understanding of total-body exposure. Radiat. Res. 2017, 187, 612–629. [Google Scholar] [CrossRef] [Green Version]
- Pannkuk, E.L.; Laiakis, E.C.; Garcia, M.; Fornace, A.J.; Singh, V.K. Nonhuman primates with acute radiation syndrome: Results from a global serum metabolomics study after 7.2 gy total-body irradiation. Radiat. Res. 2018, 190, 576–583. [Google Scholar] [CrossRef]
- Pannkuk, E.L.; Laiakis, E.C.; Gill, K.; Jain, S.K.; Mehta, K.Y.; Nishita, D.; Bujold, K.; Bakke, J.; Gahagen, J.; Authier, S.; et al. Liquid chromatography-mass spectrometry-based metabolomics of nonhuman primates after 4 gy total body radiation exposure: Global effects and targeted panels. J. Proteome Res. 2019, 18, 2260–2269. [Google Scholar] [CrossRef]
- Laiakis, E.C.; Pannkuk, E.L.; Chauthe, S.K.; Wang, Y.W.; Lian, M.; Mak, T.D.; Barker, C.A.; Astarita, G.; Fornace, A.J. A serum small molecule biosignature of radiation exposure from total body irradiated patients. J. Proteome Res. 2017, 16, 3805–3815. [Google Scholar] [CrossRef]
- Pannkuk, E.L.; Fornace, A.J.; Laiakis, E.C. Metabolomic applications in radiation biodosimetry: Exploring radiation effects through small molecules. Int. J. Radiat. Biol. 2017, 93, 1151–1176. [Google Scholar] [CrossRef]
- Goudarzi, M.; Weber, W.; Mak, T.D.; Chung, J.; Doyle-Eisele, M.; Melo, D.; Brenner, D.J.; Guilmette, R.A.; Fornace, A.J. Development of urinary biomarkers for internal exposure by cesium-137 using a metabolomics approach in mice. Radiat. Res. 2014, 181, 54–64. [Google Scholar] [CrossRef] [Green Version]
- Goudarzi, M.; Weber, W.M.; Mak, T.D.; Chung, J.; Doyle-Eisele, M.; Melo, D.R.; Brenner, D.J.; Guilmette, R.A.; Fornace, A.J. Metabolomic and lipidomic analysis of serum from mice exposed to an internal emitter, cesium-137, using a shotgun lc-ms(e) approach. J. Proteome Res. 2015, 14, 374–384. [Google Scholar] [CrossRef] [Green Version]
- Mos, J.; Olivier, B.; van Oorschot, R.; Dijkstra, H. Different test situations for measuring offensive aggression in male rats do not result in the same wound pattern. Physiol. Behav. 1984, 32, 453–456. [Google Scholar] [CrossRef]
- Tang, F.R.; Loke, W.K.; Khoo, B.C. Low-dose or low-dose-rate ionizing radiation-induced bioeffects in animal models. J. Radiat. Res. 2017, 58, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.R.; Loganovsky, K. Low dose or low dose rate ionizing radiation-induced health effect in the human. J. Environ. Radioact. 2018, 192, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Sukocheva, O.A.; Furuya, H.; Ng, M.L.; Friedemann, M.; Menschikowski, M.; Tarasov, V.V.; Chubarev, V.N.; Klochkov, S.G.; Neganova, M.E.; Mangoni, A.A.; et al. Sphingosine kinase and sphingosine-1-phosphate receptor signaling pathway in inflammatory gastrointestinal disease and cancers: A novel therapeutic target. Pharmacol. Ther. 2020, 207, 107464. [Google Scholar] [CrossRef]
- Czubowicz, K.; Jęśko, H.; Wencel, P.; Lukiw, W.J.; Strosznajder, R.P. The role of ceramide and sphingosine-1-phosphate in alzheimer’s disease and other neurodegenerative disorders. Mol. Neurobiol. 2019, 56, 5436–5455. [Google Scholar] [CrossRef] [Green Version]
- Kurano, M.; Yatomi, Y. Sphingosine 1-phosphate and atherosclerosis. J. Atheroscler. Thromb. 2018, 25, 16–26. [Google Scholar] [CrossRef] [Green Version]
- Kolesnick, R.; Fuks, Z. Radiation and ceramide-induced apoptosis. Oncogene 2003, 22, 5897–5906. [Google Scholar] [CrossRef] [Green Version]
- Bonnaud, S.; Niaudet, C.; Pottier, G.; Gaugler, M.H.; Millour, J.; Barbet, J.; Sabatier, L.; Paris, F. Sphingosine-1-phosphate protects proliferating endothelial cells from ceramide-induced apoptosis but not from DNA damage-induced mitotic death. Cancer Res. 2007, 67, 1803–1811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnaud, S.; Niaudet, C.; Legoux, F.; Corre, I.; Delpon, G.; Saulquin, X.; Fuks, Z.; Gaugler, M.H.; Kolesnick, R.; Paris, F. Sphingosine-1-phosphate activates the akt pathway to protect small intestines from radiation-induced endothelial apoptosis. Cancer Res. 2010, 70, 9905–9915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Oskouian, B.; Fyrst, H.; Zhang, M.; Paris, F.; Saba, J.D. S1p lyase regulates DNA damage responses through a novel sphingolipid feedback mechanism. Cell Death Dis. 2011, 2, e119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, W.; Wu, X.; Goudarzi, M.; Shi, J.; Song, W.; Li, C.; Liu, J.; Chen, H.; Zhang, X.; Zeng, X.; et al. Metabolomic alterations associated with behçet’s disease. Arthritis Res. Ther. 2018, 20, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mak, T.D.; Laiakis, E.C.; Goudarzi, M.; Fornace, A.J. Metabolyzer: A novel statistical workflow for analyzing postprocessed lc-ms metabolomics data. Anal. Chem. 2014, 86, 506–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Dose Rate Group | Time Point (d) | WholE-body Committed Dose (Gy) | Dose Rates (Gy/day) | Number of Samples |
|---|---|---|---|---|
| Sham | 2 | 0.00 | 0.00 | 6 |
| 3 | 0.00 | 0.00 | 6 | |
| 5 | 0.00 | 0.00 | 6 | |
| 7 | 0.00 | 0.00 | 6 | |
| 14 | 0.00 | 0.00 | 8 | |
| Low Dose Rate | 2 | 1.31 ± 0.36 | 0.61 ± 0.17 | 6 |
| 3 | 1.65 ± 0.07 | 0.47 ± 0.02 | 6 | |
| 5 | 2.42 ± 0.14 | 0.39 ± 0.03 | 6 | |
| 7 | 2.97 ± 0.31 | 0.3 ± 0.04 | 6 | |
| 14 | 4.3 ± 0.84 | 0.16 ± 0.05 | 7 | |
| Medium Dose Rate | 2 | 2.16 ± 0.17 | 0.97 ± 0.08 | 5 |
| 3 | 2.76 ± 0.45 | 0.78 ± 0.11 | 6 | |
| 5 | 4.44 ± 0.41 | 0.69 ± 0.07 | 6 | |
| 7 | 5.68 ± 0.24 | 0.58 ± 0.03 | 6 | |
| 14 | 6.81 ± 1.84 | 0.24 ± 0.11 | 6 | |
| High Dose Rate | 2 | 2.98 ± 0.14 | 1.36 ± 0.07 | 6 |
| 3 | 4.41 ± 0.32 | 1.25 ± 0.08 | 6 | |
| 5 | 5.98 ± 0.32 | 0.94 ± 0.05 | 6 | |
| 7 | 8.37 ± 1.05 | 0.85 ± 0.08 | 6 | |
| 14 | 12.31 ± 1.86 | 0.85 ± 0.08 | 6 |
| Dose Rate | Low (0.16 Gy/day) | Medium (0.69 Gy/day) | High (1.25 Gy/day) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 Day | 3 Day | 5 Day | 7 Day | 14 Day | 2 Day | 3 Day | 5 Day | 7 Day | 14 Day | 2 Day | 3 Day | 5 Day | 7 Day | 14 Day | |
| Suberic Acid | * | * | * | * | * | ||||||||||
| Sebacic Acid | * | ** | * | * | |||||||||||
| Lactic Acid | * | * | * | * | * | ** | * | ||||||||
| L-Isoleucine | * | * | * | ||||||||||||
| L-Phenylalanine | * | * | |||||||||||||
| L-Arginine | * | * | * | * | * | ||||||||||
| Oleamide | * | * | * | ||||||||||||
| Sphingosine 1-phosphate | * | ** | ** | * | * | ** | ** | * | * | * | ** | ||||
| Uric Acid | ** | * | * | * | |||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.-H.; Lin, Y.-T.; Laiakis, E.C.; Goudarzi, M.; Weber, W.; Fornace, A.J., Jr. Serum Metabolomic Alterations Associated with Cesium-137 Internal Emitter Delivered in Various Dose Rates. Metabolites 2020, 10, 270. https://doi.org/10.3390/metabo10070270
Li H-H, Lin Y-T, Laiakis EC, Goudarzi M, Weber W, Fornace AJ Jr. Serum Metabolomic Alterations Associated with Cesium-137 Internal Emitter Delivered in Various Dose Rates. Metabolites. 2020; 10(7):270. https://doi.org/10.3390/metabo10070270
Chicago/Turabian StyleLi, Heng-Hong, Yun-Tien Lin, Evagelia C. Laiakis, Maryam Goudarzi, Waylon Weber, and Albert J. Fornace, Jr. 2020. "Serum Metabolomic Alterations Associated with Cesium-137 Internal Emitter Delivered in Various Dose Rates" Metabolites 10, no. 7: 270. https://doi.org/10.3390/metabo10070270
APA StyleLi, H.-H., Lin, Y.-T., Laiakis, E. C., Goudarzi, M., Weber, W., & Fornace, A. J., Jr. (2020). Serum Metabolomic Alterations Associated with Cesium-137 Internal Emitter Delivered in Various Dose Rates. Metabolites, 10(7), 270. https://doi.org/10.3390/metabo10070270







