Restricted Nitrogen and Water Applications in the Orchard Modify the Carbohydrate and Amino Acid Composition of Nonpareil and Carmel Almond Hulls
Abstract
:1. Introduction
2. Results
2.1. Nonpareil Almond Variety
2.2. Carmel Almond Variety
3. Discussion
4. Materials and Methods
4.1. Site Description
4.2. Sample Collection
4.3. Sample Preparation for NMR
4.4. 1H NMR Spectroscopy Analysis of the Almond Hull Composition
4.5. Data Processing and Multivariate Statistical Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teviotdale, B.L.; Michailides, T.J.; Pscheidt, J.W. Compendium Of Nut Crop Diseases In Temperate Zones; American Phytopathological Society: Portland, OR, USA, 2002; Volume 26, p. 89. [Google Scholar]
- Gouk, C. Almond diseases and disorders. Acta Hortic. Int. Soc. Hortic. Sci. 2016, 1109, 249–254. [Google Scholar] [CrossRef]
- Cline, H. Hull rot is the “gout” of almond diseases. West. Farm Press 2008, 30, 8. [Google Scholar]
- Mirocha, C.J.; Wilson, E.E. Hull rot disease of almonds. Phytopathology 1961, 51, 843–847. [Google Scholar]
- Adaskaveg, J.E. Epidemiology, and control of fungal and bacterial diseases of almond. In Proceedings of the Almond Conference: Disease and Aflatoxin Management Update, Sacramento, CA, USA, 5–7 December 2017. [Google Scholar]
- Kreidl, S.; Wiechel, T.; Faulker, P.; Tesoriero, L.; Edwards, J. Hull rot of almond. In Nutshell—Autumn; Almond Board of Ausralia Inc., 2021; pp. 20–21. [Google Scholar]
- Mirocha, C.J.; DeVay, J.E.; Wilson, E.E. Role of fumaric acid in the hull rot disease of almond. Phytopathology 1961, 51, 851–860. [Google Scholar]
- Teviotdale, B.L.; Michailides, T.J.; Goldhamer, D.A.; Viveros, M.; Schmidt, L.; Hines, V. Cutting off irrigation early may reduce almond hull rot. Calif. Agric. 1994, 48, 33–36. [Google Scholar] [CrossRef]
- Teviotdale, B.L.; Michailides, T.J.; Goldhamer, D.A.; Viveros, M. Reduction of almond hull rot disease caused by Rhizopus stolonifer by early termination of preharvest irrigation. Plant Dis. 1995, 79, 402–405. [Google Scholar] [CrossRef]
- Teviotdale, B.L.; Goldhamer, D.A.; Viveros, M. Effects of deficit irrigation on hull rot disease of almond trees caused by Monilinia fructicola and Rhizopus stolonifer. Plant Dis. 2001, 85, 399–403. [Google Scholar] [CrossRef] [Green Version]
- Teviotdale, B.L. Effect of Four Level of Applied Nitrogen on Three Fungal Diseases of Almond Trees. In Proceedings of the 22nd Almond Research Conference; Almond Board of California, CA, USA, 1994. [Google Scholar]
- Saa, S.; Peach-Fine, E.; Brown, P.; Michailides, T.; Castro, S.; Bostock, R.; Laca, E. Nitrogen increases hull rot and interferes with the hull split phenology in almond (Prunus dulcis). Sci. Hortic. 2016, 199, 41–48. [Google Scholar] [CrossRef]
- Magnuson, J.K.; Lasure, L.L. Organic acid production by filamentous fungi. In Advances in Fungal Biotechnology for Industry, Agriculture, and Medicine; Tkacz, J.S., Lange, L., Eds.; Springer: Boston, MA, USA, 2004; pp. 307–340. [Google Scholar]
- Goldberg, I.; Rokem, J.S.; Pines, O. Organic acids: Old metabolites, new themes. J. Chem. Technol. Biotechnol. 2006, 81, 1601–1611. [Google Scholar] [CrossRef]
- Ward, G.E.; Lockwood, L.B.; May, O.E.; Herrick, H.T. Biochemical studies in the genus Rhizopus. I. The production of dextro-lactic acid. J. Am. Chem. Soc. 1936, 58, 1286–1288. [Google Scholar] [CrossRef]
- Foster, J.W.; Waksman, S.A. The production of fumaric acid by molds belonging to the genus Rhizopus. J. Am. Chem. Soc. 1939, 61, 127–135. [Google Scholar] [CrossRef]
- Maas, R.H.W.; Bakker, R.R.; Eggink, G.; Weusthuis, R.A. Lactic acid production from xylose by the fungus Rhizopus oryzae. Appl. Microbiol. Biotechnol. 2006, 72, 861–868. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Jin, B.; Kelly, J.M. Production of lactic acid from renewable materials by Rhizopus fungi. Biochem. Eng. J. 2007, 35, 251–263. [Google Scholar] [CrossRef]
- Nwokoro, O. Studies on the production of citric acid by Rhizopus stolonifer. Am. Chem. Sci. J. 2015, 6, 205–212. [Google Scholar] [CrossRef]
- Roa Engel, C.; Straathof, A.; Zijlmans, T.; Gulik, W.; Wielen, L. Fumaric acid production by fermentation. Appl. Microbiol. Biotechnol. 2008, 78, 379–389. [Google Scholar] [CrossRef] [Green Version]
- Lennartsson, P.R.; Taherzadeh, M.J.; Edebo, L. Rhizopus. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 284–290. [Google Scholar]
- Kapilan, R. Enzyme production by Rhizophus stolonifer isolated from bread and kinetic properties of the extracellular amylase. Jacobs J. Enzymol. Enzym. Eng. 2015, 1, 1–5. [Google Scholar]
- Papadaki, A.; Androutsopoulos, N.; Patsalou, M.; Koutinas, M.; Kopsahelis, N.; Machado de Castro, A.; Papanikolaou, S.; Koutinas, A. Biotechnological production of fumaric acid: The effect of morphology of Rhizopus arrhizus NRRL 2582. Fermentation 2017, 3, 33. [Google Scholar] [CrossRef] [Green Version]
- Martin-Dominguez, V.; Estevez, J.; Ojembarrena, D.F.; Santos, E.V.; Ladero, M. Fumaric acid production: A biorefinery perspective. Fermentation 2018, 4, 33. [Google Scholar] [CrossRef] [Green Version]
- Sequeira, R.M.; Lew, R.B. Carbohydrate composition of almond hulls. J. Agric. Food Chem. 1970, 18, 950–951. [Google Scholar] [CrossRef] [PubMed]
- Calixto, F.S.; Cañellas, J. Chemical composition of hulls of the sweet almond (Prunus amygdalus). J. Sci. Food Agric. 1982, 33, 336–339. [Google Scholar] [CrossRef]
- Calixto, F.S.; Canellas, J.; Garcia-Raso, A. Gas chromatographic analysis of sugars and sugar-alcohols in the mesocarp, endocarp, and kernel of almond fruit. J. Agric. Food Chem. 1984, 32, 1018–1020. [Google Scholar] [CrossRef]
- DePeters, E.J.; Swanson, K.L.; Bill, H.M.; Asmus, J.; Heguy, J.M. Nutritional composition of almond hulls. Appl. Anim. Sci. 2020, 36, 761–770. [Google Scholar] [CrossRef]
- Aguilar, A.A.; Smith, N.E.; Baldwin, R.L. Nutritional value of almond hulls for dairy cows. J. Dairy Sci. 1984, 67, 97–103. [Google Scholar] [CrossRef]
- Abreu, A.C.; Fernández, I. NMR metabolomics applied on the discrimination of variables influencing tomato (Solanum lycopersicum). Molecules 2020, 25, 3738. [Google Scholar] [CrossRef]
- Goldhamer, D.A.; Viveros, M. Effects of preharvest irrigation cutoff durations and postharvest water deprivation on almond tree performance. Irrig. Sci. 2000, 19, 125–131. [Google Scholar] [CrossRef]
- Meussen, B.; Graaff, L.; Sanders, J.; Weusthuis, R. Metabolic engineering of Rhizopus oryzae for the production of platform chemicals. Appl. Microbiol. Biotechnol. 2012, 94, 875–886. [Google Scholar] [CrossRef] [Green Version]
- Das, R.K.; Brar, S.K.; Verma, M. Fumaric Acid: Production and Application Aspects. In Platform Chemical Biorefinery; Kaur Brar, S., Jyoti Sarma, S., Pakshirajan, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 133–157. [Google Scholar]
- Kowalczyk, S.; Komoń-Janczara, E.; Glibowska, A.; Kuzdraliński, A.; Czernecki, T.; Targoński, Z. A co-utilization strategy to consume glycerol and monosaccharides by Rhizopus strains for fumaric acid production. AMB Express 2018, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kenealy, W.; Zaady, E.; du Preez, J.C.; Stieglitz, B.; Goldberg, I. Biochemical aspects of fumaric acid accumulation by Rhizopus arrihzus. Appl. Environ. Microbiol. 1986, 52, 128. [Google Scholar] [CrossRef] [Green Version]
- Moon, S.K.; Wee, Y.J.; Yun, J.S.; Ryu, H.W. Production of fumaric acid using rice bran and subsequent conversion to succinic acid through a two-step process. Appl. Biochem. Biotechnol. 2004, 113–116, 843–855. [Google Scholar] [CrossRef]
- Stocks, P.K.; Ward, B.Q. Utilization of amino acids by Rhizopus nigricans. Can. J. Microbiol. 1962, 8, 761–767. [Google Scholar] [CrossRef]
- Sorenson, W.G.; Hesseltine, C.W. Carbon and nitrogen utilization by Rhizopus oligosporus. Mycologia 1966, 58, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Thanh, N.V.; Rombouts, F.M.; Nout, M.J.R. Effect of individual amino acids and glucose on activation and germination of Rhizopus oligosporus sporangiospores in tempe starter. J. Appl. Microbiol. 2005, 99, 1204–1214. [Google Scholar] [CrossRef] [PubMed]
- Clarke, K.G. Microbial kinetics during batch, countinuous and fed-batch processes. In Bioprocess Engineering: An Introductory Engineering and Life Science Approach; Elsevier Science & Technology: Jordon Hill, UK, 2013. [Google Scholar]

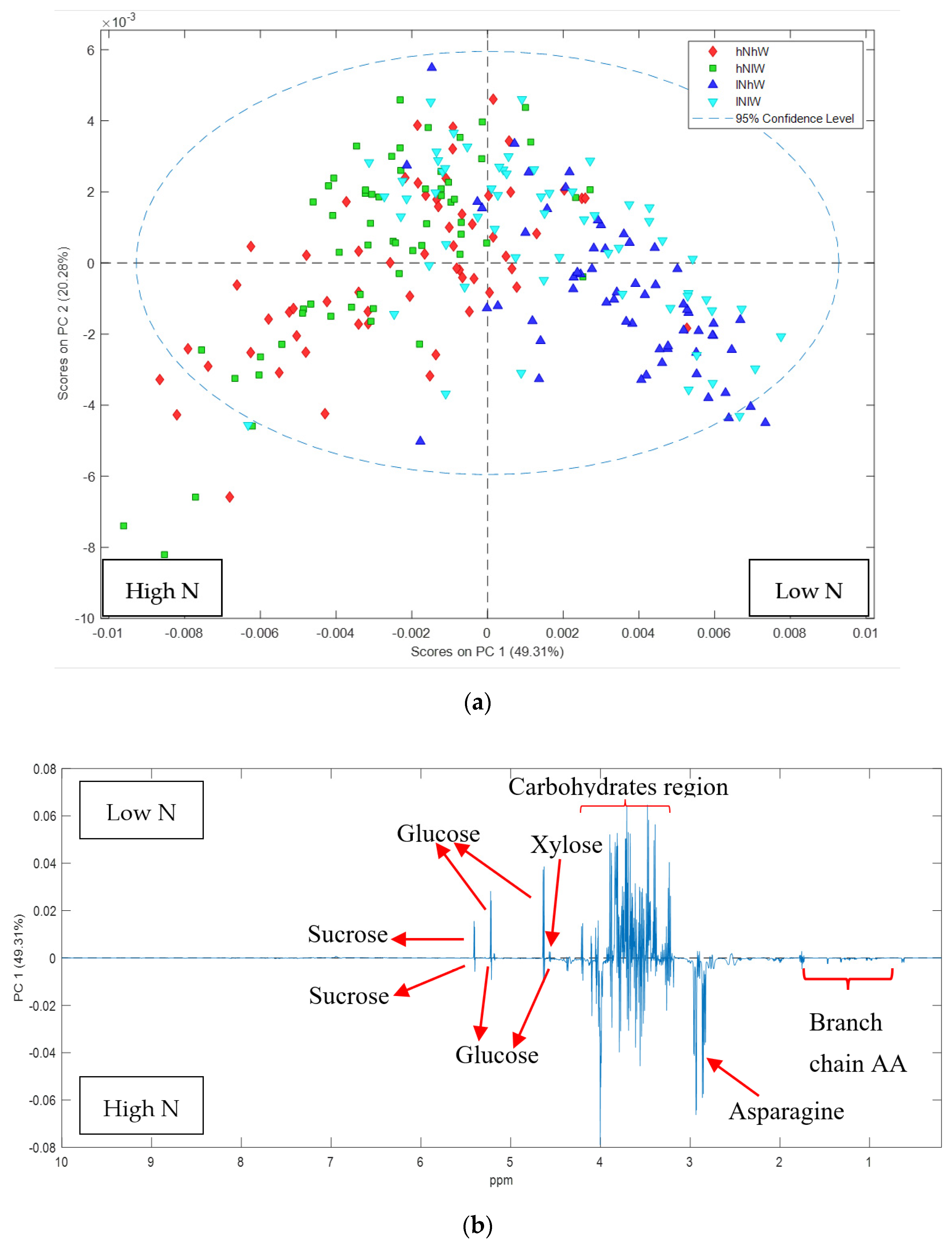







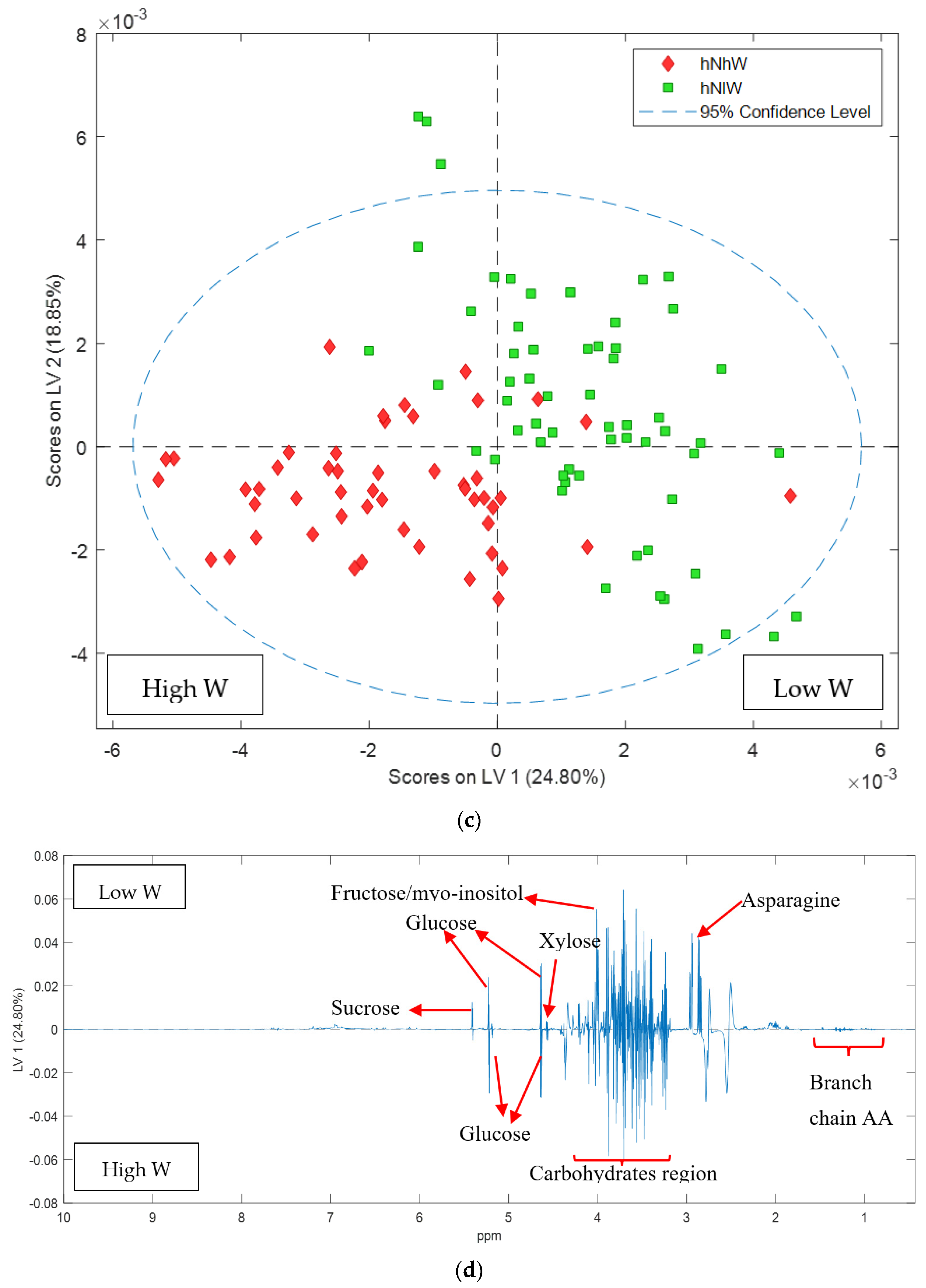
| Treatments | Nonpareil | Carmel |
|---|---|---|
| High N High W | Glucose | |
| Asparagine | ||
| High N Low W | Sucrose | Sucrose Asparagine |
| Low N High W | Glucose | Asparagine |
| Low N Low W | Sucrose | Sucrose Glucose |
| Asparagine |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaveri, A.; Edwards, J.; Rochfort, S. Restricted Nitrogen and Water Applications in the Orchard Modify the Carbohydrate and Amino Acid Composition of Nonpareil and Carmel Almond Hulls. Metabolites 2021, 11, 674. https://doi.org/10.3390/metabo11100674
Zaveri A, Edwards J, Rochfort S. Restricted Nitrogen and Water Applications in the Orchard Modify the Carbohydrate and Amino Acid Composition of Nonpareil and Carmel Almond Hulls. Metabolites. 2021; 11(10):674. https://doi.org/10.3390/metabo11100674
Chicago/Turabian StyleZaveri, Anjali, Jacqueline Edwards, and Simone Rochfort. 2021. "Restricted Nitrogen and Water Applications in the Orchard Modify the Carbohydrate and Amino Acid Composition of Nonpareil and Carmel Almond Hulls" Metabolites 11, no. 10: 674. https://doi.org/10.3390/metabo11100674
APA StyleZaveri, A., Edwards, J., & Rochfort, S. (2021). Restricted Nitrogen and Water Applications in the Orchard Modify the Carbohydrate and Amino Acid Composition of Nonpareil and Carmel Almond Hulls. Metabolites, 11(10), 674. https://doi.org/10.3390/metabo11100674







