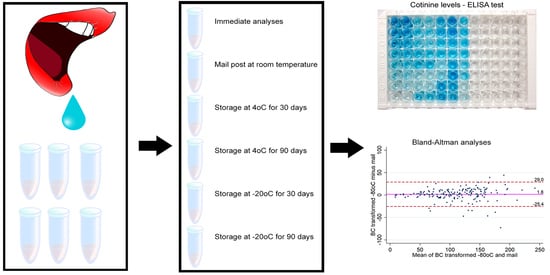
Effect of Sample Storage Conditions on Measurements of Salivary Cotinine Levels
Abstract
:1. Introduction
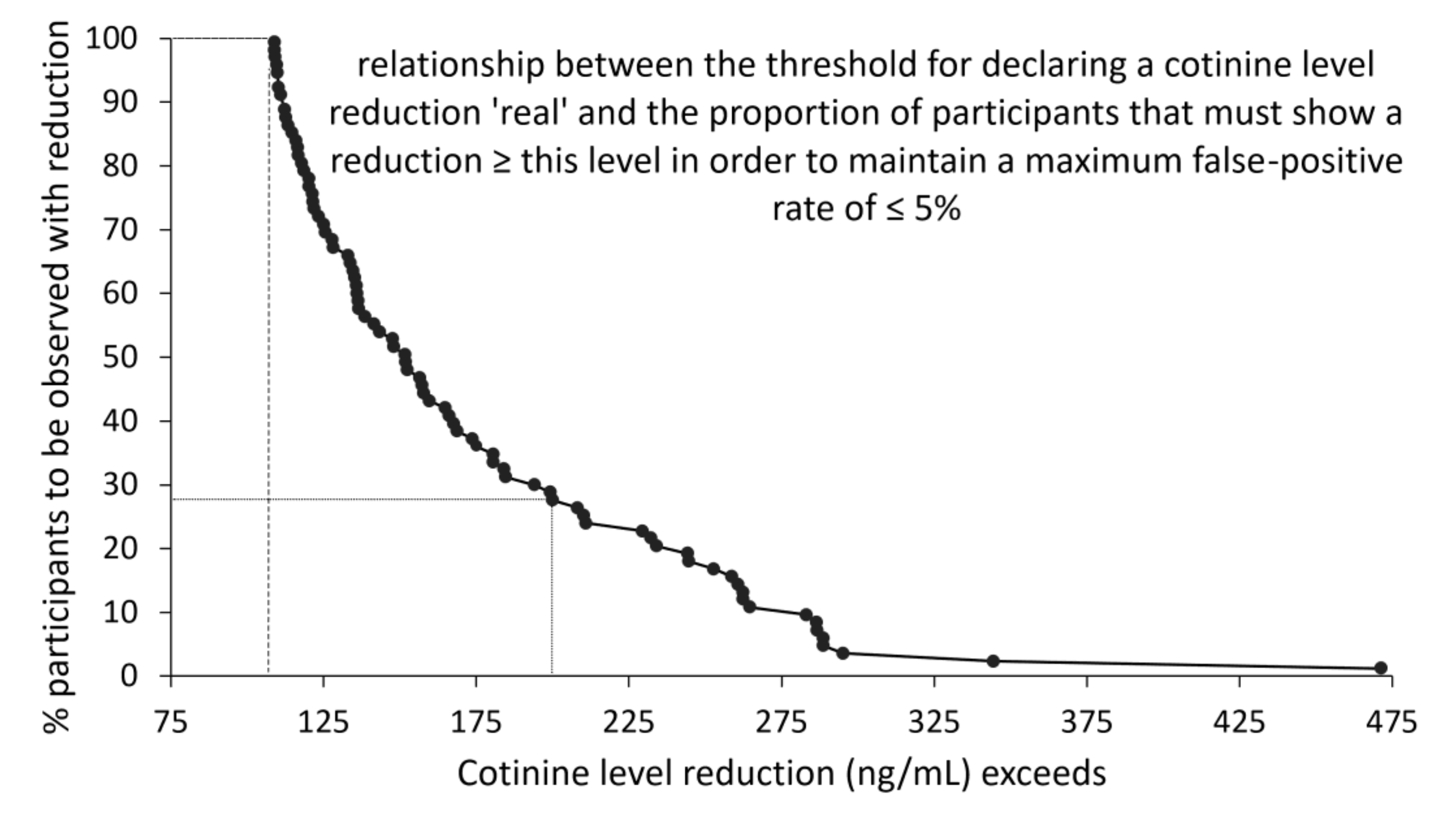
2. Results
3. Discussion
4. Materials and Methods
4.1. Sample Processing and Cotinine Analyses
4.2. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Johnston, R.; Doma, K.; Crowe, M. Nicotine effects on exercise performance and physiological responses in nicotine-naive individuals: A systematic review. Clin. Physiol. Funct. Imaging 2018, 38, 527–538. [Google Scholar] [CrossRef]
- Mundel, T. Nicotine: Sporting friend or foe? A review of athlete use, performance consequences and other considerations. Sports Med. 2017, 47, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Cope, G.F.; Nayyar, P.; Holder, R. Feedback from a point-of-care test for nicotine intake to reduce smoking during pregnancy. Ann. Clin. Biochem. 2003, 40, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.J.; Jones, J.W. Drug Testing in a Drug Court Environment: Common Issues to Address; U.S. Department of Justice: Washington, DC, USA, 2000; p. 27. [Google Scholar]
- Berman, M.; Crane, R. Mandating a tobacco-free workforce: A convergence of business and public health interests. Wm. Mitchell L. Rev. 2008, 34, 1651–1674. [Google Scholar]
- Moore, M.R.; Mason, M.J.; Brown, A.R.; Garcia, C.M.; Seibers, A.D.; Stephens, C.J. Remote biochemical verification of tobacco use: Reducing costs and improving methodological rigor with mailed oral cotinine swabs. Addict. Behav. 2018, 87, 151–154. [Google Scholar] [CrossRef]
- Hindocha, C.; Freeman, T.P.; Curran, H.V. Anatomy of a joint: Comparing self-reported and actual dose of cannabis and tobacco in a joint, and how these are influenced by controlled acute administration. Cannabis Cannabinoid. Res. 2017, 2, 217–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goniewicz, M.L.; Smith, D.M. Are some e-cigarette users “blowing smoke”? Assessing the accuracy of self-reported smoking abstinence in exclusive e-cigarette users. Nicotine Tob. Res. 2019, 21, 699–700. [Google Scholar] [CrossRef]
- Johnson-Kozlow, M.; Wahlgren, D.R.; Hovell, M.F.; Flores, D.M.; Liles, S.; Hofstetter, C.R.; Zellner, J.; Zakarian, J.M. Adolescents validly report their exposure to secondhand smoke. J. Clin. Epidemiol. 2010, 63, 914–919. [Google Scholar] [CrossRef] [Green Version]
- Moran, V.E. Cotinine: Beyond that expected, more than a biomarker of tobacco consumption. Front. Pharmacol. 2012, 3, 173. [Google Scholar] [CrossRef] [Green Version]
- Yuki, D.; Kikuchi, A.; Miura, N.; Kakehi, A.; Onozawa, M. Good relationship between saliva cotinine kinetics and plasma cotinine kinetics after smoking one cigarette. Regul. Toxicol. Pharmacol. 2013, 67, 240–245. [Google Scholar] [CrossRef]
- Murray, D.M.; McBride, C.; Lindquist, R.; Belcher, J.D. Sensitivity and specificity of saliva thiocyanate and cotinine for cigarette smoking: A comparison of two collection methods. Addict. Behav. 1991, 16, 161–166. [Google Scholar] [CrossRef]
- Greeley, D.A.; Valois, R.F.; Bernstein, D.A. Stability of salivary cotinine sent through the U.S. Mail for verification of smoking status. Addict. Behav. 1992, 17, 291–296. [Google Scholar] [CrossRef]
- Eisenber, D.T.A.; Kuzawa, C.W.; Hayes, M.G. Improving qPCR telomere length assays: Controlling for well position effects increases statistical power. Am. J. Hum. Biol. 2015, 27, 570–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Drug Administration Home Page. Bioanalytical Method Validation Guidance for Industry. Available online: http://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry (accessed on 31 August 2020).
- Wong, L.P.; Mohd Salim, S.N.; Alias, H.; Aghamohammadi, N.; Hoe, V.C.W.; Isahak, M.; Ali Mohd, M. The association between e-cigarette use behaviors and saliva cotinine concentration among healthy e-cigarette users in Malaysia. J. Addict. Nurs. 2020, 31, 102–109. [Google Scholar] [CrossRef] [PubMed]
- SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob. Res. 2002, 4, 149–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietz, P.M.; Homa, D.; England, L.J.; Burley, K.; Tong, V.T.; Dube, S.R.; Bernert, J.T. Estimates of nondisclosure of cigarette smoking among pregnant and nonpregnant women of reproductive age in the united states. Am. J. Epidemiol. 2011, 173, 355–359. [Google Scholar] [CrossRef] [Green Version]
- Florescu, A.A.P.A.; Ferrence, R.; Einarson, T.; Selby, P.; Soldin, O.; Koren, G. Methods for quantification of exposure to cigarette smoking and environmental tobacco smoke: Focus on developmental toxicology. Ther. Drug Monit. 2009, 31, 14–30. [Google Scholar] [CrossRef] [Green Version]
- Pell, J.P.; Cobbe, S.M.; Haw, S.J.; Newby, D.E.; Pell, A.C.; Oldroyd, K.G.; Borland, W.; Murdoch, D.L.; Pringle, S.D.; Dunn, F.G.; et al. Validity of self-reported smoking status: Comparison of patients admitted to hospital with acute coronary syndrome and the general population. Nicotine Tob. Res. 2008, 10, 861–866. [Google Scholar] [CrossRef] [Green Version]
- Lewis, S.J.; Cherry, N.M.; Mc, L.N.R.; Barber, P.V.; Wilde, K.; Povey, A.C. Cotinine levels and self-reported smoking status in patients attending a bronchoscopy clinic. Biomarkers 2003, 8, 218–228. [Google Scholar] [CrossRef]
- Stram, D.O.; Huberman, M.; Wu, A.H. Is residual confounding a reasonable explanation for the apparent protective effects of beta-carotene found in epidemiologic studies of lung cancer in smokers? Am. J. Epidemiol. 2002, 155, 622–628. [Google Scholar] [CrossRef] [Green Version]
- Leite, F.R.M.; Nascimento, G.G.; Scheutz, F.; Lopez, R. Effect of smoking on periodontitis: A systematic review and meta-regression. Am. J. Prev. Med. 2018, 54, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Groenwold, R.H.; Klungel, O.H.; Altman, D.G.; van der Graaf, Y.; Hoes, A.W.; Moons, K.G.; Protect, W.P. Adjustment for continuous confounders: An example of how to prevent residual confounding. CMAJ 2013, 185, 401–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renehan, A.G.; Leitzmann, M.F.; Zwahlen, M. Re: Body mass index and risk of lung cancer among never, former, and current smokers. J. Natl. Cancer. Inst. 2012, 104, 1680–1681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hujoel, P.P.; Drangsholt, M.; Spiekerman, C.; DeRouen, T.A. Periodontitis-systemic disease associations in the presence of smoking--causal or coincidental? Periodontology 2000 2002, 30, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Blettner, M. Controlling for continuous confounders in epidemiologic research. Epidemiology 1997, 8, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Stokes, A.; Preston, S.H. How dangerous is obesity? Issues in measurement and interpretation. Popul. Dev. Rev. 2016, 42, 595–614. [Google Scholar] [CrossRef]
- Okuyemi, K.S.; Ahluwalia, J.S.; Richter, K.P.; Mayo, M.S.; Resnicow, K. Differences among african american light, moderate, and heavy smokers. Nicotine Tob. Res. 2001, 3, 45–50. [Google Scholar] [CrossRef]
- D’Agostino, R.B.; Belanger, A.; D’Agostino, R.B., Jr. A suggestion for using powerful and informative tests of normality. Am. Stat. 1990, 44, 316–321. [Google Scholar]
- Giavarina, D. Understanding Bland Altman analyses. Biochem. Med. (Zagreb) 2015, 25, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef]
- Newson, R. Confidence intervals for rank statistics: Percentile slopes, differences, and ratios. Stata J. 2006, 6, 497–520. [Google Scholar] [CrossRef] [Green Version]
- Gunsolley, J.C.; Best, A.M. Change in attachment level. J. Periodontol. 1988, 59, 450–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Storage Conditions | Mean ± SD | Median | Range |
|---|---|---|---|
| Immediate | 456.6 ± 241.9 | 435.5 | 11.3–1318.0 |
| 446.4 ± 234.3 | 436.9 | 16.8–1208.0 | |
| 4 °C for 30 days | 467.7 ± 248.0 | 455.9 | 9.8–1209.0 |
| 4 °C for 90 days | 438.8 ± 215.6 | 422.5 | 20.7–1023.0 |
| −20 °C for 30 days | 432.8 ± 230.3 | 411.2 | 9.4–1215.0 |
| −20 °C for 90 days | 443.9 ± 230.4 | 418.9 | 17.3–1166.0 |
| Self-Reported Smoking Exposure | Mean (95% CI) | Standard Error |
|---|---|---|
| Light (<10 cigarettes per day) | 346.4 (253.1–439.8) | 45.7 |
| Moderate (10–19 cigarettes per day) | 416.0 (360.9–471.1) | 27.6 |
| Heavy (≥20 cigarettes per day) | 542.0 (488.6–595.3) | 26.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leite, F.R.M.; Baelum, V.; Pajaniaye, J.B.; Abildtrup, L.A.; López, R. Effect of Sample Storage Conditions on Measurements of Salivary Cotinine Levels. Metabolites 2020, 10, 365. https://doi.org/10.3390/metabo10090365
Leite FRM, Baelum V, Pajaniaye JB, Abildtrup LA, López R. Effect of Sample Storage Conditions on Measurements of Salivary Cotinine Levels. Metabolites. 2020; 10(9):365. https://doi.org/10.3390/metabo10090365
Chicago/Turabian StyleLeite, Fábio Renato Manzolli, Vibeke Baelum, Julie Becker Pajaniaye, Lisbeth Ann Abildtrup, and Rodrigo López. 2020. "Effect of Sample Storage Conditions on Measurements of Salivary Cotinine Levels" Metabolites 10, no. 9: 365. https://doi.org/10.3390/metabo10090365
APA StyleLeite, F. R. M., Baelum, V., Pajaniaye, J. B., Abildtrup, L. A., & López, R. (2020). Effect of Sample Storage Conditions on Measurements of Salivary Cotinine Levels. Metabolites, 10(9), 365. https://doi.org/10.3390/metabo10090365