Branched-Chain Amino Acids and Branched-Chain Keto Acids in Hyperammonemic States: Metabolism and as Supplements
Abstract
:1. Introduction
2. Amination of BCKAs to BCAAs under Physiological Conditions
3. BCAA Synthesis from BCKAs under Hyperammonemic Conditions
4. BCAA Synthesis from Exogenous BCKAs
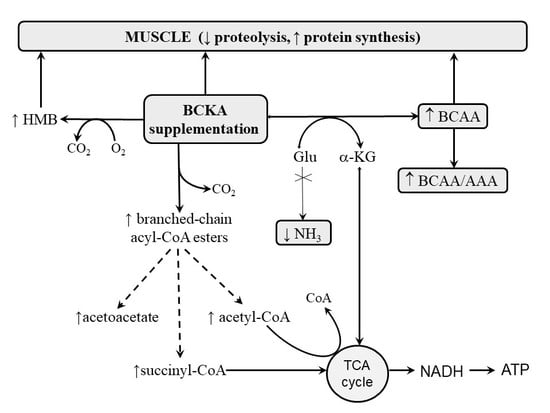
5. Benefits of BCKA-Containing Supplements in Hyperammonemic States
- Decreased ammonia production;
- The attenuation of cataplerosis;
- A supply of anaplerotic agents;
- The correction of amino acid imbalance;
- Nitrogen sparing and protein anabolism.
5.1. Decreased Ammonia Production
5.2. Attenuation of Cataplerosis
5.3. Supply of Anaplerotic Agents
5.4. Correction of Amino Acid Imbalance
5.5. Nitrogen Sparing and Protein Anabolism
6. Effects of BCKAs in Subjects with Liver Disease
7. Effects of KAEAA on UCDs
8. Effects of BCKAs and KAEAAs in Exercise
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Banister, E.W.; Cameron, B.J. Exercise-induced hyperammonemia: Peripheral and central effects. Int. J. Sports Med. 1990, 11, S129–S142. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.J.; Smeeton, N.J.; Watt, P.W. Ammonia metabolism, the brain and fatigue; revisiting the link. Prog. Neurobiol. 2010, 91, 200–219. [Google Scholar] [CrossRef] [PubMed]
- Graham, T.E.; MacLean, D.A. Ammonia and amino acid metabolism in human skeletal muscle during exercise. Can. J. Physiol. Pharmacol. 1992, 70, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Leweling, H.; Breitkreutz, R.; Behne, F.; Staedt, U.; Striebel, J.P.; Holm, E. Hyperammonemia-induced depletion of glutamate and branched-chain amino acids in muscle and plasma. J. Hepatol. 1996, 25, 756–762. [Google Scholar] [CrossRef]
- Holeček, M.; Šprongl, L.; Tichý, M. Effect of hyperammonemia on leucine and protein metabolism in rats. Metabolism 2000, 49, 1330–1334. [Google Scholar] [CrossRef]
- Holecek, M.; Kandar, R.; Sispera, L.; Kovarik, M. Acute hyperammonemia activates branched-chain amino acid catabolism and decreases their extracellular concentrations: Different sensitivity of red and white muscle. Amino Acids 2011, 40, 575–584. [Google Scholar] [CrossRef]
- Holeček, M.; Vodeničarovová, M. Effects of branched-chain amino acids on muscles under hyperammonemic conditions. J. Physiol. Biochem. 2018, 74, 523–530. [Google Scholar] [CrossRef]
- Fischer, J.E.; Baldessarini, R.J. False neurotransmitters and hepatic failure. Lancet 1971, 298, 75–80. [Google Scholar] [CrossRef]
- Herlong, H.F.; Maddrey, W.C.; Walser, M. The use of ornithine salts of branched-chain ketoacids in portal-systemic encephalopathy. Ann. Intern. Med. 1980, 93, 545–550. [Google Scholar] [CrossRef]
- Holeček, M.; Mráz, J.; Tilšer, I. Plasma amino acids in four models of experimental liver injury in rats. Amino Acids 1996, 10, 229–241. [Google Scholar] [CrossRef]
- Holeček, M.; Vodeničarovová, M. Muscle wasting and branched-chain amino acid, alpha-ketoglutarate, and ATP depletion in a rat model of liver cirrhosis. Int. J. Exp. Pathol. 2018, 99, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Batshaw, M.L.; Brusilow, S.; Walser, M. Long-term management of a case of carbamyl phosphate synthetase deficiency using ketanalogues and hydroxyanalogues of essential amino acids. Pediatrics 1976, 58, 227–235. [Google Scholar] [PubMed]
- Rodney, S.; Boneh, A. Amino acid profiles in patients with urea cycle disorders at admission to hospital due to metabolic decompensation. JIMD Rep. 2013, 9, 97–104. [Google Scholar] [PubMed] [Green Version]
- Als-Nielsen, B.; Koretz, R.L.; Kjaergard, L.L.; Gluud, C. Branched-chain amino acids for hepatic encephalopathy. Cochrane Database Syst. Rev. 2003. [Google Scholar] [CrossRef]
- Gluud, L.L.; Dam, G.; Les, I.; Córdoba, J.; Marchesini, G.; Borre, M.; Aagaard, N.K.; Vilstrup, H. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Wolfe, R.R.; Goodenough, R.D.; Wolfe, M.H.; Royle, G.T.; Nadel, E.R. Isotopic analysis of leucine and urea metabolism in exercising humans. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1982, 52, 458–466. [Google Scholar] [CrossRef]
- Knapik, J.; Meredith, C.; Jones, B.; Fielding, R.; Young, V.; Evans, W. Leucine metabolism during fasting and exercise. J. Appl. Physiol. (1985) 1991, 70, 43–47. [Google Scholar] [CrossRef]
- Bassini, A.; Magalhães-Neto, A.M.; Sweet, E.; Bottino, A.; Veiga, C.; Tozzi, M.B.; Pickard, M.B.; Cameron, L.-C. Caffeine decreases systemic urea in elite soccer players during intermittent exercise. Med. Sci. Sports Exerc. 2013, 45, 683–690. [Google Scholar] [CrossRef]
- De Palo, E.F.; Gatti, R.; Bigon, L.; Previti, O.; De Palo, C.B. Branched-chainα-amino acid chronic treatment: Responses of plasma α-keto-related compounds and ammonia when used in physical exercise performance. Amino Acids 1996, 10, 317–332. [Google Scholar] [CrossRef]
- Madsen, K.; MacLean, D.A.; Kiens, B.; Christensen, D. Effects of glucose, glucose plus branched-chain amino acids, or placebo on bike performance over 100 km. J. Appl. Physiol. (1985) 1996, 81, 2644–2650. [Google Scholar] [CrossRef] [Green Version]
- MacLean, D.A.; Graham, T.E.; Saltin, B. Stimulation of muscle ammonia production during exercise following branched-chain amino acid supplementation in humans. J. Physiol. 1996, 493, 909–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, P.; Shirreffs, S.M.; Maughan, R.J. The effect of acute branched-chain amino acid supplementation on prolonged exercise capacity in a warm environment. Eur. J. Appl. Physiol. 2004, 93, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Falavigna, G.; de Araújo, A.J.; Rogero, M.M.; Pires, I.S.; Pedrosa, R.G.; Martins, E.; de Castro, I.A.; Tirapegui, J. Effects of diets supplemented with branched-chain amino acids on the performance and fatigue mechanisms of rats submitted to prolonged physical exercise. Nutrients 2012, 4, 1767–1780. [Google Scholar] [CrossRef] [Green Version]
- Wagenmakers, A.J.; Coakley, J.H.; Edwards, R.H. Metabolism of branched-chain amino acids and ammonia during exercise: Clues from McArdle’s disease. Int. J. Sports Med. 1990, 11, S101–S113. [Google Scholar] [CrossRef] [PubMed]
- Holecek, M. Evidence of a vicious cycle in glutamine synthesis and breakdown in pathogenesis of hepatic encephalopathy-therapeutic perspectives. Metab. Brain Dis. 2014, 29, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holeček, M. Branched-chain amino acid supplementation in treatment of liver cirrhosis: Updated views on how to attenuate their harmful effects on cataplerosis and ammonia formation. Nutrition 2017, 41, 80–85. [Google Scholar] [CrossRef]
- Watanabe, A.; Shiota, T.; Okita, M.; Nagashima, H. Effect of a branched chain amino acid-enriched nutritional product on the pathophysiology of the liver and nutritional state of patients with liver cirrhosis. Acta Med. Okayama 1983, 37, 321–333. [Google Scholar]
- Nishikawa, Y.; Ukida, M.; Matsuo, R.; Morimoto, Y.; Omori, N.; Mikami, M.; Tsuji, T. Administration of a branched-chain amino acid preparation during hepatic failure: A study emphasizing ammonia metabolism. Acta Med. Okayama 1994, 48, 25–30. [Google Scholar]
- Dam, G.; Keiding, S.; Munk, O.L.; Ott, P.; Buhl, M.; Vilstrup, H.; Bak, L.K.; Waagepetersen, H.S.; Schousboe, A.; Møller, N.; et al. Branched-chain amino acids increase arterial blood ammonia in spite of enhanced intrinsic muscle ammonia metabolism in patients with cirrhosis and healthy subjects. Am. J. Physiol. 2011, 301, G269–G277. [Google Scholar] [CrossRef] [Green Version]
- Meyer, H.P.; Chamuleau, R.A.; Legemate, D.A.; Mol, J.A.; Rothuizen, J. Effects of a branched-chain amino acid-enriched diet on chronic hepatic encephalopathy in dogs. Metab. Brain Dis. 1999, 14, 103–115. [Google Scholar] [CrossRef]
- Jungers, P.; Chauveau, P. Amino acids and keto acids in the treatment of chronic renal failure. Blood Purif. 1988, 6, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Walser, M.; Mitch, W.E.; Abras, E. Supplements containing amino acids and keto acids in the treatment of chronic uremia. Kidney Int. Suppl. 1983, 16, S285–S289. [Google Scholar]
- Teplan, V.; Schück, O.; Horácková, M.; Skibová, J.; Holecek, M. Effect of a keto acid-amino acid supplement on the metabolism and renal elimination of branched-chain amino acids in patients with chronic renal insufficiency on a low protein diet. Wien. Klin. Wochenschr. 2000, 112, 876–881. [Google Scholar]
- Harper, A.E.; Miller, R.H.; Block, K.P. Branched-chain amino acid metabolism. Annu. Rev. Nutr. 1984, 4, 409–454. [Google Scholar] [CrossRef] [PubMed]
- Imura, K.; Shiota, T.; Swain, L.M.; Walser, M. Utilization for protein synthesis of 2-ketoisocaproate relative to utilization of leucine, as estimated from exhalation of labelled CO2. Clin. Sci. (Lond.) 1988, 75, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Matthews, D.E.; Walser, M. Nitrogen sparing by 2-ketoisocaproate in parenterally fed rats. Am. J. Physiol. 1990, 259, E633–E638. [Google Scholar] [CrossRef]
- Holecek, M.; Sprongl, L.; Tilser, I. Metabolism of branched-chain amino acids in starved rats: The role of hepatic tissue. Physiol. Res. 2001, 50, 25–33. [Google Scholar] [PubMed]
- Holecek, M. The BCAA-BCKA cycle: Its relation to alanine and glutamine synthesis and protein balance. Nutrition 2001, 17, 70. [Google Scholar] [CrossRef]
- Holecek, M.; Rysava, R.; Safranek, R.; Kadlcikova, J.; Sprongl, L. Acute effects of decreased glutamine supply on protein and amino acid metabolism in hepatic tissue: A study using isolated perfused rat liver. Metabolism 2003, 52, 1062–1067. [Google Scholar] [CrossRef]
- Muñoz, S.; Walser, M. Effect of experimental liver disease on the utilization for protein synthesis of orally administered alpha-ketoisocaproate. Hepatology 1986, 6, 472–476. [Google Scholar] [CrossRef]
- Walser, M.; Lund, P.; Ruderman, N.B.; Coulter, A.W. Synthesis of essential amino acids from their alpha-keto analogues by perfused rat liver and muscle. J. Clin. Invest. 1973, 52, 2865–2877. [Google Scholar] [CrossRef] [PubMed]
- Brand, K. Metabolism of 2-oxoacid analogues of leucine, valine and phenylalanine by heart muscle, brain and kidney of the rat. Biochim. Biophys. Acta 1981, 677, 126–132. [Google Scholar] [CrossRef]
- Holecek, M.; Sprongl, L.; Tichy, M.; Pecka, M. Leucine metabolism in rat liver after a bolus injection of endotoxin. Metabolism 1998, 47, 681–685. [Google Scholar] [CrossRef]
- Holeček, M.; Muthný, T.; Kovařík, M.; Šišpera, L. Simultaneous infusion of glutamine and branched-chain amino acids (BCAA) to septic rats does not have more favorable effect on protein synthesis in muscle, liver, and small intestine than separate infusions. JPEN J. Parenter. Enter. Nutr. 2006, 30, 467–473. [Google Scholar] [CrossRef]
- Abumrad, N.N.; Wise, K.L.; Williams, P.E.; Abumrad, N.A.; Lacy, W.W. Disposal of alpha-ketoisocaproate: Roles of liver, gut, and kidneys. Am. J. Physiol. 1982, 243, E123–E131. [Google Scholar] [CrossRef]
- Khatra, B.S.; Chawla, R.K.; Sewell, C.W.; Rudman, D. Distribution of branched-chain alpha-keto acid dehydrogenases in primate tissues. J. Clin. Investig. 1977, 59, 558–564. [Google Scholar] [CrossRef]
- Okita, M.; Watanabe, A.; Takei, N.; Nagashima, H.; Ubuka, T. Effects of branched-chain alpha-keto acids on plasma amino acid concentrations in carbon tetrachloride-intoxicated rats. J. Nutr. 1984, 114, 1235–1241. [Google Scholar] [CrossRef]
- Schauder, P. Pharmacokinetic and metabolic interrelationships among branched-chain keto and amino acids in humans. J. Lab. Clin. Med. 1985, 106, 701–707. [Google Scholar]
- Mitch, W.E.; Walser, M.; Sapir, D.G. Nitrogen sparing induced by leucine compared with that induced by its keto analogue, alpha-ketoisocaproate, in fasting obese man. J. Clin. Investig. 1981, 67, 553–562. [Google Scholar] [CrossRef]
- Tischler, M.E.; Desautels, M.; Goldberg, A.L. Does leucine, leucyl-tRNA, or some metabolite of leucine regulate protein synthesis and degradation in skeletal and cardiac muscle? J. Biol. Chem. 1982, 257, 1613–1621. [Google Scholar]
- Stewart, P.M.; Walser, M.; Drachman, D.B. Branched-chain ketoacids reduce muscle protein degradation in Duchenne muscular dystrophy. Muscle Nerve 1982, 5, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Sapir, D.G.; Stewart, P.M.; Walser, M.; Moreadith, C.; Moyer, E.D.; Imbembo, A.L.; Rosenshein, N.B.; Munoz, S. Effects of alpha-ketoisocaproate and of leucine on nitrogen metabolism in postoperative patients. Lancet 1983, 1, 1010–1014. [Google Scholar] [CrossRef]
- Sherwin, R.S. The effect of ketone bodies and dietary carbohydrate intake on protein metabolism. Acta Chir. Scand. Suppl. 1981, 507, 30–40. [Google Scholar] [PubMed]
- Holeček, M. Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. J. Cachexia Sarcopenia Muscle 2017, 8, 529–541. [Google Scholar] [CrossRef]
- Maddrey, W.C.; Weber, F.L., Jr.; Coulter, A.W.; Chura, C.M.; Chapanis, N.P.; Walser, M. Effects of keto analogues of essential amino acids in portal-systemic encephalopathy. Gastroenterology 1976, 71, 190–195. [Google Scholar] [CrossRef]
- Eriksson, L.S.; Hagenfeldt, L.; Wahren, J. Intravenous infusion of alpha-oxoisocaproate: Influence on amino acid and nitrogen metabolism in patients with liver cirrhosis. Clin. Sci. (Lond.) 1982, 62, 285–293. [Google Scholar] [CrossRef]
- Walker, S.; Götz, R.; Czygan, P.; Stiehl, A.; Lanzinger, G.; Sieg, A.; Raedsch, R.; Kommerell, B. Oral keto analogs of branched-chain amino acids in hyperammonemia in patients with cirrhosis of the liver. A double-blind crossover study. Digestion 1982, 24, 105–111. [Google Scholar] [CrossRef]
- Holecek, M.; Siman, P.; Vodenicarovova, M.; Kandar, R. Alterations in protein and amino acid metabolism in rats fed a branched-chain amino acid- or leucine-enriched diet during postprandial and postabsorptive states. Nutr. Metab. (Lond.) 2016, 13, 12. [Google Scholar] [CrossRef] [Green Version]
- Scaglia, F.; Carter, S.; O’Brien, W.E.; Lee, B. Effect of alternative pathway therapy on branched chain amino acid metabolism in urea cycle disorder patients. Mol. Genet. Metab. 2004, 81, S79–S85. [Google Scholar] [CrossRef]
- Brunetti-Pierri, N.; Lanpher, B.; Erez, A.; Ananieva, E.A.; Islam, M.; Marini, J.C.; Sun, Q.; Yu, C.; Hegde, M.; Li, J.; et al. Phenylbutyrate therapy for maple syrup urine disease. Hum. Mol. Genet. 2011, 20, 631–640. [Google Scholar] [CrossRef] [Green Version]
- Holecek, M.; Vodenicarovova, M. Phenylbutyrate exerts adverse effects on liver regeneration and amino acid concentrations in partially hepatectomized rats. Int. J. Exp. Pathol. 2016, 97, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Batshaw, M.; Brusilow, S.; Walser, M. Treatment of carbamyl phosphate synthetase deficiency with keto analogues of essential amino acids. N. Engl. J. Med. 1975, 292, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Thoene, J.; Batshaw, M.; Spector, E.; Kulovich, S.; Brusilow, S.; Walser, M.; Nyhan, W. Neonatal citrllinemia: Treatment with keto-analogues of essential amino acids. J. Pediatr. 1977, 90, 218–224. [Google Scholar] [CrossRef]
- Walser, M.; Batshaw, M.; Sherwood, G.; Robinson, B.; Brusilow, S. Nitrogen metabolism in neonatal citrullinaemia. Clin. Sci. Mol. Med. 1977, 53, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Glasgow, A.M.; Kraegel, J.H.; Schulman, J.D. Studies of the cause and treatment of hyperammonemia in females with ornithine transcarbamylase deficiency. Pediatrics 1978, 62, 30–37. [Google Scholar]
- McReynolds, J.W.; Mantagos, S.; Brusilow, S.; Rosenberg, L.E. Treatment of complete ornithine transcarbamylase deficiency with nitrogen-free analogues of essential amino acids. J. Pediatr. 1978, 93, 421–427. [Google Scholar] [CrossRef]
- Mero, A. Leucine supplementation and intensive training. Sports Med. 1999, 27, 347–358. [Google Scholar] [CrossRef]
- Graham, T.E.; Bangsbo, J.; Gollnick, P.D.; Juel, C.; Saltin, B. Ammonia metabolism during intense dynamic exercise and recovery in humans. Am. J. Physiol. 1990, 259, E170–E176. [Google Scholar] [CrossRef]
- Hellsten, Y. The effect of muscle contraction on the regulation of adenosine formation in rat skeletal muscle cells. J. Physiol. 1999, 518, 761–768. [Google Scholar] [CrossRef]
- Shimomura, Y.; Murakami, T.; Nakai, N.; Nagasaki, M.; Harris, R.A. Exercise promotes BCAA catabolism: Effects of BCAA supplementation on skeletal muscle during exercise. J. Nutr. 2004, 134, S1583–S1587. [Google Scholar] [CrossRef] [Green Version]
- Fouré, A.; Bendahan, D. Is branched-chain amino acids supplementation an efficient nutritional strategy to alleviate skeletal muscle damage? A systematic review. Nutrients 2017, 9, 10. [Google Scholar]
- Greer, B.K.; White, J.P.; Arguello, E.M.; Haymes, E.M. Branched-chain amino acid supplementation lowers perceived exertion but does not affect performance in untrained males. J. Strength Cond. Res. 2011, 25, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Negro, M.; Giardina, S.; Marzani, B.; Marzatico, F. Branched-chain amino acid supplementation does not enhance athletic performance but affects muscle recovery and the immune system. J. Sports Med. Phys. Fitness 2008, 48, 347–351. [Google Scholar] [PubMed]
- Kephart, W.C.; Mumford, P.W.; McCloskey, A.E.; Holland, A.M.; Shake, J.J.; Mobley, C.B.; Jagodinsky, A.E.; Weimar, W.H.; Oliver, G.D.; Young, K.C.; et al. Post-exercise branched chain amino acid supplementation does not affect recovery markers following three consecutive high intensity resistance training bouts compared to carbohydrate supplementation. J. Int. Soc. Sports Nutr. 2016, 13, 30. [Google Scholar] [CrossRef] [Green Version]
- de Almeida, R.D.; Prado, E.S.; Llosa, C.D.; Magalhães-Neto, A.; Cameron, L.C. Acute supplementation with keto analogues and amino acids in rats during resistance exercise. Br. J. Nutr. 2010, 104, 1438–1442. [Google Scholar] [CrossRef] [Green Version]
- Prado, E.S.; de Rezende Neto, J.M.; de Almeida, R.D.; Dória de Melo, M.G.; Cameron, L.C. Keto analogue and amino acid supplementation affects the ammonaemia response during exercise under ketogenic conditions. Br. J. Nutr. 2011, 105, 1729–1733. [Google Scholar] [CrossRef]
- Liu, Y.; Lange, R.; Langanky, J.; Hamma, T.; Yang, B.; Steinacker, J.M. Improved training tolerance by supplementation with α-Keto acids in untrained young adults: A randomized, double blind, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2012, 9, 37. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Spreng, T.; Lehr, M.; Yang, B.; Karau, A.; Gebhardt, H.; Steinacker, J.M. The supportive effect of supplementation with α-keto acids on physical training in type 2 diabetes mellitus. Food Funct. 2015, 6, 2224–2230. [Google Scholar] [CrossRef]
- Camerino, S.R.; Lima, R.C.; França, T.C.; Herculano Ede, A.; Rodrigues, D.S.; Gouveia, M.G.; Cameron, L.C.; Prado, E.S. Keto analogue and amino acid supplementation and its effects on ammonemia and performance under thermoneutral conditions. Food Funct. 2016, 7, 872–880. [Google Scholar] [CrossRef]
- Lima, R.C.P.; Camerino, S.R.A.S.; França, T.C.L.; Rodrigues, D.S.A.; Gouveia, M.G.S.; Ximenes-da-Silva, A.; Bassini, A.; Prado, E.S.; Cameron, L.C. Keto analogues and amino acids supplementation induces a decrease of white blood cell counts and a reduction of muscle damage during intense exercise under thermoneutral conditions. Food Funct. 2017, 8, 1519–1525. [Google Scholar] [CrossRef]



| Study Design | Results | Reference |
|---|---|---|
| Patients with cirrhosis and PSE; AA + KAEAA, infusion (1–5 days) or orally (3–12 days). | ↑ BCAA, methionine and phenylalanine, ↓ glutamine. No effect on ammonia levels. Improvement in mental status and psychological testing. | [55] |
| Patients with cirrhosis and PSE; BCAA, ornithine, or calcium salts of BCKA orally for 7–10 days; double-blind crossover comparison. | Combination of ornithine and BCKA improved EEG and clinical signs of PSE more than BCAA or components given separately. | [9] |
| Patients with cirrhosis and healthy controls; KIC infusion (300 µmol/min for 150 min). | ↑ leucine and ammonia; ↓ urea, valine, isoleucine, methionine, phenylalanine, and GLN. | [56] |
| Patients with cirrhosis and PSE; lactulose and protein restriction; BCKA (15 g/day) or placebo for 4 weeks in a crossover regimen. | No effect on ammonia and BCAA levels, EEG, number connection test, and clinical state. | [57] * |
| Rats, acute liver injury (CCl4); BCKA (sodium or ornithine salts); infusion (60 min) or intragastric administration. | Higher BCAA levels after BCKA in CCl4-treated animals than in controls after infusion. Only slight increases in BCAAs after gavage of BCKAs. | [47] |
| Study Design | Results | Reference |
|---|---|---|
| Carbamoyl phosphate synthetase deficiency; KAEAAs administered by infusion or orally. | Infusion: ↓ ammonia and ↑ BCAAs, methionine, and phenylalanine. Oral intake: ↓ ammonia and alanine. | [62] |
| Carbamoyl phosphate synthetase deficiency; AA + KAEAAs orally for one year. | ↓ ammonia and ↑ BCAAs, methionine, and phenylalanine; improved clinical status. ↑ ammonia, GLN, and alanine after withdrawal. | [12] |
| Citrullinemia; EAA + KAEAAs orally for 8 months. | ↓ ammonia and citrulline until death due to diarrhea and dehydration. | [63] |
| Citrullinemia; AA + KAEAAs orally for 7 months. | ↓ ammonia until death due to acute hyperammonemic crisis. | [64] |
| Ornithine transcarbamylase deficiency; compared effects of LPD + KAEAAs, LPD + EAAs, and LPD + lactulose. | LPD + KAEAAs better than LPD + EAAs or LPD + lactulose. | [65] |
| Ornithine transcarbamylase deficiency; KAEAAs orally. | Ammonia, growth, and development maintained near normal from 2nd day until death at 5 months. | [66] |
| Study Design | Results | Reference |
|---|---|---|
| Male patients with McArdle’s disease; BCAAs or BCKAs prior to start cycling exercise. | After BCAAs: deterioration of exercise performance and ↑ in ammonia. After BCKAs: improved exercise performance and smaller ↑ in ammonia. | [24] |
| Rats; AA+KAEAAs (0.3 g/kg) or saline orally 1 h before exercise. | Attenuated increase in ammonia; ↓ urea. | [75] |
| Cyclists; ketogenic diet for 2 days before experiment, AA+KAEAAs or lactose orally 1 h before cycling (2 h). | Attenuated increase in ammonia induced by exercise. | [76] |
| Male untrained volunteers; α-KG or BCKAs (0.2 g/kg/d) for 4 weeks during endurance training (running). | α-KG or BCKAs improved training effects and recovery state. | [77] |
| Patients with type 2 diabetes; training on cycle ergometer and mixture of α-KG and BCKAs (0.2 g/kg orally) for 6 weeks or placebo (glucose, sodium and calcium salts). | Positive effects on physical training (higher VO2max, endurance capacity, and power output). | [78] |
| Cyclists; ketogenic diet for 2 days before experiment, AA+KAEAAs or lactose, cycling session (2 h) followed by a maximum test. | ↑ (~70%) ammonia in placebo, not in AA+KAEAAs group. No difference in physical or cognitive performance. | [79] |
| Cyclists, ketogenic diet for 2 days before experiment, AA+KAEAAs or lactose orally 1 h before cycling (2 h). | ↑ ammonia, creatine kinase, lactate dehydrogenase, and AST in placebo group. No significant changes in AA+KAAAs group. | [80] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holeček, M. Branched-Chain Amino Acids and Branched-Chain Keto Acids in Hyperammonemic States: Metabolism and as Supplements. Metabolites 2020, 10, 324. https://doi.org/10.3390/metabo10080324
Holeček M. Branched-Chain Amino Acids and Branched-Chain Keto Acids in Hyperammonemic States: Metabolism and as Supplements. Metabolites. 2020; 10(8):324. https://doi.org/10.3390/metabo10080324
Chicago/Turabian StyleHoleček, Milan. 2020. "Branched-Chain Amino Acids and Branched-Chain Keto Acids in Hyperammonemic States: Metabolism and as Supplements" Metabolites 10, no. 8: 324. https://doi.org/10.3390/metabo10080324
APA StyleHoleček, M. (2020). Branched-Chain Amino Acids and Branched-Chain Keto Acids in Hyperammonemic States: Metabolism and as Supplements. Metabolites, 10(8), 324. https://doi.org/10.3390/metabo10080324