Optimizing Secondary Electrospray Ionization High-Resolution Mass Spectrometry (SESI-HRMS) for the Analysis of Volatile Fatty Acids from Gut Microbiome
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimizing SESI Conditions for the Best Performance of Gas-Phase VFA Analysis
2.1.1. Identification of Six Representative m/z Values
2.1.2. Optimization of Essential SESI Parameters
Electrospray Solvents
Concentration of Formic Acid as an Additive in Electrospray Solvent
Sample Inlet and Ionization Chamber Temperature
SESI Spray Pressure
2.1.3. Calibration Curve of Representative VFAs
2.2. SESI-HRMS Based Evaluation of Gas-Phase VFAs from Gut Microbial Culture
2.2.1. Comparison of Optimized and the Least Optimized Parameters
2.2.2. SCFA Analysis Using HPLC-HESI-MS/MS
3. Materials and Methods
3.1. Chemicals
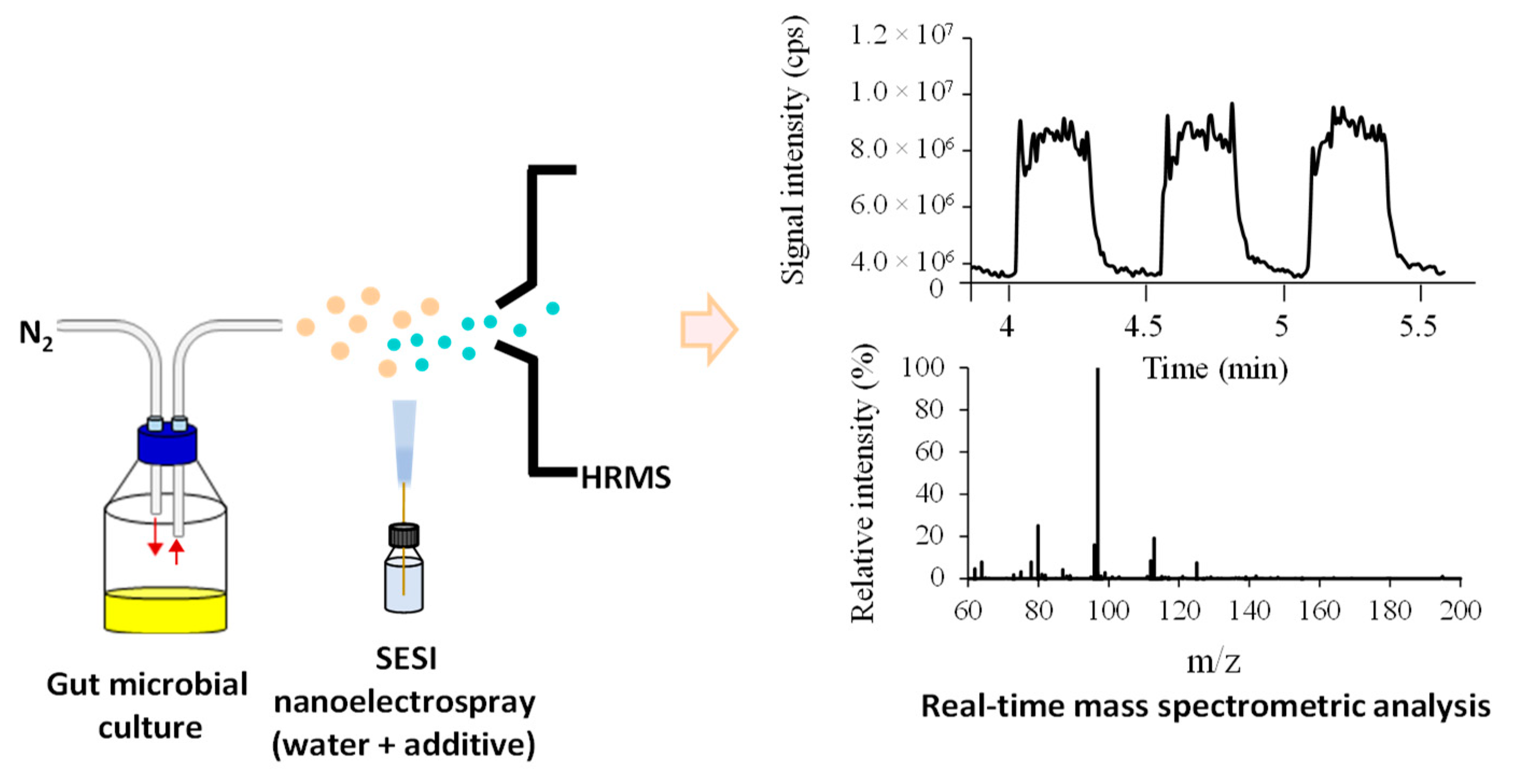
3.2. Setup and Optimization of Secondary Electrospray Ionization (SESI)
3.3. High-Resolution Mass Spectrometry (HRMS)
3.4. Growth Condition of Microbial Cultures and Antibiotics Treatment
3.5. Real-Time Analysis of Headspace Volatile in Gut Microbial Cultures
3.6. HPLC-HESI-MS/MS Analysis of Microbial Cultures
3.6.1. Extraction of C2–C7 Volatile Fatty Acids (VFAs) of Microbial Cultures
3.6.2. HPLC-HESI-MS/MS Analysis
3.7. Data Processing and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Crespo, E.; Cristescu, S.M.; de Ronde, H.; Kuijper, S.; Kolk, A.H.; Anthony, R.M.; Harren, F.J. Proton Transfer Reaction Mass Spectrometry detects rapid changes in volatile metabolite emission by Mycobacterium smegmatis after the addition of specific antimicrobial agents. J. Microbiol. Methods 2011, 86, 8–15. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, M.; Mayhew, C. A preliminary comparison of volatile organic compounds in the headspace of cultures of Staphylococcus aureus grown in nutrient, dextrose and brain heart bovine broths measured using a proton transfer reaction mass spectrometer. J. Breath Res. 2009, 3, 027001. [Google Scholar] [CrossRef] [PubMed]
- Slade, E.A.; Thorn, R.; Lovering, A.M.; Young, A.; Reynolds, D.M. In vitro discrimination of wound-associated bacteria by volatile compound profiling using selected ion flow tube-mass spectrometry. J. Appl. Microbiol. 2017, 123, 233–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.J.; Jayaprakasha, G.K.; Rush, C.M.; Crosby, K.M.; Patil, B.S. Production system influences volatile biomarkers in tomato. Metabolomics 2018, 14, 99. [Google Scholar] [CrossRef]
- Li, H.; Zhu, J. Differentiating antibiotic-resistant staphylococcus aureus using secondary electrospray ionization tandem mass spectrometry. Anal. Chem. 2018, 90, 12108–12115. [Google Scholar] [CrossRef]
- Rioseras, A.T.; Gomez, D.G.; Ebert, B.E.; Blank, L.M.; Ibáñez, A.J.; Sinues, P.M. Comprehensive real-time analysis of the yeast Volatilome. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Farrell, R.R.; Fahrentrapp, J.; García-Gómez, D.; Sinues, P.M.-L.; Zenobi, R. Rapid fingerprinting of grape volatile composition using secondary electrospray ionization orbitrap mass spectrometry: A preliminary study of grape ripening. Food Control 2017, 81, 107–112. [Google Scholar] [CrossRef]
- Weber, R.; Haas, N.; Baghdasaryan, A.; Bruderer, T.; Inci, D.; Micic, S.; Perkins, N.; Spinas, R.; Zenobi, R.; Moeller, A. Volatile organic compound breath signatures of children with cystic fibrosis by real-time SESI-HRMS. ERJ Open Res 2020, 6. [Google Scholar] [CrossRef]
- Martínez-Lozano, P.; Rus, J.; de la Mora, G.F.; Hernández, M.; de la Mora, J.F. Secondary electrospray ionization (SESI) of ambient vapors for explosive detection at concentrations below parts per trillion. J. Am. Soc. Mass Spectr. 2009, 20, 287–294. [Google Scholar] [CrossRef] [Green Version]
- Gaugg, M.T.; Gomez, D.G.; Barrios-Collado, C.; Vidal-de-Miguel, G.; Kohler, M.; Zenobi, R.; Martinez-Lozano Sinues, P. Expanding metabolite coverage of real-time breath analysis by coupling a universal secondary electrospray ionization source and high resolution mass spectrometry—A pilot study on tobacco smokers. J. Breath Res. 2016, 10, 016010. [Google Scholar] [CrossRef]
- Fenn, J.B.; Mann, M.; Meng, C.K.; Wong, S.F.; Whitehouse, C.M. Electrospray ionization for mass spectrometry of large biomolecules. Science 1989, 246, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Hill Jr, H.H.; Wittmer, D.P. Analytical merit of electrospray ion mobility spectrometry as a chromatographic detector. J. Microcolumn Sep. 1994, 6, 515–524. [Google Scholar] [CrossRef]
- Vidal-de-Miguel, G.; Macia, M.; Pinacho, P.; Blanco, J. Low-sample flow secondary electrospray ionization: Improving vapor ionization efficiency. Anal. Chem. 2012, 84, 8475–8479. [Google Scholar] [CrossRef] [PubMed]
- Barrios-Collado, C.; Vidal-de-Miguel, G.; Sinues, P.M.-L. Numerical modeling and experimental validation of a universal secondary electrospray ionization source for mass spectrometric gas analysis in real-time. Sens. Actuators B Chem. 2016, 223, 217–225. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Lin, L.; Yu, Q.; Wang, X. Exploiting the native inspiratory ability of a mass spectrometer to improve analysis efficiency. RSC Adv. 2020, 10, 4103–4109. [Google Scholar] [CrossRef] [Green Version]
- Lavelle, A.; Sokol, H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 1–15. [Google Scholar] [CrossRef]
- Ho, K.M.; Kalgudi, S.; Corbett, J.M.; Litton, E. Gut microbiota in surgical and critically ill patients. Anaesth. Intensive Care 2020, 48, 179–195. [Google Scholar] [CrossRef]
- Holota, Y.; Dovbynchuk, T.; Kaji, I.; Vareniuk, I.; Dzyubenko, N.; Chervinska, T.; Zakordonets, L.; Stetska, V.; Ostapchenko, L.; Serhiychuk, T.; et al. The long-term consequences of antibiotic therapy: Role of colonic short-chain fatty acids (SCFA) system and intestinal barrier integrity. PLoS ONE 2019, 14, e0220642. [Google Scholar] [CrossRef]
- Raposo, F.; Borja, R.; Cacho, J.; Mumme, J.; Orupõld, K.; Esteves, S.; Noguerol-Arias, J.; Picard, S.; Nielfa, A.; Scherer, P. First international comparative study of volatile fatty acids in aqueous samples by chromatographic techniques: Evaluating sources of error. Trends Anal. Chem. 2013, 51, 127–143. [Google Scholar] [CrossRef]
- Lamichhane, S.; Sen, P.; Dickens, A.M.; Orešič, M.; Bertram, H.C. Gut metabolome meets microbiome: A methodological perspective to understand the relationship between host and microbe. Methods 2018, 149, 3–12. [Google Scholar] [CrossRef]
- Harsch, I.A.; Konturek, P.C. The role of gut microbiota in obesity and type 2 and type 1 diabetes mellitus: New insights into “old” diseases. Med. Sci. 2018, 6, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velikonja, A.; Lipoglavšek, L.; Zorec, M.; Avguštin, G. Alterations in gut microbiota composition and metabolic parameters after dietary intervention with barley beta glucans in patients with high risk for metabolic syndrome development. Anaerobe 2019, 55, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Vetrani, C.; Costabile, G.; Luongo, D.; Naviglio, D.; Rivellese, A.A.; Riccardi, G.; Giacco, R. Effects of whole-grain cereal foods on plasma short chain fatty acid concentrations in individuals with the metabolic syndrome. Nutrition 2016, 32, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Hald, S.; Schioldan, A.G.; Moore, M.E.; Dige, A.; Lærke, H.N.; Agnholt, J.; Bach Knudsen, K.E.; Hermansen, K.; Marco, M.L.; Gregersen, S. Effects of arabinoxylan and resistant starch on intestinal microbiota and short-chain fatty acids in subjects with metabolic syndrome: A randomised crossover study. PLoS ONE 2016, 11, e0159223. [Google Scholar] [CrossRef] [PubMed]
- Kasote, D.M.; Jayaprakasha, G.; Patil, B.S. Encapsulation of Polyphenols: An Effective Way to Enhance Their Bioavailability for Gut Health. In Advances in Plant Phenolics: From Chemistry to Human Health; ACS Publications: Washington, DC, USA, 2018; pp. 239–259. [Google Scholar] [CrossRef]
- Manuzak, J.A.; Zevin, A.S.; Cheu, R.; Richardson, B.; Modesitt, J.; Hensley-McBain, T.; Miller, C.; Gustin, A.T.; Coronado, E.; Gott, T. Antibiotic-induced microbiome perturbations are associated with significant alterations to colonic mucosal immunity in rhesus macaques. Mucosal Immunol. 2020, 13, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Graversen, K.B.; Bahl, M.I.; Larsen, J.M.; Ballegaard, A.R.; Licht, T.R.; Bogh, K.L. Short-Term Amoxicillin-Induced Perturbation of the Gut Microbiota Promotes Acute Intestinal Immune Regulation in Brown Norway Rats. Front. Microbiol. 2020, 11, 496. [Google Scholar] [CrossRef] [Green Version]
- Bruderer, T.; Gaisl, T.; Gaugg, M.T.; Nowak, N.; Streckenbach, B.; Müller, S.; Moeller, A.; Kohler, M.; Zenobi, R. On-line analysis of exhaled breath: Focus review. Chem. Rev. 2019, 119, 10803–10828. [Google Scholar] [CrossRef]
- Kiontke, A.; Oliveira-Birkmeier, A.; Opitz, A.; Birkemeyer, C. Electrospray ionization efficiency is dependent on different molecular descriptors with respect to solvent pH and instrumental configuration. PLoS ONE 2016, 11, e0167502. [Google Scholar] [CrossRef]
- Tejero Rioseras, A.; Singh, K.D.; Nowak, N.; Gaugg, M.T.; Bruderer, T.; Zenobi, R.; Sinues, P.M. Real-Time Monitoring of Tricarboxylic Acid Metabolites in Exhaled Breath. Anal. Chem. 2018, 90, 6453–6460. [Google Scholar] [CrossRef]
- Meier, L.; Berchtold, C.; Schmid, S.; Zenobi, R. Sensitive detection of drug vapors using an ion funnel interface for secondary electrospray ionization mass spectrometry. J. Mass Spectrom. 2012, 47, 555–559. [Google Scholar] [CrossRef]
- Meier, L.; Berchtold, C.; Schmid, S.; Zenobi, R. High mass resolution breath analysis using secondary electrospray ionization mass spectrometry assisted by an ion funnel. J. Mass Spectrom. 2012, 47, 1571–1575. [Google Scholar] [CrossRef] [PubMed]
- Sinues, P.M.-L.; Criado, E.; Vidal, G. Mechanistic study on the ionization of trace gases by an electrospray plume. Int. J. Mass Spectrom. 2012, 313, 21–29. [Google Scholar] [CrossRef]
- Singh, K.D.; Tancev, G.; Decrue, F.; Usemann, J.; Appenzeller, R.; Barreiro, P.; Jauma, G.; Macia Santiago, M.; Vidal de Miguel, G.; Frey, U.; et al. Standardization procedures for real-time breath analysis by secondary electrospray ionization high-resolution mass spectrometry. Anal. Bioanal. Chem. 2019, 411, 4883–4898. [Google Scholar] [CrossRef] [Green Version]
- Rioseras, A.T.; Gaugg, M.T.; Sinues, P.M.-L. Secondary electrospray ionization proceeds via gas-phase chemical ionization. Anal. Methods 2017, 9, 5052–5057. [Google Scholar] [CrossRef] [Green Version]
- Sinues, P.M.; Kohler, M.; Zenobi, R. Monitoring diurnal changes in exhaled human breath. Anal. Chem. 2013, 85, 369–373. [Google Scholar] [CrossRef]
- Bean, H.D.; Mellors, T.R.; Zhu, J.; Hill, J.E. Profiling aged artisanal Cheddar cheese using secondary electrospray ionization mass spectrometry. J. Agric. Food Chem. 2015, 63, 4386–4392. [Google Scholar] [CrossRef]
- Wolf, J.-C.; Schaer, M.; Siegenthaler, P.; Zenobi, R. Direct quantification of chemical warfare agents and related compounds at low ppt levels: Comparing active capillary dielectric barrier discharge plasma ionization and secondary electrospray ionization mass spectrometry. Anal. Chem. 2015, 87, 723–729. [Google Scholar] [CrossRef]
- Cristoni, S.; Rossi Bernardi, L.; Guidugli, F.; Tubaro, M.; Traldi, P. The role of different phenomena in surface-activated chemical ionization (SACI) performance. J. Mass Spectrom. 2005, 40, 1550–1557. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Nian, L.; Kwok, L.; Sun, T. Reduction in fecal microbiota diversity and short-chain fatty acid producers in methicillin-resistant Staphylococcus aureus infected individuals as revealed by PacBio single molecule, real-time sequencing technology. Eur. J. Clin. Microbiol. Infectious Dis. 2017, 36, 1463–1472. [Google Scholar] [CrossRef]
- Canani, R.B.; Di Costanzo, M.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. WJG 2011, 17, 1519. [Google Scholar] [CrossRef]
- Jung, T.-H.; Park, J.H.; Jeon, W.-M.; Han, K.-S. Butyrate modulates bacterial adherence on LS174T human colorectal cells by stimulating mucin secretion and MAPK signaling pathway. Nutr. Res. Pract. 2015, 9, 343–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, L.; Li, Z.-R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Weir, T.L.; Manter, D.K.; Sheflin, A.M.; Barnett, B.A.; Heuberger, A.L.; Ryan, E.P. Stool microbiome and metabolome differences between colorectal cancer patients and healthy adults. PLoS ONE 2013, 8, e70803. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-J.; Li, S.; Gan, R.-Y.; Zhou, T.; Xu, D.-P.; Li, H.-B. Impacts of gut bacteria on human health and diseases. Int. J. Mol. Sci. 2015, 16, 7493–7519. [Google Scholar] [CrossRef] [PubMed]
- Høverstad, T.; Carlstedt-Duke, B.; Lingaas, E.; Midtvedt, T.; Norin, K.; Saxerholt, H.; Steinbakk, M. Influence of ampicillin, clindamycin, and metronidazole on faecal excretion of short-chain fatty acids in healthy subjects. Scand. J. Gastroenterol. 1986, 21, 621–626. [Google Scholar] [CrossRef]
- Willing, B.P.; Russell, S.L.; Finlay, B.B. Shifting the balance: Antibiotic effects on host–microbiota mutualism. Nat. Rev. Microbiology 2011, 9, 233–243. [Google Scholar] [CrossRef]
- Zhao, X.; Jiang, Z.; Yang, F.; Wang, Y.; Gao, X.; Wang, Y.; Chai, X.; Pan, G.; Zhu, Y. Sensitive and simplified detection of antibiotic influence on the dynamic and versatile changes of fecal short-chain fatty acids. PLoS ONE 2016, 11, e0167032. [Google Scholar] [CrossRef]
- El-Ansary, A.; Bhat, R.S.; Al-Daihan, S.; Al Dbass, A.M. The neurotoxic effects of ampicillin-associated gut bacterial imbalances compared to those of orally administered propionic acid in the etiology of persistent autistic features in rat pups: Effects of various dietary regimens. Gut Pathog. 2015, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Tait, E.; Perry, J.D.; Stanforth, S.P.; Dean, J.R. Identification of volatile organic compounds produced by bacteria using HS-SPME-GC–MS. J. Chromatogr. Sci. 2014, 52, 363–373. [Google Scholar] [CrossRef] [Green Version]
- Timm, C.M.; Lloyd, E.P.; Egan, A.; Mariner, R.; Karig, D. Direct Growth of Bacteria in Headspace Vials Allows for Screening of Volatiles by Gas Chromatography Mass Spectrometry. Front. Microbiol. 2018, 9, 491. [Google Scholar] [CrossRef]
- Lapthorn, C.; Pullen, F.; Chowdhry, B.Z. Ion mobility spectrometry-mass spectrometry (IMS-MS) of small molecules: Separating and assigning structures to ions. Mass Spectrom. Rev. 2013, 32, 43–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodges, J.K.; Zhu, J.; Yu, Z.; Vodovotz, Y.; Brock, G.; Sasaki, G.Y.; Dey, P.; Bruno, R.S. Intestinal-level anti-inflammatory bioactivities of catechin-rich green tea: Rationale, design, and methods of a double-blind, randomized, placebo-controlled crossover trial in metabolic syndrome and healthy adults. Contemp. Clin. Trials Commun. 2020, 17, 100495. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lin, K.; Sequeira, C.; Borchers, C.H. An isotope-labeled chemical derivatization method for the quantitation of short-chain fatty acids in human feces by liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2015, 854, 86–94. [Google Scholar] [CrossRef] [PubMed]






| Compounds a | Formula | Theoretical m/z [M-H]− | Measured m/z [M-H]− | Mass Error (ppm) |
|---|---|---|---|---|
| Acetic acid (C2-VFA) | C2H4O2 | 59.0139 | 59.0138 | −0.9 |
| Propionic acid (C3-VFA) | C3H6O2 | 73.0295 | 73.0296 | 1.3 |
| Butyric acid/Isobutyric acid (C4-VFA) | C4H8O2 | 87.0452 | 87.0453 | 1.7 |
| Valeric acid/Isovaleric acid (C5-VFA) | C5H10O2 | 101.0608 | 101.0609 | 1.0 |
| Hexanoic acid/Isocarporic acid (C6-VFA) | C6H12O2 | 115.0765 | 115.0765 | 0.4 |
| Heptanoic acid (C7-VFA) | C7H14O2 | 129.0921 | 129.0922 | 0.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.H.J.; Zhu, J. Optimizing Secondary Electrospray Ionization High-Resolution Mass Spectrometry (SESI-HRMS) for the Analysis of Volatile Fatty Acids from Gut Microbiome. Metabolites 2020, 10, 351. https://doi.org/10.3390/metabo10090351
Lee JHJ, Zhu J. Optimizing Secondary Electrospray Ionization High-Resolution Mass Spectrometry (SESI-HRMS) for the Analysis of Volatile Fatty Acids from Gut Microbiome. Metabolites. 2020; 10(9):351. https://doi.org/10.3390/metabo10090351
Chicago/Turabian StyleLee, Jisun H. J., and Jiangjiang Zhu. 2020. "Optimizing Secondary Electrospray Ionization High-Resolution Mass Spectrometry (SESI-HRMS) for the Analysis of Volatile Fatty Acids from Gut Microbiome" Metabolites 10, no. 9: 351. https://doi.org/10.3390/metabo10090351
APA StyleLee, J. H. J., & Zhu, J. (2020). Optimizing Secondary Electrospray Ionization High-Resolution Mass Spectrometry (SESI-HRMS) for the Analysis of Volatile Fatty Acids from Gut Microbiome. Metabolites, 10(9), 351. https://doi.org/10.3390/metabo10090351



_Zhu.png)

