Metabolomics Comparison of Hanwoo (Bos taurus coreanae) Biofluids Using Proton Nuclear Magnetic Resonance Spectroscopy
Abstract
:1. Introduction
2. Results
2.1. Rumen Fluid Metabolites
2.2. Serum Metabolites
2.3. Urine Metabolites and Commonly Quantified Metabolites from the Three Biofluids
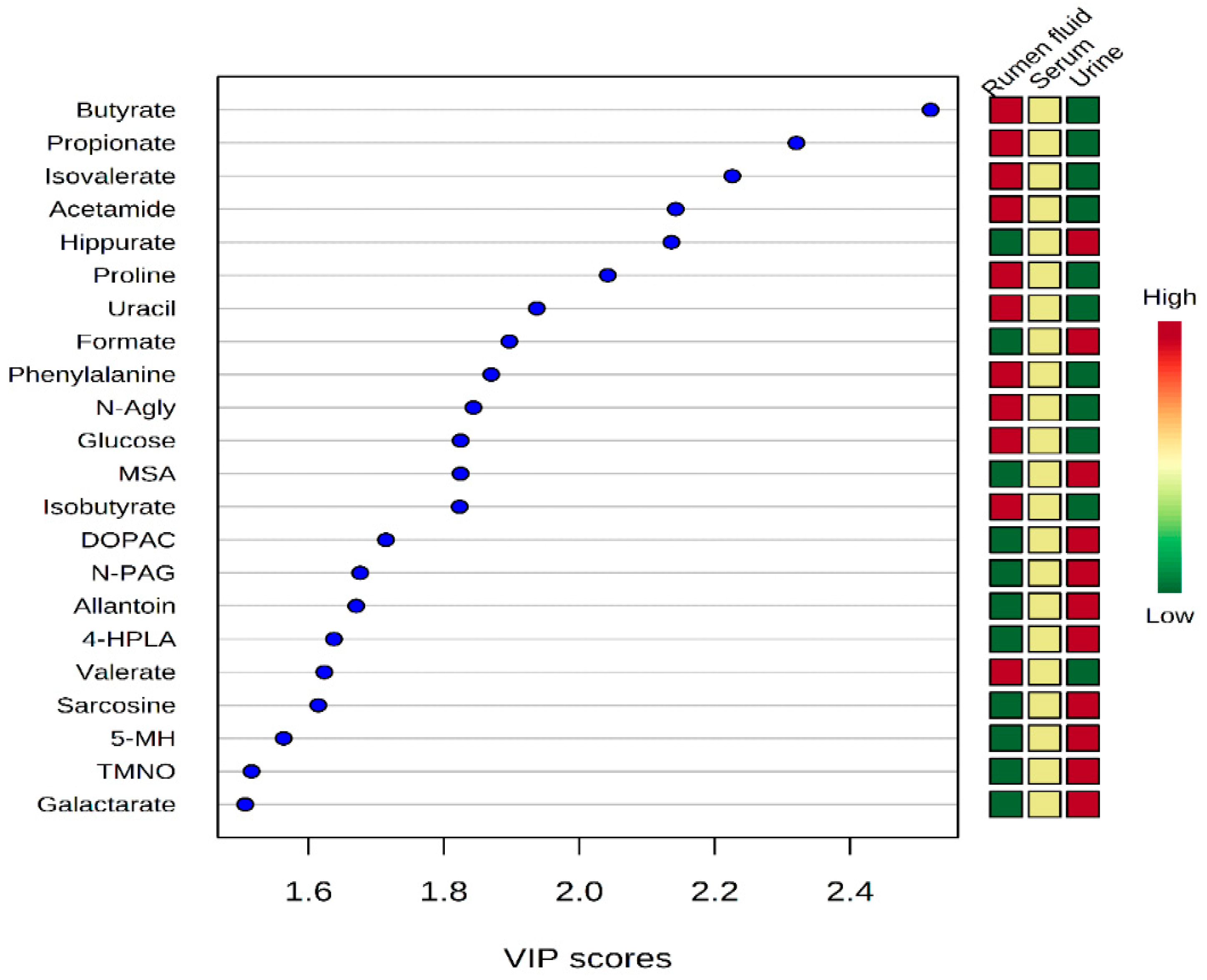
2.4. Statistical Analysis from Three Biofluids
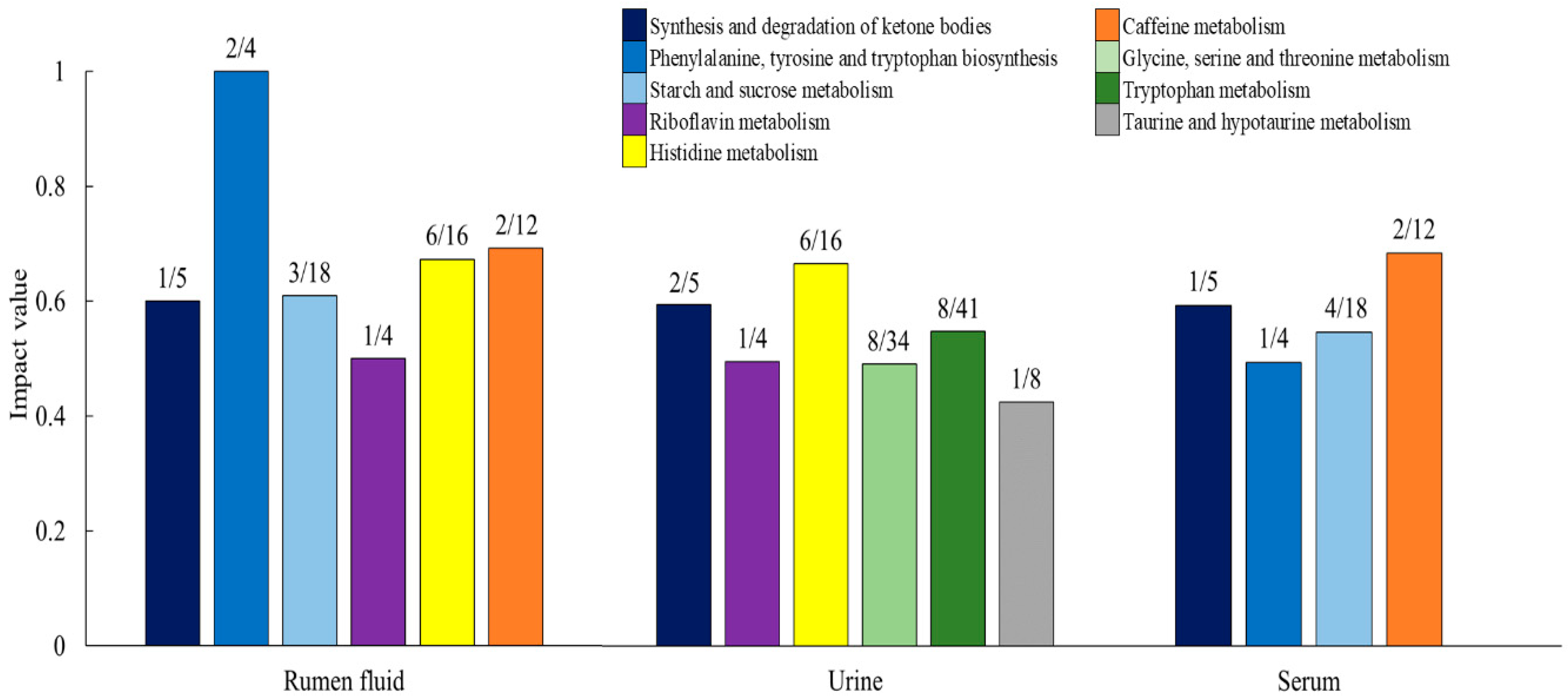
2.5. Metabolic Pathway Analysis
3. Discussion
4. Materials and Methods
4.1. Animals and Collected Samples
4.2. Sample Preparation for 1H-NMR Spectroscopy
4.3. 1H-NMR Spectroscopy Data and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Foroutan, A.; Goldansaz, S.A.; Lipfert, M.; Wishart, D.S. Protocols for NMR analysis in livestock metabolomics. In Metabolomics; Humana Press: New York, NY, USA, 2019; pp. 311–324. [Google Scholar]
- Belloque, J.; Ramos, M. Application of NMR spectroscopy to milk and dairy products. Trends Food Sci. Technol. 1999, 10, 313–320. [Google Scholar] [CrossRef]
- Song, Z.; Wang, H.; Yin, X.; Deng, P.; Jiang, W. Application of NMR metabolomics to search for human disease biomarkers in blood. Clin. Chem. Lab. Med. 2019, 57, 417–441. [Google Scholar] [CrossRef] [PubMed]
- De Castro, F.; Benedetti, M.; Del Coco, L.; Fanizzi, F.P. NMR-based metabolomics in metal-based drug research. Molecules 2019, 24, 2240. [Google Scholar] [CrossRef] [Green Version]
- Bo, Y.; Feng, J.; Xu, J.J.; Huang, Y.; Cai, H.; Cui, X.; Dong, J.; Ding, S.; Chen, Z. High-resolution pure shift NMR spectroscopy offers better metabolite discrimination in food quality analysis. Food Res. Int. 2019, 125, 108574. [Google Scholar] [CrossRef] [PubMed]
- Pontes, J.G.M.; Brasil, A.J.M.; Cruz, G.C.F.; De Souza, R.N.; Tasic, L. NMR-based metabolomics strategies: Plants, animals and humans. Anal. Methods 2017, 9, 1078–1096. [Google Scholar] [CrossRef]
- Van Soest, P.J. Nutritional Ecology of the Ruminant, 2nd ed.; Cornell University Press: Ithaca, NY, USA, 2019. [Google Scholar]
- Saleem, F.; Bouatra, S.; Guo, A.C.; Psychogios, N.; Mandal, R.; Dunn, S.M.; Ametaj, B.N.; Wishart, D.S. The bovine ruminal fluid metabolome. Metabolomics 2013, 9, 360–378. [Google Scholar] [CrossRef]
- Chandan, R.C.; Cullen, J.; Chapman, D. Physicochemical analyses of the bovine milk fat globule membrane. III. Proton magnetic resonance spectroscopy. J. Dairy Sci. 1972, 55, 1232–1236. [Google Scholar] [CrossRef]
- Foroutan, A.; Fitzsimmons, C.; Mandal, R.; Piri-Moghadam, H.; Zheng, J.; Guo, A.; Li, C.; Guan, L.L.; Wishart, D.S. The bovine metabolome. Metabolites 2020, 10, 233. [Google Scholar] [CrossRef]
- Zhao, S.; Zhao, J.; Bu, D.; Sun, P.; Wang, J.; Dong, Z. Metabolomics analysis reveals large effect of roughage types on rumen microbial metabolic profile in dairy cows. Lett. Appl. Microbiol. 2014, 59, 79–85. [Google Scholar] [CrossRef]
- O’Callaghan, T.F.; Vázquez-Fresno, R.; Serra-Cayuela, A.; Dong, E.; Mandal, R.; Hennessy, D.; McAuliffe, S.; Dillon, P.; Wishart, D.S.; Stanton, C.; et al. Pasture feeding changes the bovine rumen and milk metabolome. Metabolites 2018, 8, 27. [Google Scholar] [CrossRef] [Green Version]
- Min, L.; Zhao, S.; Tian, H.; Zhou, X.; Zhang, Y.; Li, S.; Yang, H.; Zheng, N.; Wang, J. Metabolic responses and “omics” technologies for elucidating the effects of heat stress in dairy cows. Int. J. Biometeorol. 2017, 61, 1149–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, H.; Zheng, N.; Wang, W.; Cheng, J.; Li, S.; Zhang, Y.; Wang, J. Integrated metabolomics study of the milk of heat-stressed lactating dairy cows. Sci. Rep. 2016, 6, 24208. [Google Scholar] [CrossRef] [PubMed]
- Van Gastelen, S.; Antunes-Fernandes, E.C.; Hettinga, K.A.; Dijkstra, J. Relationships between methane emission of Holstein Friesian dairy cows and fatty acids, volatile metabolites and non-volatile metabolites in milk. Animal 2017, 11, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Bica, R.; Kew, W.; Uhrin, D.; Pacheco, D.; Macrae, A. Nuclear magnetic resonance to detect rumen metabolites associated with enteric methane emissions from beef cattle. Sci. Rep. 2020, 10, 5578. [Google Scholar] [CrossRef] [Green Version]
- Ametaj, B.N.; Zebeli, Q.; Saleem, F.; Psychogios, N.; Lewis, M.J.; Dunn, S.M.; Xia, J.; Wishart, D.S. Metabolomics reveals unhealthy alterations in rumen metabolism with increased proportion of cereal grain in the diet of dairy cows. Metabolomics 2010, 6, 583–594. [Google Scholar] [CrossRef]
- Maher, A.D.; Hayes, B.; Cocks, B.; Marett, L.; Wales, W.J.; Rochfort, S.J. Latent biochemical relationships in the blood-milk metabolic axis of dairy cows revealed by statistical integration of 1H NMR spectroscopic data. J. Proteome Res. 2013, 12, 1428–1435. [Google Scholar] [CrossRef]
- Nyberg, N.T.; Nielsen, M.O.; Jaroszewski, J.W. Metabolic trajectories based on 1H NMR spectra of urines from sheep exposed to nutritional challenges during prenatal and early postnatal life. Metabolomics 2010, 6, 489–496. [Google Scholar] [CrossRef]
- Lee, S.-H.; Park, B.-H.; Sharma, A.; Dang, C.-G.; Lee, S.-S.; Choi, T.-J.; Choy, Y.-H.; Kim, H.-C.; Jeon, K.-J.; Kim, S.-D.; et al. Hanwoo cattle: Origin, domestication, breeding strategies and genomic selection. J. Anim. Sci. Technol. 2014, 56, 2. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Lee, S.J.; Oh, D.S.; Lee, I.D.; Eom, J.S.; Park, H.Y.; Choi, S.H.; Lee, S.S. In vitro evaluation of Rhus succedanea extracts for ruminants. Asian-Austral. J. Anim. Sci. 2018, 31, 1635–1642. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.S.; Lee, S.J.; Lee, S.S.; Kim, E.T.; Jeong, J.S.; Lee, J.H.; Jeong, J.; Park, J.K.; Park, B.Y.; Jeong, H.Y.; et al. Effect of optimal sodium stearoyl-2-lactylate supplementation on growth performance and blood and carcass characteristics in Hanwoo steers during the early fattening period. Asian-Austral. J. Anim. Sci. 2018, 31, 1442–1448. [Google Scholar] [CrossRef]
- Eom, J.S.; Lee, S.J.; Lee, Y.G.; Lee, S.S. Comparison of volatile fatty acids, monosaccharide analysis and metabolic profiling in rumen fluid according to feeding methods. J. Korea Acad. Ind. Coop. Soc. 2018, 19, 814–824. [Google Scholar]
- Lee, J.; Choi, M.; Kang, J.; Chung, Y.; Jin, Y.; Kim, M.; Lee, Y.; Chang, Y.H. Physicochemical structural, pasting, and rheological properties of potato starch isolated from different cultivars. Korean J. Food. Sci. Technol. 2017, 49, 360–368. [Google Scholar]
- Jeong, J.-Y.; Hwang, G.-S.; Park, J.-C.; Kim, D.-H.; Ha, M. 1H NMR-based urinary metabolic profiling of gender and diurnal variation in healthy Korean subjects. Env. Heal. Toxicol. 2010, 25, 295–306. [Google Scholar]
- Jeong, J.Y.; Kim, M.S.; Jung, H.J.; Kim, M.J.; Lee, H.J.; Lee, S.D. Screening of the liver, serum, and urine of piglets fed zearalenone using a NMR-based metabolomic approach. Korean J. Environ. Agric. 2018, 45, 447–454. [Google Scholar]
- MacLeod, N.A.; Ørskov, E.R. Absorption and utilization of volatile fatty acids in ruminants. Can. J. Anim. Sci. 1984, 64, 354–355. [Google Scholar] [CrossRef]
- Gäbel, P.G.; Bestmann, M.; Martens, H. Influences of diet, short-chain fatty acids, lactate and chloride on bicarbonate movement across the reticulo-rumen wall of sheep. J. Vet. Med. Ser. A 1991, 38, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Owens, F.N.; Secrist, D.S.; Hill, W.J.; Gill, D.R. Acidosis in cattle: A review. J. Anim. Sci. 1998, 76, 275–286. [Google Scholar] [CrossRef]
- Saleem, F.; Ametaj, B.N.; Bouatra, S.; Mandal, R.; Zebeli, Q.; Dunn, S.M.; Wishart, D.S. A metabolomics approach to uncover the effects of grain diets on rumen health in dairy cows. J. Dairy Sci. 2012, 95, 6606–6623. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.S.; Zhang, R.Y.; Zhu, W.Y.; Mao, S.Y. Effects of subacute ruminal acidosis challenges on fermentation and biogenic amines in the rumen of dairy cows. Livest. Sci. 2013, 155, 262–272. [Google Scholar] [CrossRef]
- Lee, D.H. Methods for genetic parameter estimations of carcass weight, longissimus muscle area and marbling score in Korean cattle. J. Anim. Sci. Technol. 2004, 46, 509–516. [Google Scholar]
- Beauchemin, K.A.; Kreuzer, M.; O’Mara, F.; McAllister, T.A. Nutritional management for enteric methane abatement: A review. Aust. J. Exp. Agric. 2008, 48, 21–27. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014. Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014; ISBN 9789291691432. [Google Scholar]
- Johnson, K.A.; Johnson, D.E. Methane emissions from cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Tapio, I.; Snelling, T.J.; Strozzi, F.; Wallace, R.J. The ruminal microbiome associated with methane emissions from ruminant livestock. J. Anim. Sci. Biotechnol. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Morgavi, D.P.; Doreau, M. Methane mitigation in ruminants: From microbe to the farm scale. Animal 2010, 4, 351–365. [Google Scholar] [CrossRef] [Green Version]
- Balch, W.E.; Fox, G.E.; Magrum, L.J.; Woese, C.R.; Wolfe, R.S. Methanogens: Reevaluation of a unique biological group. Microbiol. Rev. 1979, 43, 260–296. [Google Scholar] [CrossRef] [Green Version]
- Hayward, H.R.; Stadtman, T.C. Anaerobic degradation of choline I., Vibrio cholinicusn. sp: Fermentation of choline by an anaerobic, cytochrome-producing bacterium1. J. Bacteriol. 1959, 78, 557–561. [Google Scholar] [CrossRef] [Green Version]
- Baker, F.D.; Papiska, H.R.; Campbell, L.L. Choline fermentation by Desulfovibrio desulfuricans. J. Bacteriol. 1962, 84, 973–978. [Google Scholar] [CrossRef] [Green Version]
- Neill, A.R.; Grime, D.W.; Dawson, R.M.C. Conversion of choline methyl groups through trimethylamine into methane in the rumen. Biochem. J. 1978, 170, 529–535. [Google Scholar] [CrossRef] [Green Version]
- Asanuma, N.; Iwamoto, M.; Hino, T. Formate metabolism by ruminal microorganisms in relation to methanogenesis. Nihon Chikusan Gakkaiho 1998, 69, 576–584. [Google Scholar] [CrossRef]
- Asanuma, N.; Iwamoto, M.; Hino, T. Effect of the addition of fumarate on methane production by ruminal microorganisms in vitro. J. Dairy Sci. 1999, 82, 780–787. [Google Scholar] [CrossRef]
- Lopez, S.; McIntosh, F.M.; Wallace, R.J.; Newbold, C.J. Effect of adding acetogenic bacteria on methane production by mixed rumen microorganisms. Anim. Feed Sci. Technol. 1999, 78, 1–9. [Google Scholar] [CrossRef]
- Ungerfeld, E.M.; Rust, S.R.; Burnett, R. Attempts to inhibit ruminai methanogenesis by blocking pyruvate oxidative decarboxylation. Can. J. Microbiol. 2003, 49, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Callaway, T.R.; Martin, S.A. Effects of cellobiose and monensin on in vitro fermentation of organic acids by mixed ruminal bacteria. J. Dairy Sci. 1997, 80, 1126–1135. [Google Scholar] [CrossRef]
- Martin, S.A.; Park, C.M. Effect of extracellular hydrogen on organic acid utilization by the ruminai bacterium Selenomonas ruminantium. Curr. Microbiol. 1996, 32, 327–331. [Google Scholar] [CrossRef]
- Newbold, C.J.; López, S.; Nelson, N.; Ouda, J.O.; Wallace, R.J.; Moss, A.R. Propionate precursors and other metabolic intermediates as possible alternative electron acceptors to methanogenesis in ruminal fermentation in vitro. Br. J. Nutr. 2005, 94, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Grummer, R.R. Etiology of lipid-related metabolic disorders in periparturient dairy cows. J. Dairy Sci. 1993, 76, 3882–3896. [Google Scholar] [CrossRef]
- Butler, W.R.; Smith, R.D. Interrelationships between energy balance and postpartum reproductive function in dairy cattle. J. Dairy Sci. 1989, 72, 767–783. [Google Scholar] [CrossRef]
- Collard, B.L.; Boettcher, P.J.; Dekkers, J.C.M.; Petitclerc, D.; Schaeffer, L.R. Relationships between energy balance and health traits of dairy cattle in early lactation. J. Dairy Sci. 2000, 83, 2683–2690. [Google Scholar] [CrossRef]
- Van der Drift, S.G.A.; Houweling, M.; Schonewille, J.T.; Tielens, A.G.M.; Jorritsma, R. Protein and fat mobilization and associations with serum β-hydroxybutyrate concentrations in dairy cows. J. Dairy Sci. 2012, 95, 4911–4920. [Google Scholar] [CrossRef]
- Weikard, R.; Altmaier, E.; Suhre, K.; Weinberger, K.M.; Hammon, H.M.; Albrecht, E.; Setoguchi, K.; Takasuga, A.; Kühn, C. Metabolomic profiles indicate distinct physiological pathways affected by two loci with major divergent effect on Bos taurus growth and lipid deposition. Physiol. Genom. 2010, 42A, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Vervoort, J.; Saccenti, E.; van Hoeij, R.; Kemp, B.; van Knegsel, A. Milk metabolomics data reveal the energy balance of individual dairy cows in early lactation. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hoeij, R.J.; Dijkstra, J.; Bruckmaier, R.M.; Gross, J.J.; Lam, T.J.G.M.; Remmelink, G.J.; Kemp, B.; van Knegsel, A.T.M. The effect of dry period length and postpartum level of concentrate on milk production, energy balance, and plasma metabolites of dairy cows across the dry period and in early lactation. J. Dairy Sci. 2017, 100, 5863–5879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Vervoort, J.; Saccenti, E.; Kemp, B.; van Hoeij, R.J.; van Knegsel, A.T.M. Relationship between energy balance and metabolic profiles in plasma and milk of dairy cows in early lactation. J. Dairy Sci. 2020, 103, 4795–4805. [Google Scholar] [CrossRef] [PubMed]
- Blakebrough-Hall, C.; Dona, A.; D’occhio, M.J.; McMeniman, J.; González, L.A. Diagnosis of bovine respiratory disease in feedlot cattle using blood 1H NMR metabolomics. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Cusack, P.M.V.; McMeniman, N.; Lean, I.J. The medicine and epidemiology of bovine respiratory disease in feedlots. Aust. Vet. J. 2003, 81, 480–487. [Google Scholar] [CrossRef]
- Basoglu, A.; Baspinar, N.; Tenori, L.; Vignoli, A.; Yildiz, R. Plasma metabolomics in calves with acute bronchopneumonia. Metabolomics 2016, 12, 128. [Google Scholar] [CrossRef]
- Dervishi, E.; Zhang, G.; Hailemariam, D.; Goldansaz, S.A.; Deng, Q.; Dunn, S.M.; Ametaj, B.N. Alterations in innate immunity reactants and carbohydrate and lipid metabolism precede occurrence of metritis in transition dairy cows. Res. Vet. Sci. 2016, 104, 30–39. [Google Scholar] [CrossRef]
- Dervishi, E.; Zhang, G.; Hailemariam, D.; Mandal, R.; Wishart, D.S.; Ametaj, B.N. Urine metabolic fingerprinting can be used to predict the risk of metritis and highlight the pathobiology of the disease in dairy cows. Metabolomics 2018, 14, 83. [Google Scholar] [CrossRef]
- Van Nuffel, A.; Zwertvaegher, I.; Pluym, L.; Van Weyenberg, S.; Thorup, V.M.; Pastell, M.; Sonck, B.; Saeys, W. Lameness detection in dairy cows: Part 1. How to distinguish between non-lame and lame cows based on differences in locomotion or behavior. Animals 2015, 5, 838–860. [Google Scholar] [CrossRef] [Green Version]
- Garbarino, E.J.; Hernandez, J.A.; Shearer, J.K.; Risco, C.A.; Thatcher, W.W. Effect of lameness on ovarian activity in postpartum Holstein cows. J. Dairy Sci. 2004, 87, 4123–4131. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Dervishi, E.; Zwierzchowski, G.; Mandal, R.; Wishart, D.S.; Ametaj, B.N. Urinary metabolomics around parturition identifies metabolite alterations in dairy cows affected postpartum by pameness: Preliminary study. Dairy 2020, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- AOAC International. Association of the Official Analytical Chemists (AOAC): Official Methods of Analysis, 20th ed.; AOAC: Washington, DC, USA, 2016. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, C.; Li, C.; Xia, C.; Xu, C.; Wu, L.; Zhang, H. Characterization of the serum metabolic profile of dairy cows with milk fever using 1H-NMR spectroscopy. Vet. Q. 2014, 34, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Bertram, H.C.; Yde, C.C.; Zhang, X.; Kristensen, N.B. Effect of dietary nitrogen content on the urine metabolite profile of dairy cows assessed by nuclear magnetic resonance (NMR)-based metabolomics. J. Agric. Food Chem. 2011, 59, 12499–12505. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, I.Y.; Sung, H.R.; Nam, M.; Kim, Y.J.; Kyung, D.S.; Seong, J.K.; Hwang, G.S. Metabolic dysfunction following weight regain compared to initial weight gain in a high-fat diet-induced obese mouse model. J. Nutr. Biochem. 2019, 69, 44–52. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Li, C.; Wang, Y.; Laghi, L. Characterization of yak common biofluids metabolome by means of proton nuclear magnetic resonance spectroscopy. Metabolites 2019, 9, 41. [Google Scholar] [CrossRef] [Green Version]

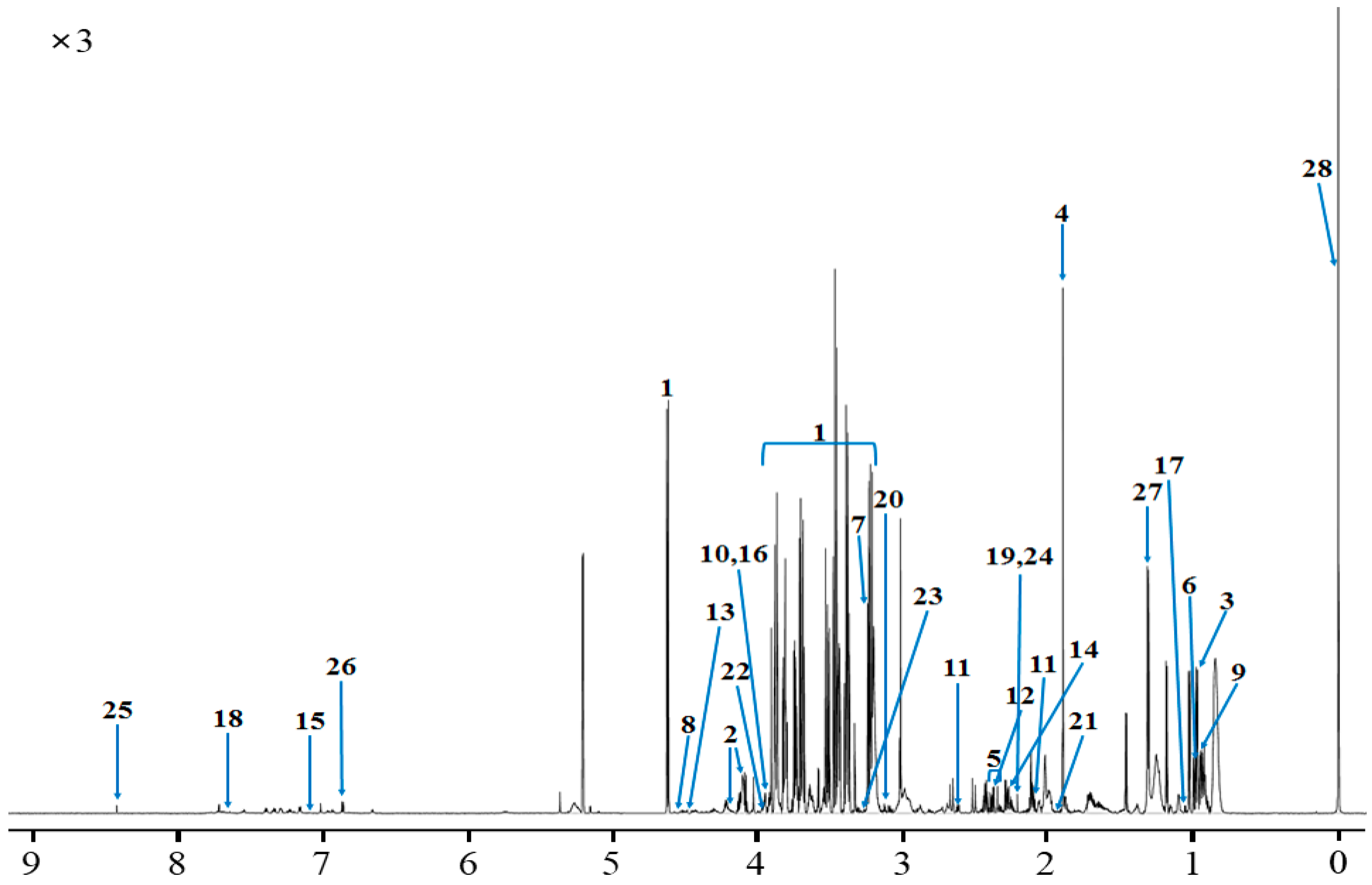

| Metabolites | Class | Concentration (μM/L) |
|---|---|---|
| Acetate | Organic acids | 28,172.77 ± 4924.54 |
| Propionate | Organic acids | 8126.70 ± 1341.28 |
| Butyrate | Organic acids | 6021.97 ± 1140.22 |
| Valerate | Organic acids | 940.82 ± 187.60 |
| Glucose | Carbohydrates | 632.42 ± 387.16 |
| Isobutyrate | Organic acids | 495.55 ± 168.30 |
| Isovalerate | Organic acids | 470.08 ± 144.26 |
| Acetamide | Organic acids | 237.18 ± 47.79 |
| Ribose | Carbohydrates | 231.48 ± 50.74 |
| 3-phenylpropionate | Others | 223.40 ± 55.69 |
| Phenylacetate | Organic acids | 220.22 ± 49.20 |
| 3-methylglutarate | Lipids | 214.75 ± 67.05 |
| Alanine | Amino acids | 195.65 ± 51.88 |
| Maltose | Carbohydrates | 178.13 ± 278.73 |
| Caprate | Lipids | 160.80 ± 58.72 |
| Proline | Amino acids | 119.20 ± 40.69 |
| N-acetylglucosamine | Carbohydrates | 112.55 ± 16.91 |
| Urea | Aliphatic acylic compounds | 86.56 ± 55.54 |
| N-carbamoylaspartate | Carboxylic acids | 86.07 ± 33.83 |
| Xanthine | Nucleosides, nucleotides | 70.03 ± 12.87 |
| N-acetylglycine | Carboxylic acids | 61.42 ± 64.75 |
| Glycine | Amino acids | 61.26 ± 41.85 |
| Uracil | Nucleosides, nucleotides | 58.87 ± 17.19 |
| Threonine | Amino acids | 57.50 ± 12.14 |
| Isoleucine | Amino acids | 57.47 ± 22.42 |
| Glycerate | Amino acids | 52.57 ± 42.20 |
| Lactulose | Carbohydrates | 52.36 ± 40.70 |
| Pyruvate | Carbohydrates | 51.98 ± 27.61 |
| 3-hydroxy-3-methylglutarate | Lipids | 51.50 ± 33.29 |
| N-alpha-acetyllysine | Carboxylic acids | 51.08 ± 53.04 |
| Metabolites | Class | Concentration (μM/L) |
|---|---|---|
| Glucose | Carbohydrates | 603.60 ± 143.82 |
| Lactate | Organic acids | 223.53 ± 40.57 |
| 2-hydroxyisovalerate | Lipids | 96.33 ± 23.44 |
| Acetate | Organic acids | 73.38 ± 25.19 |
| 3-hydroxybutyrate | Lipids | 63.00 ± 20.58 |
| Isoleucine | Amino acids | 36.62 ± 7.19 |
| Creatinine | Imidazolinones | 30.23 ± 10.07 |
| Leucine | Amino acids | 25.63 ± 5.77 |
| Gluconate | Organic acids | 21.63 ± 11.66 |
| sn-glycero-3-phosphocholine | Others | 21.22 ± 5.58 |
| trans-4-hydroxy-L-proline | Carboxylic acids | 15.98 ± 4.73 |
| Carnitine | Lipids | 13.20 ± 10.06 |
| 3-hydroxyisovalerate | Carboxylic acids | 8.63 ± 8.41 |
| Creatine phosphate | Carboxylic acids | 8.03 ± 5.17 |
| Glycylproline | Carboxylic acids | 7.96 ± 3.84 |
| Acetoacetate | Carbohydrates | 7.58 ± 4.70 |
| Lactulose | Carbohydrates | 7.03 ± 4.81 |
| Ascorbate | Others | 5.98 ± 1.58 |
| Malonate | Carboxylic acids | 5.85 ± 1.56 |
| Creatine | Amino acids | 5.70 ± 1.17 |
| Valine | Amino acids | 5.17 ± 0.21 |
| 3-methylhistidine | Others | 4.30 ± 1.71 |
| Glycolate | Lipids | 4.32 ± 2.91 |
| Levulinate | Others | 3.90 ± 0.59 |
| Acetoin | Others | 3.72 ± 2.17 |
| Succinylacetone | Organic acids | 3.58 ± 1.31 |
| 2-hydroxyphenylacetate | Others | 3.30 ± 1.71 |
| 5-aminolevulinate | Carboxylic acids | 2.70 ± 1.50 |
| N-acetylglucosamine | Carbohydrates | 2.47 ± 0.06 |
| N-nitrosodimethylamine | Organic acids | 2.20 ± 1.67 |
| Metabolites | Class | Concentration (μM/L) |
|---|---|---|
| Urea | Aliphatic acylic compounds | 51,262.08 ± 28,840.87 |
| Hippurate | Amino acids | 8332.20 ± 7592.61 |
| N-phenylacetylglycine | Amino acids | 5273.43 ± 2722.36 |
| Glycolate | Lipids | 1721.83 ± 2935.46 |
| Trimethylamine N-oxide | Aliphatic acylic compounds | 938.30 ± 811.19 |
| Allantoin | Imidazolinones | 769.23 ± 1019.92 |
| 2-hydroxyvalerate | Lipids | 509.77 ± 354.91 |
| Ribose | Carbohydrates | 442.70 ± 312.08 |
| Benzoate | Organic acids | 427.80 ± 86.02 |
| Glycine | Amino acids | 402.90 ± 153.29 |
| Acetate | Organic acids | 310.50 ± 161.75 |
| Guanidoacetate | Carboxylic acids | 258.45 ± 239.55 |
| Creatine | Amino acids | 257.65 ± 374.33 |
| Glucuronate | Carbohydrates | 255.87 ± 178.89 |
| Galactarate | Others | 178.25 ± 119.45 |
| Xanthine | Nucleosides, nucleotides | 175.48 ± 101.52 |
| Dimethylamine | Amines | 169.97 ± 151.11 |
| Formate | Organic acids | 153.53 ± 64.46 |
| 3-indoxylsulfate | Indoles | 133.60 ± 76.22 |
| Xylitol | Carbohydrates | 121.90 ± 71.35 |
| 2-methylglutarate | Lipids | 105.37 ± 49.52 |
| cis-aconitate | Carboxylic acids | 102.73 ± 85.26 |
| Glycylproline | Carboxylic acids | 97.35 ± 58.11 |
| 2-hydroxyisocaproate | Lipids | 94.97 ± 41.50 |
| Mandelate | Benzoic acids | 93.70 ± 31.86 |
| Kynurenine | Amines | 82.60 ± 57.39 |
| Gentisate | Benzoic acids | 78.60 ± 18.26 |
| Phenylacetate | Organic acids | 76.63 ± 30.07 |
| Salicylurate | Benzoic acids | 74.90 ± 81.77 |
| 3-phenylpropionate | Others | 70.97 ± 46.43 |
| Metabolites a | Class b | Rumen Fluid (μM/L) | Serum (μM/L) | Urine (μM/L) |
|---|---|---|---|---|
| 2-HPA | Others | 17.55 ± 10.11 | 3.30 ± 1.71 | 31.08 ± 16.22 |
| 3-HIV | COOH | 26.68 ± 22.45 | 8.63 ± 8.41 | 22.15 ± 17.84 |
| VMA | BZA | 1.37 ± 0.12 | 1.06 ± 0.22 | 19.75 ± 12.83 |
| 4-Pyridoxate | Others | 6.56 ± 5.52 | 0.85 ± 0.19 | 11.98 ± 9.00 |
| 5-HIAA | Indoles | 11.10 ± 6.37 | 2.37 ± 0.90 | 36.27 ± 21.16 |
| Acetate | OA | 28,172.77 ± 4924.54 | 73.38 ± 25.19 | 310.50 ± 161.75 |
| Acetoacetate | CHO | 10.85 ± 7.03 | 7.58 ± 4.70 | 60.75 ± 56.84 |
| Anserine | AA | 24.65 ± 10.43 | 2.18 ± 1.52 | 20.50 ± 5.64 |
| Betaine | Others | 1.37 ± 0.75 | 0.52 ± 0.30 | 60.43 ± 33.89 |
| Carnitine | Lipids | 14.40 ± 14.20 | 13.20 ± 10.06 | 21.13 ± 20.32 |
| Glycylproline | COOH | 45.17 ± 23.34 | 7.96 ± 3.84 | 97.35 ± 58.11 |
| Guanidoacetate | COOH | 25.90 ± 12.47 | 2.15 ± 2.14 | 258.45 ± 239.55 |
| Isoleucine | AA | 57.47 ± 22.42 | 36.62 ± 7.19 | 14.07 ± 2.72 |
| Malonate | COOH | 15.78 ± 7.15 | 5.85 ± 1.56 | 46.70 ± 44.64 |
| NDMA | OA | 12.17 ± 5.40 | 2.20 ± 1.67 | 18.95 ± 4.27 |
| Pantothenate | COOH | 7.93 ± 3.07 | 1.20 ± 0.26 | 29.35 ± 9.43 |
| Succinylacetone | OA | 8.30 ± 5.41 | 3.58 ± 1.31 | 42.15 ± 26.69 |
| Syringate | BZA | 2.63 ± 0.29 | 0.38 ± 0.08 | 11.15 ± 19.38 |
| Thymol | Lipids | 14.33 ± 4.86 | 1.97 ± 0.32 | 27.63 ± 13.40 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eom, J.S.; Lee, S.J.; Kim, H.S.; Choi, Y.Y.; Kim, S.H.; Lee, Y.G.; Lee, S.S. Metabolomics Comparison of Hanwoo (Bos taurus coreanae) Biofluids Using Proton Nuclear Magnetic Resonance Spectroscopy. Metabolites 2020, 10, 333. https://doi.org/10.3390/metabo10080333
Eom JS, Lee SJ, Kim HS, Choi YY, Kim SH, Lee YG, Lee SS. Metabolomics Comparison of Hanwoo (Bos taurus coreanae) Biofluids Using Proton Nuclear Magnetic Resonance Spectroscopy. Metabolites. 2020; 10(8):333. https://doi.org/10.3390/metabo10080333
Chicago/Turabian StyleEom, Jun Sik, Shin Ja Lee, Hyun Sang Kim, You Young Choi, Sang Ho Kim, Yoo Gyung Lee, and Sung Sill Lee. 2020. "Metabolomics Comparison of Hanwoo (Bos taurus coreanae) Biofluids Using Proton Nuclear Magnetic Resonance Spectroscopy" Metabolites 10, no. 8: 333. https://doi.org/10.3390/metabo10080333





