Reproducibility of Targeted Lipidome Analyses (Lipidyzer) in Plasma and Erythrocytes over a 6-Week Period
Abstract
1. Introduction
2. Results
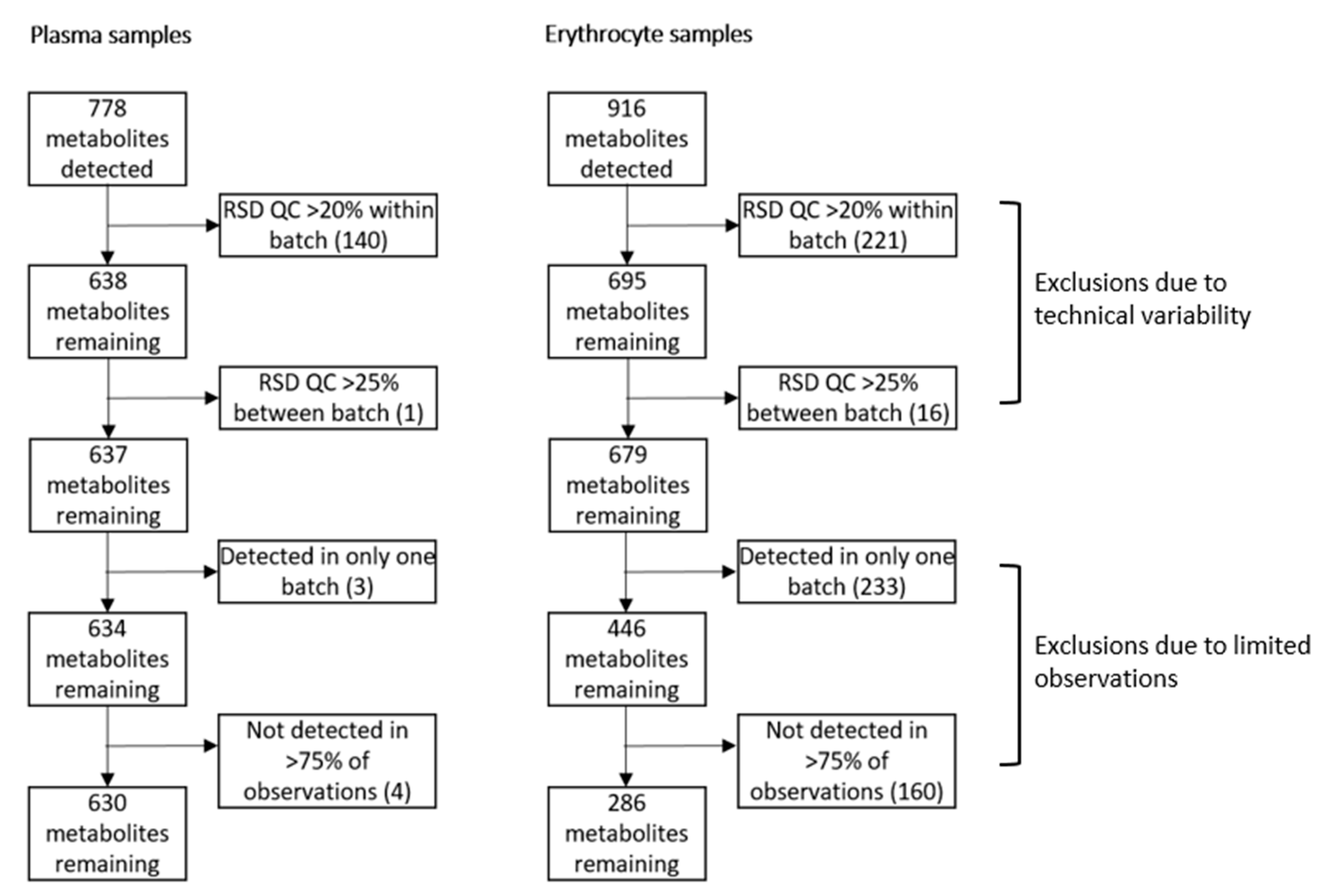
2.1. Study Population and Samples
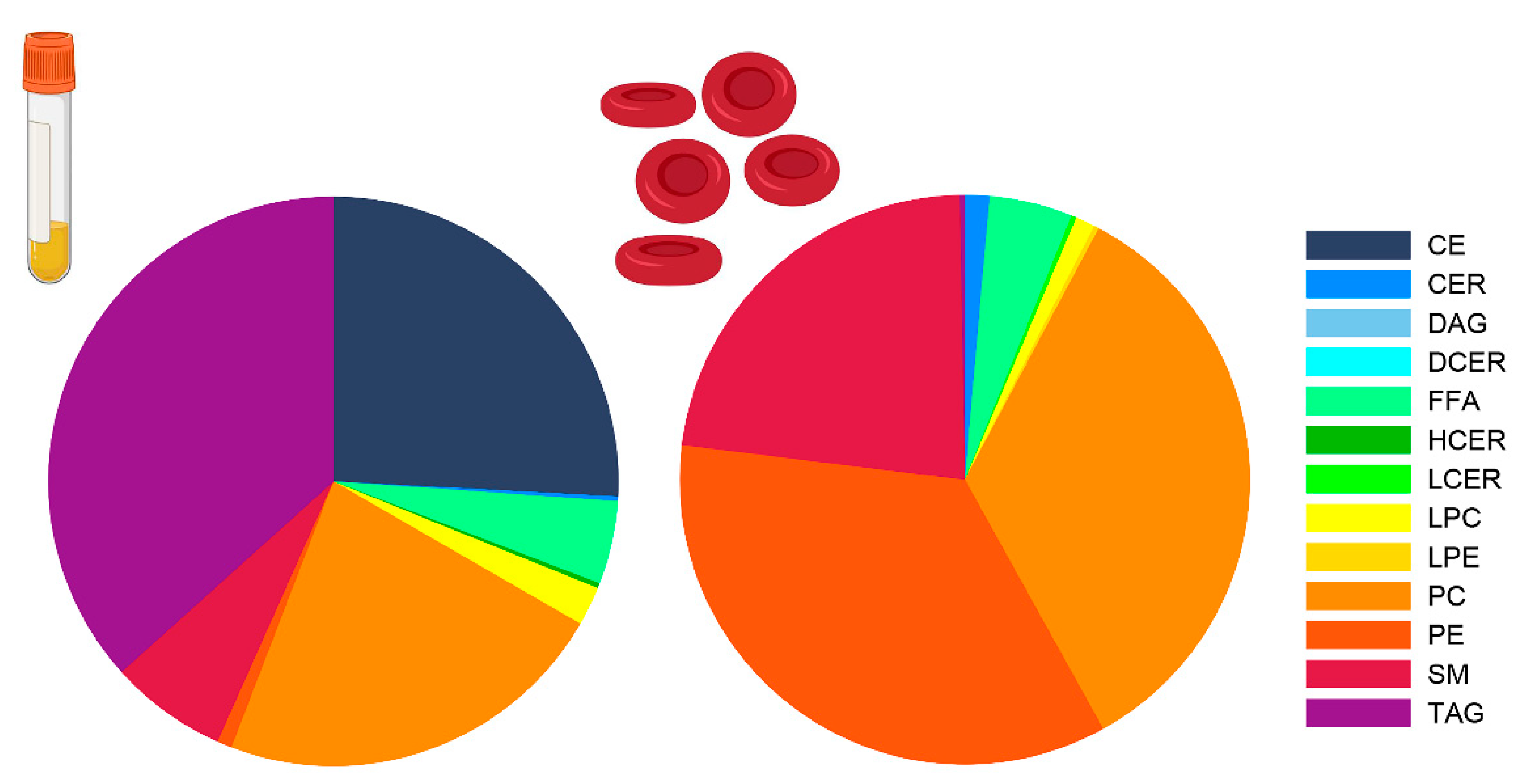
2.2. Lipid Composition in Plasma and Erythrocytes
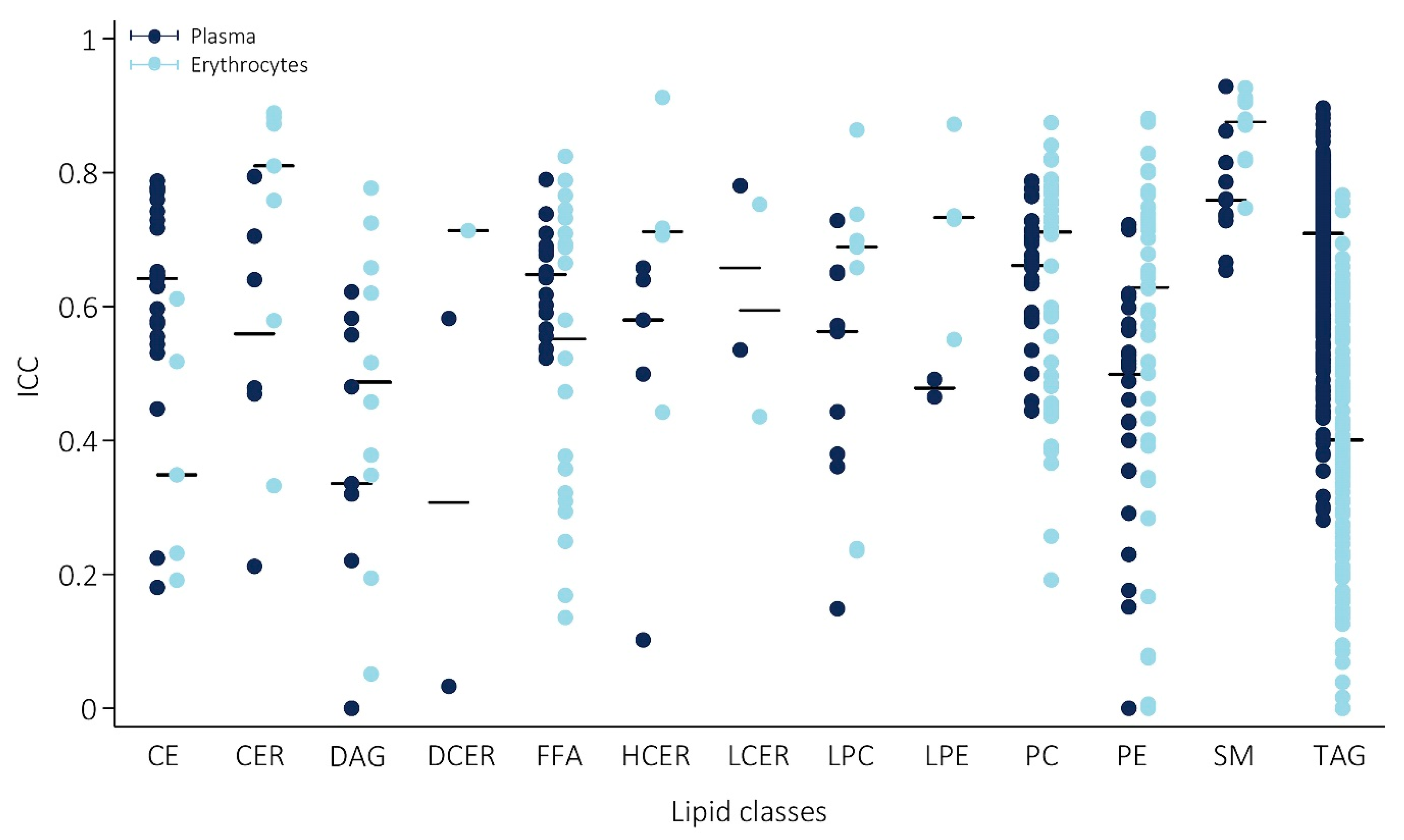
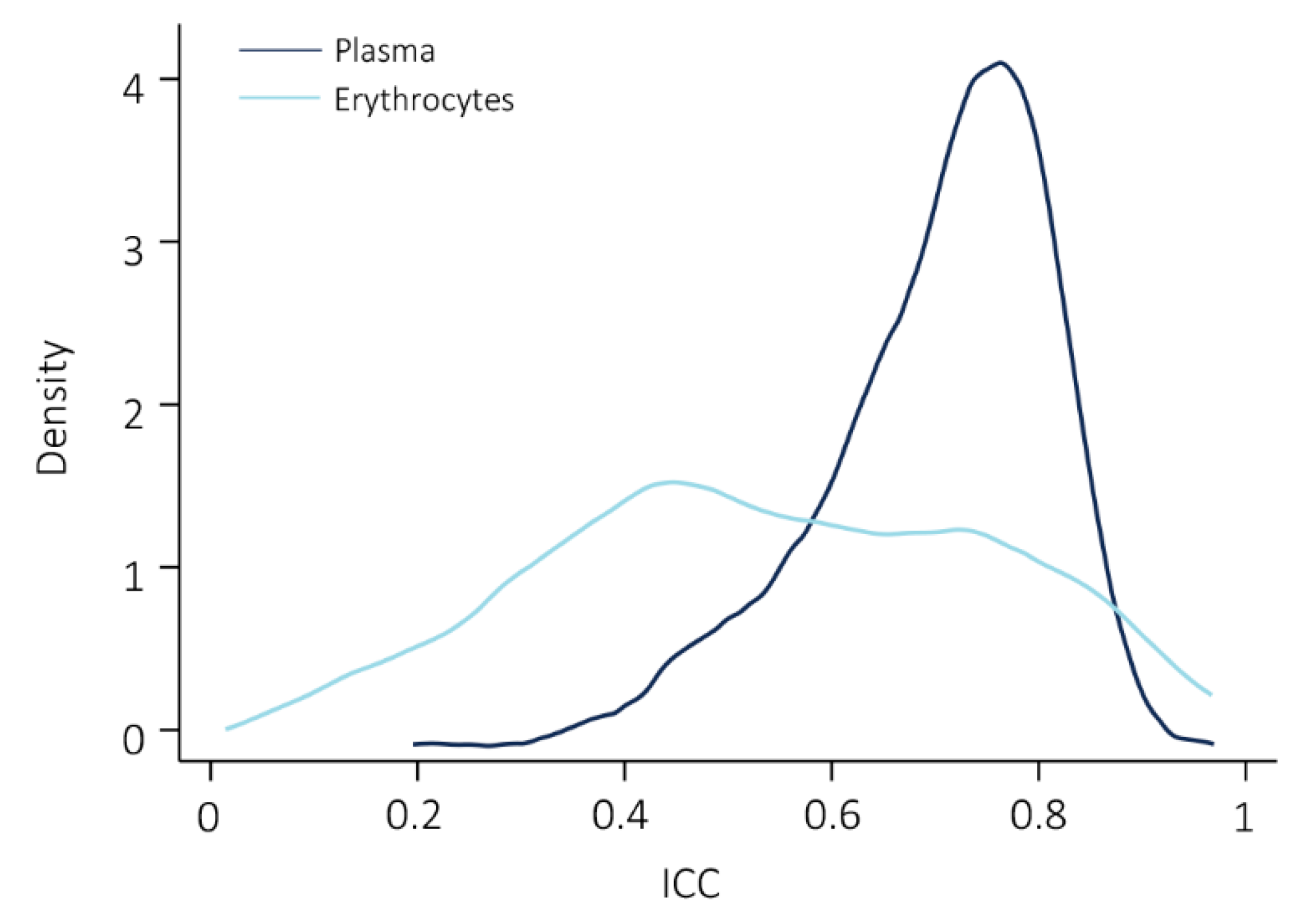
2.3. The Majority of Lipid Species Showed Good Reproducibility in Plasma
2.4. Comparison of Erythrocyte Lipid Reproducibility
3. Discussion
4. Materials and Methods
4.1. Study Design and Patients
4.2. Blood Sampling and Lipidomics Analysis
4.3. Data Processing
4.4. Statistical Analyses
4.5. Data Availability and Lipid Nomenclature
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wymann, M.P.; Schneiter, R. Lipid signalling in disease. Nat. Rev. Mol. Cell Biol. 2008, 9, 162–176. [Google Scholar] [CrossRef]
- Laaksonen, R. Identifying new Risk Markers and Potential Targets for Coronary Artery Disease: The Value of the Lipidome and Metabolome. Cardiovasc. Drugs Ther. 2016, 30, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. The BEST Resource: Harmonizing Biomarker Terminology; 2016. Available online: https://www.fda.gov/media/99221/download (accessed on 28 December 2020).
- Kohler, I.; Verhoeven, A.; Derks, R.J.; Giera, M. Analytical pitfalls and challenges in clinical metabolomics. Bioanalysis 2016, 8, 1509–1532. [Google Scholar] [CrossRef] [PubMed]
- Copeland, K.T.; Checkoway, H.; McMichael, A.J.; Holbrook, R.H. Bias due to misclassification in the estimation of relative risk. Am. J. Epidemiol. 1977, 105, 488–495. [Google Scholar] [CrossRef]
- Breier, M.; Wahl, S.; Prehn, C.; Fugmann, M.; Ferrari, U.; Weise, M.; Banning, F.; Seissler, J.; Grallert, H.; Adamski, J.; et al. Targeted metabolomics identifies reliable and stable metabolites in human serum and plasma samples. PLoS ONE 2014, 9, e89728. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Folsom, A.R.; Eckfeldt, J.H.; Lewis, L.; Chambless, L.E. Short- and long-term repeatability of fatty acid composition of human plasma phospholipids and cholesterol esters. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Am. J. Clin. Nutr. 1995, 62, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; Drogan, D.; Wang-Sattler, R.; Prehn, C.; Illig, T.; Adamski, J.; Joost, H.-G.; Boeing, H.; Pischon, T. Reliability of serum metabolite concentrations over a 4-month period using a targeted metabolomic approach. PLoS ONE 2011, 6, e21103. [Google Scholar] [CrossRef]
- Yu, Z.; Kastenmüller, G.; He, Y.; Belcredi, P.; Möller, G.; Prehn, C.; Mendes, J.; Wahl, S.; Roemisch-Margl, W.; Ceglarek, U.; et al. Differences between human plasma and serum metabolite profiles. PLoS ONE 2011, 6, e21230. [Google Scholar] [CrossRef]
- Khan, M.J.; Codreanu, S.G.; Goyal, S.; Wages, P.A.; Gorti, S.K.K.; Pearson, M.J.; Uribe, I.; Sherrod, S.D.; McLean, J.A.; Porter, N.A.; et al. Evaluating a targeted multiple reaction monitoring approach to global untargeted lipidomic analyses of human plasma. Rapid Commun. Mass Spectrom. 2020, 34, e8911. [Google Scholar] [CrossRef]
- Li-Gao, R.; Hughes, D.A.; le Cessie, S.; de Mutsert, R.; den Heijer, M.; Rosendaal, F.R.; van Dijk, K.W.; Timpson, N.J.; Mook-Kanamori, D.O. Assessment of reproducibility and biological variability of fasting and postprandial plasma metabolite concentrations using 1H NMR spectroscopy. PLoS ONE 2019, 14, e0218549. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.A.; Heckert, A.; Ulmer, C.Z.; Jones, C.M.; Koelmel, J.P.; Abdullah, L.; Ahonen, L.; Alnouti, Y.; Armando, A.M.; Asara, J.M.; et al. Harmonizing lipidomics: NIST interlaboratory comparison exercise for lipidomics using SRM 1950-Metabolites in Frozen Human Plasma. J. Lipid Res. 2017, 58, 2275–2288. [Google Scholar] [CrossRef] [PubMed]
- Cajka, T.; Smilowitz, J.T.; Fiehn, O. Validating Quantitative Untargeted Lipidomics Across Nine Liquid Chromatography-High-Resolution Mass Spectrometry Platforms. Anal. Chem. 2017, 89, 12360–12368. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.W.; Adams, K.J.; Adamski, J.; Asad, Y.; Borts, D.; Bowden, J.A.; Byram, G.; Dang, V.; Dunn, W.B.; Fernandez, F.; et al. International Ring Trial of a High Resolution Targeted Metabolomics and Lipidomics Platform for Serum and Plasma Analysis. Anal. Chem. 2019, 91, 14407–14416. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.S. Plasma triglyceride metabolism. J. Clin. Pathol. 1973, s1-5, 5–10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grundy, S.M.; Denke, M.A. Dietary influences on serum lipids and lipoproteins. J. Lipid Res. 1990, 31, 1149–1172. [Google Scholar] [PubMed]
- Leidl, K.; Liebisch, G.; Richter, D.; Schmitz, G. Mass spectrometric analysis of lipid species of human circulating blood cells. Biochim. Biophys. Acta 2008, 1781, 655–664. [Google Scholar] [CrossRef]
- Han, X. Lipidomics for studying metabolism. Nat. Rev. Endocrinol. 2016, 12, 668–679. [Google Scholar] [CrossRef]
- Kroon, F.P.B.; Kortekaas, M.C.; Boonen, A.; Böhringer, S.; Reijnierse, M.; Rosendaal, F.R.; Riyazi, N.; Starmans, M.; Turkstra, F.; van Zeben, J.; et al. Results of a 6-week treatment with 10 mg prednisolone in patients with hand osteoarthritis (HOPE): A double-blind, randomised, placebo-controlled trial. Lancet 2019, 394, 1993–2001. [Google Scholar] [CrossRef]
- Altman, R.; Alarcón, G.; Appelrouth, D.; Bloch, D.; Borenstein, D.; Brandt, K.; Brown, C.; Cooke, T.D.; Daniel, W.; Gray, R. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis Rheum. 1990, 33, 1601–1610. [Google Scholar] [CrossRef]
- Jonasdottir, H.S.; Brouwers, H.; Toes, R.E.M.; Ioan-Facsinay, A.; Giera, M. Effects of anticoagulants and storage conditions on clinical oxylipid levels in human plasma. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Matyash, V.; Liebisch, G.; Kurzchalia, T.V.; Shevchenko, A.; Schwudke, D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 2008, 49, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Chouvarine, P.; Giera, M.; Kastenmüller, G.; Artati, A.; Adamski, J.; Bertram, H.; Hansmann, G. Trans-right ventricle and transpulmonary metabolite gradients in human pulmonary arterial hypertension. Heart 2020, 106, 1332–1341. [Google Scholar] [CrossRef] [PubMed]
- Alarcon-Barrera, J.C.; von Hegedus, J.H.; Brouwers, H.; Steenvoorden, E.; Ioan-Facsinay, A.; Mayboroda, O.A.; Ondo-Mendez, A.; Giera, M. Lipid metabolism of leukocytes in the unstimulated and activated states. Anal. Bioanal. Chem. 2020, 412, 2353–2363. [Google Scholar] [CrossRef] [PubMed]
- Contrepois, K.; Mahmoudi, S.; Ubhi, B.K.; Papsdorf, K.; Hornburg, D.; Brunet, A.; Snyder, M. Cross-Platform Comparison of Untargeted and Targeted Lipidomics Approaches on Aging Mouse Plasma. Sci. Rep. 2018, 8, 17747. [Google Scholar] [CrossRef] [PubMed]
- Liebisch, G.; Vizcaíno, J.A.; Köfeler, H.; Trötzmüller, M.; Griffiths, W.J.; Schmitz, G.; Spener, F.; Wakelam, M.J.O. Shorthand notation for lipid structures derived from mass spectrometry. J. Lipid Res. 2013, 54, 1523–1530. [Google Scholar] [CrossRef]
| Plasma | Erythrocytes | |||
|---|---|---|---|---|
| Number of Lipid Species | Class Concentration (nmol/mL) | Number of Lipid Species | Class Concentration (nmol/mL) | |
| Triacylglycerols | 482 | 1579.4 (1064.9–3195.2) | 134 | 6.5 (5.6–9.4) |
| Diacylglycerols | 9 | 13.3 (8.4–22.2) | 10 | 5.8 (4.7–6.2) |
| Free fatty acids | 20 | 745.3 (552.0–1202.9) | 20 | 486.9 (379.2–669.2) |
| Cholesteryl esters | 24 | 4571.6 (4065.1–5521.3) | 5 | 1.2 (0.9–1.7) |
| Phosphatidylcholines | 31 | 4013.7 (3203.1–4661.6) | 42 | 3899.2 (3723.0–4296.6) |
| Phosphatidylethanolamines | 26 | 156.2 (120.9–180.3) | 42 | 3954.6 (3721.9–4323.3) |
| Lysophosphatidylcholines | 9 | 385.9 (335.6–442.9) | 7 | 119.8 (109.7–168.9) |
| Lysophosphatidylethanolamines | 2 | 4.2 (3.5–4.9) | 4 | 8.6 (6.8–9.7) |
| Sphingomyelins | 12 | 1204.6 (1037.0–1351.9) | 8 | 2695.8 (2434.8–2815.6) |
| Ceramides | 6 | 14.1 (11.9–17.4) | 7 | 163.0 (133.3–186.4) |
| Dihydroceramides | 2 | 1.0 (0.8–1.3) | 1 | 1.8 (1.4–2.1) |
| Hexosylceramides | 5 | 5.1 (4.7–5.9) | 4 | 5.6 (5.0–7.4) |
| Lactosylceramides | 2 | 3.4 (2.7–3.8) | 2 | 23.8 (20.6–33.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loef, M.; von Hegedus, J.H.; Ghorasaini, M.; Kroon, F.P.B.; Giera, M.; Ioan-Facsinay, A.; Kloppenburg, M. Reproducibility of Targeted Lipidome Analyses (Lipidyzer) in Plasma and Erythrocytes over a 6-Week Period. Metabolites 2021, 11, 26. https://doi.org/10.3390/metabo11010026
Loef M, von Hegedus JH, Ghorasaini M, Kroon FPB, Giera M, Ioan-Facsinay A, Kloppenburg M. Reproducibility of Targeted Lipidome Analyses (Lipidyzer) in Plasma and Erythrocytes over a 6-Week Period. Metabolites. 2021; 11(1):26. https://doi.org/10.3390/metabo11010026
Chicago/Turabian StyleLoef, Marieke, Johannes H. von Hegedus, Mohan Ghorasaini, Féline P. B. Kroon, Martin Giera, Andreea Ioan-Facsinay, and Margreet Kloppenburg. 2021. "Reproducibility of Targeted Lipidome Analyses (Lipidyzer) in Plasma and Erythrocytes over a 6-Week Period" Metabolites 11, no. 1: 26. https://doi.org/10.3390/metabo11010026
APA StyleLoef, M., von Hegedus, J. H., Ghorasaini, M., Kroon, F. P. B., Giera, M., Ioan-Facsinay, A., & Kloppenburg, M. (2021). Reproducibility of Targeted Lipidome Analyses (Lipidyzer) in Plasma and Erythrocytes over a 6-Week Period. Metabolites, 11(1), 26. https://doi.org/10.3390/metabo11010026






