A Metabolomics Investigation of the Metabolic Changes of Raji B Lymphoma Cells Undergoing Apoptosis Induced by Zinc Ions
Abstract
:1. Introduction
2. Results
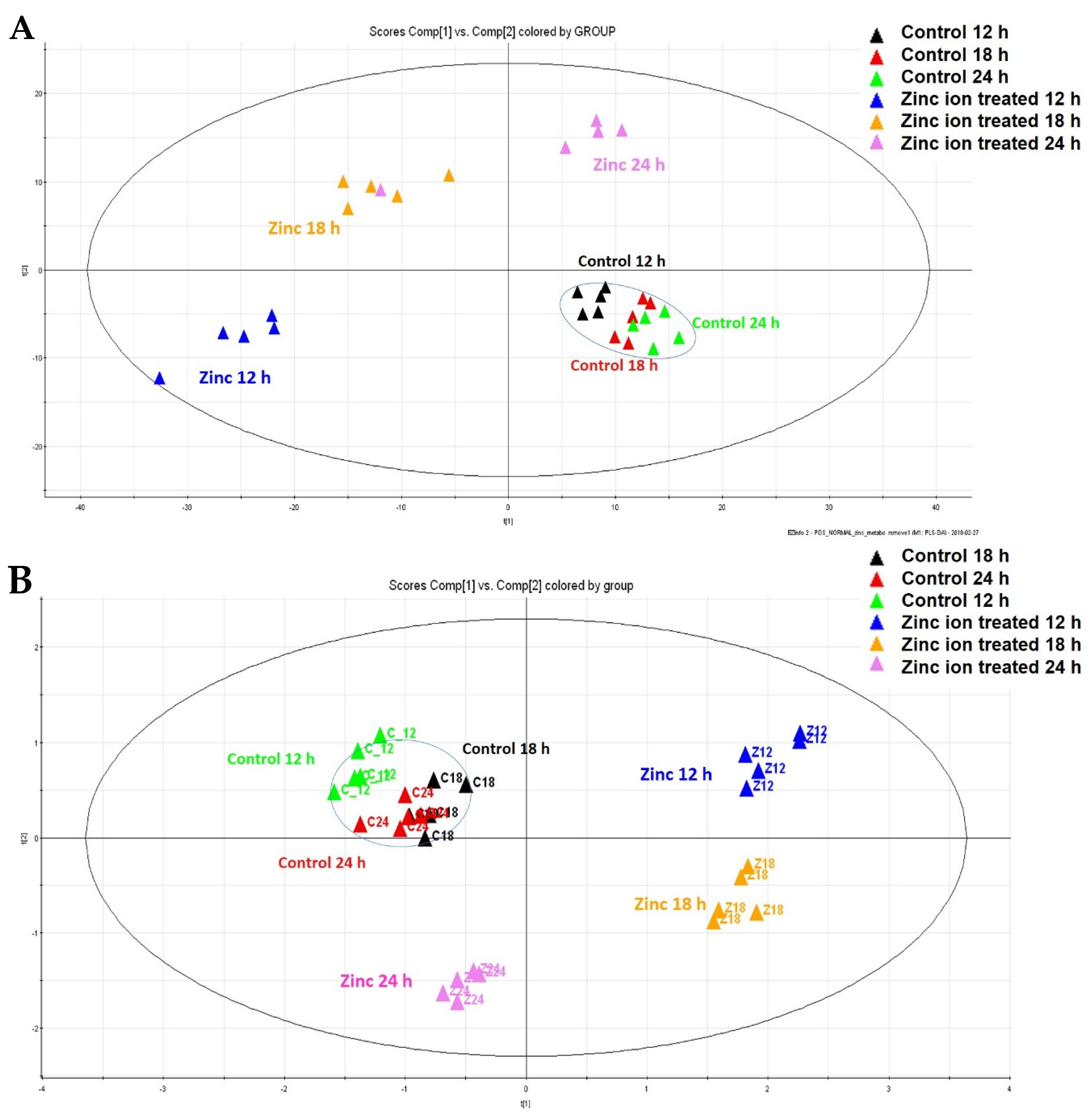
2.1. Identification of the Metabolic Changes by Metabolic Profiling after Zn Ion Treatment of Raji Cells
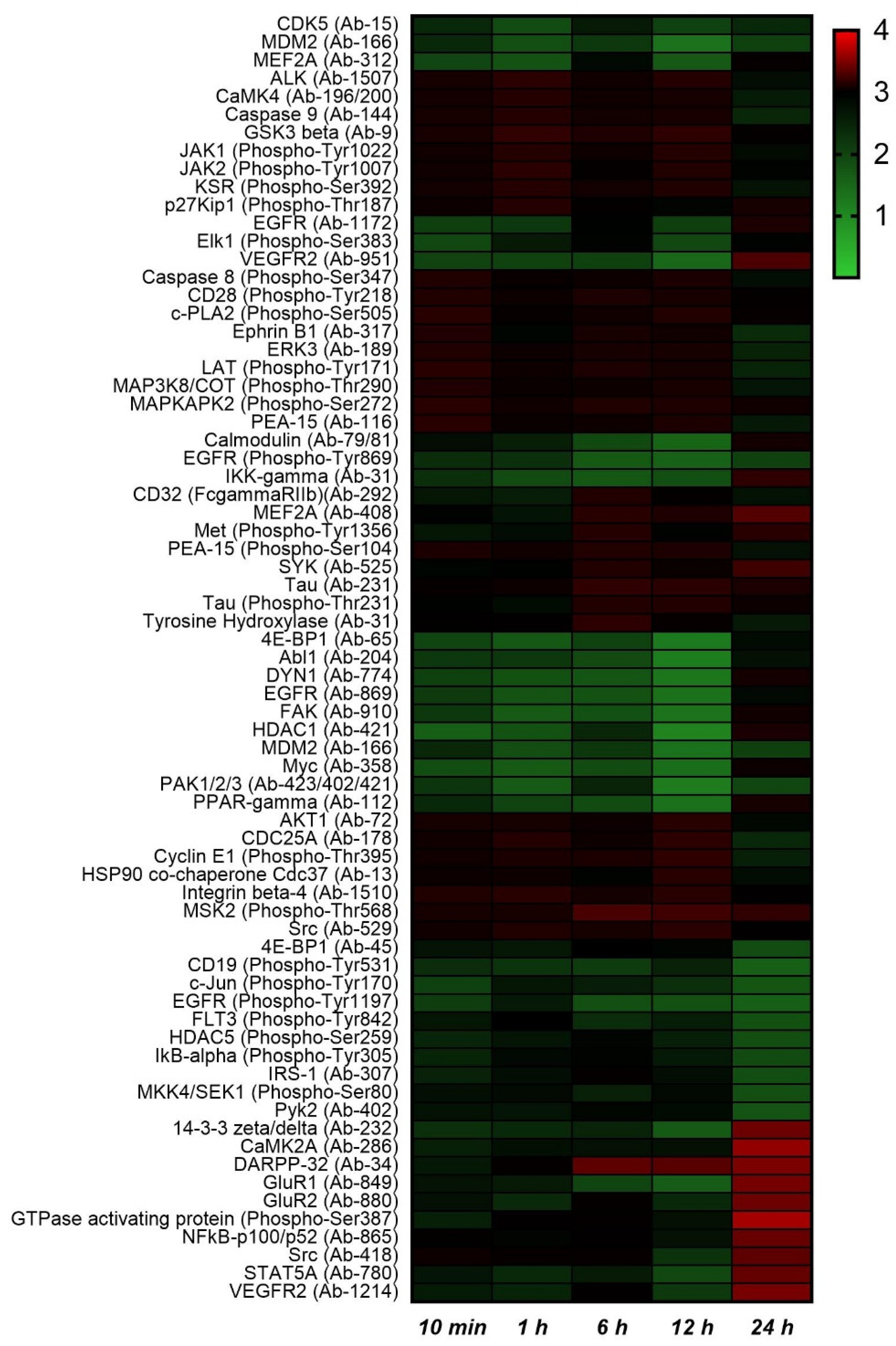
2.2. Protein Phosphorylation Analysis
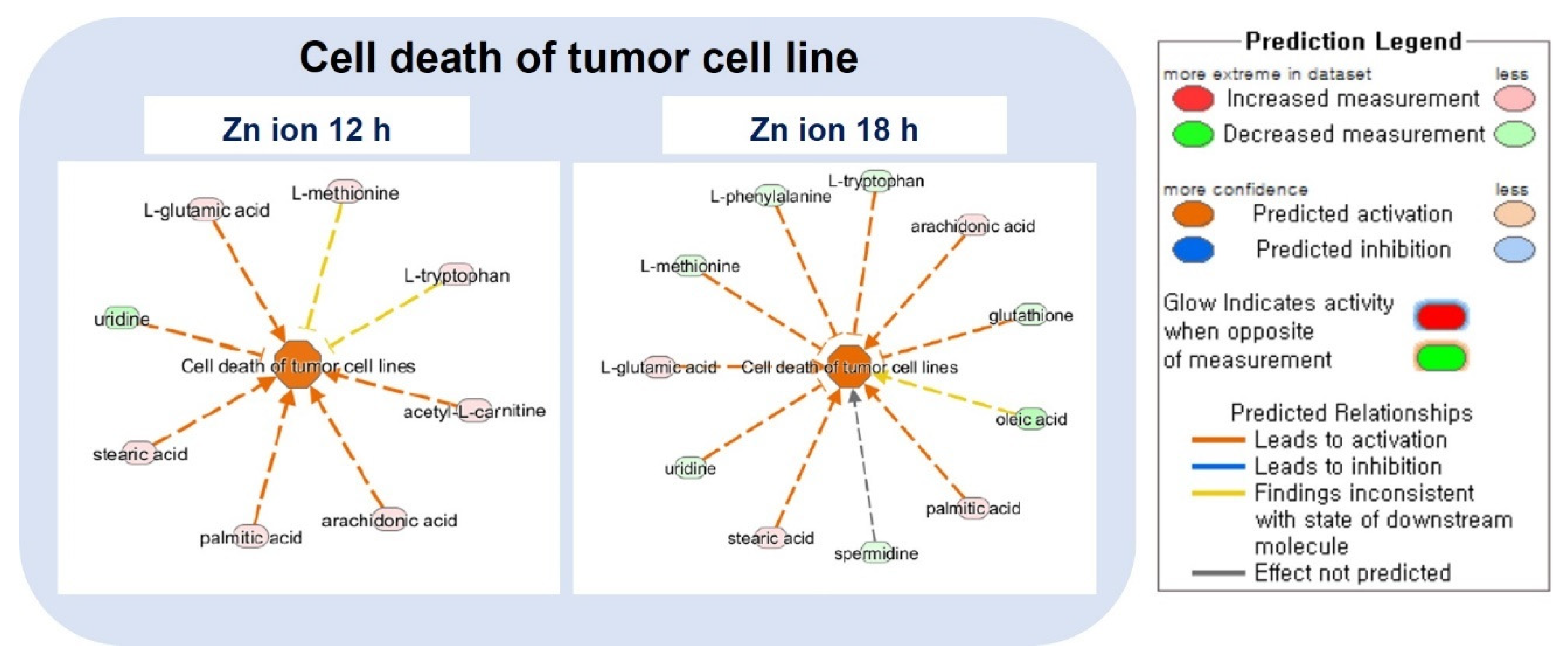
2.3. Ingenuity Pathway Analysis (IPA) and Metabolic Pathway Analysis by KEGG
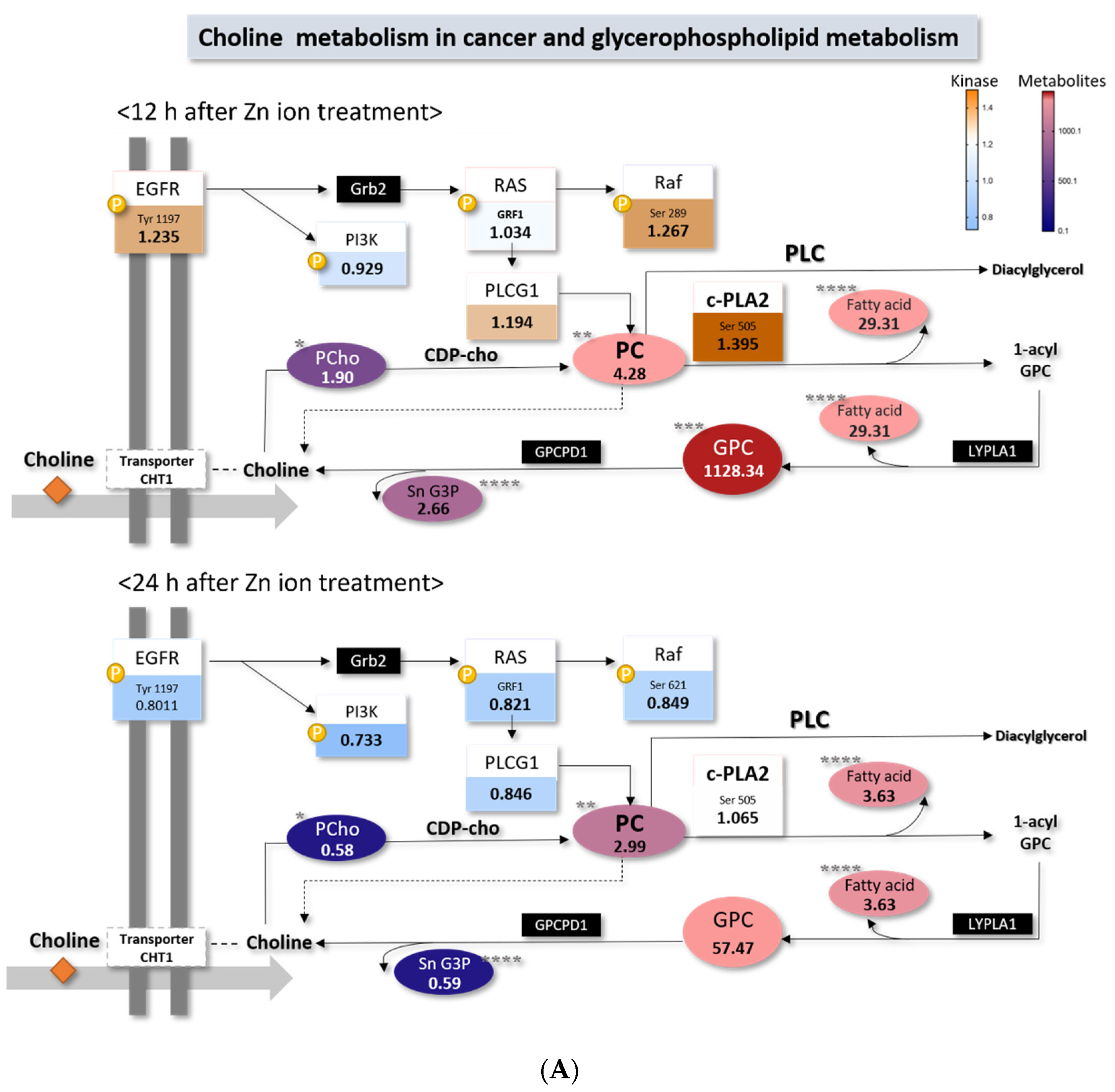
2.4. Choline Metabolism in Cancer Altered by Zn Ions
2.5. Cell Survival with Fatty Acid Treatment
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Metabolite Extraction
4.4. Metabolomic Analysis
4.5. Kinase Arrays
4.6. Data Preprocessing and Statistical Analysis for the Identification of Metabolites
4.7. Quantitative Analysis of Choline in the Cells
4.8. Quantitative Analysis of Arachidonic Acid in the Cells
4.9. Cell Viability Assay
4.10. Western Blot Analysis
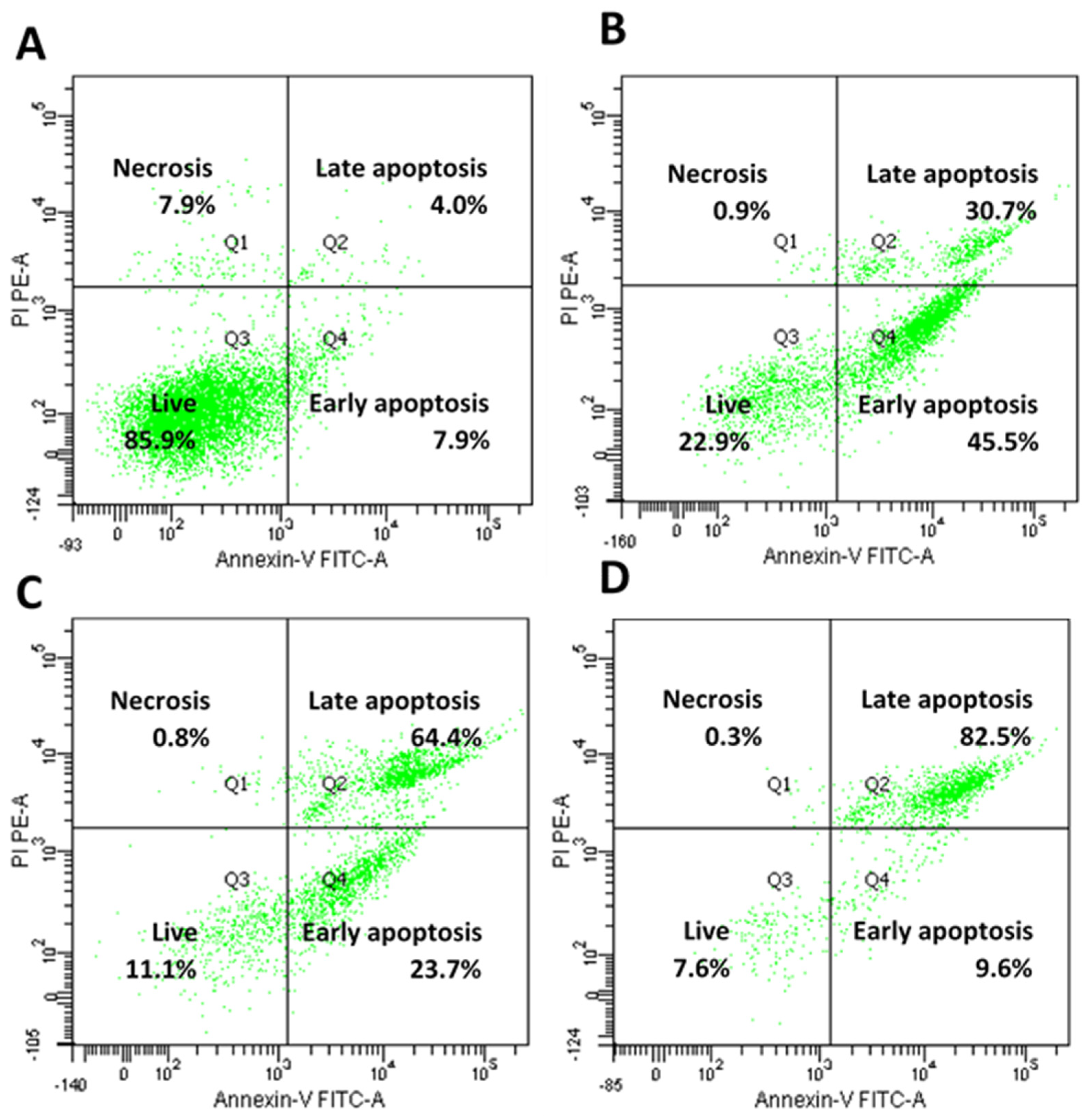
4.11. Annexin-V/Propidium Iodide Staining Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chimienti, F.; Aouffen, M.; Favier, A.; Seve, M. Zinc homeostasis-regulating proteins: New drug targets for triggering cell fate. Curr. Drug Targets 2003, 4, 323–338. [Google Scholar] [CrossRef]
- Franklin, R.B.; Costello, L.C. The important role of the apoptotic effects of zinc in the development of cancers. J. Cell. Biochem. 2009, 106, 750–757. [Google Scholar] [CrossRef] [Green Version]
- Kocdor, H.; Ates, H.; Aydin, S.; Cehreli, R.; Soyarat, F.; Kemanli, P.; Harmanci, D.; Cengiz, H.; Kocdor, M.A. Zinc supplementation induces apoptosis and enhances antitumor efficacy of docetaxel in non-small-cell lung cancer. Drug Des. Dev. Ther. 2015, 9, 3899–3909. [Google Scholar] [CrossRef] [Green Version]
- Feng, P.; Li, T.; Guan, Z.; Franklin, R.B.; Costello, L.C. The involvement of bax in zinc-induced mitochondrial apoptogenesis in malignant prostate cells. Mol. Cancer 2008, 7, 25. [Google Scholar] [CrossRef] [Green Version]
- Schrantz, N.; Auffredou, M.T.; Bourgeade, M.F.; Besnault, L.; Leca, G.; Vazquez, A. Zinc-mediated regulation of caspases activity: Dose-dependent inhibition or activation of caspase-3 in the human Burkitt lymphoma B cells (Ramos). Cell Death Differ. 2001, 8, 152–161. [Google Scholar] [CrossRef] [Green Version]
- Moon, S.-H.; Choi, W.J.; Choi, S.-W.; Kim, E.H.; Kim, J.; Lee, J.-O.; Kim, S.H. Anti-cancer activity of ZnO chips by sustained zinc ion release. Toxicol. Rep. 2016, 3, 430–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guijas, C.; Montenegro-Burke, J.R.; Warth, B.; Spilker, M.E.; Siuzdak, G. Metabolomics activity screening for identifying metabolites that modulate phenotype. Nat. Biotechnol. 2018, 36, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Halama, A.; Riesen, N.; Möller, G.; de Angelis, M.H.; Adamski, J. Identification of biomarkers for apoptosis in cancer cell lines using metabolomics: Tools for individualized medicine. J. Intern. Med. 2013, 274, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Rainaldi, G.; Romano, R.; Indovina, P.; Ferrante, A.; Motta, A.; Indovina, P.L.; Santini, M.T. Metabolomics using1H-NMR of apoptosis and necrosis in HL60 leukemia cells: Differences between the two types of cell death and independence from the stimulus of apoptosis used. Radiat. Res. 2008, 169, 170–180. [Google Scholar] [CrossRef]
- Egawa-Takata, T.; Endo, H.; Fujita, M.; Ueda, Y.; Miyatake, T.; Okuyama, H.; Yoshino, K.; Kamiura, S.; Enomoto, T.; Kimura, T.; et al. Early reduction of glucose uptake after cisplatin treatment is a marker of cisplatin sensitivity in ovarian cancer. Cancer Sci. 2010, 101, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, D.K.; Chadha, V.D. Zinc: A promising agent in dietary chemoprevention of cancer. Indian J. Med. Res. 2010, 132, 676–682. [Google Scholar]
- Witkiewicz-Kucharczyk, A.; Bal, W. Damage of zinc fingers in DNA repair proteins, a novel molecular mechanism in carcinogenesis. Toxicol. Lett. 2006, 162, 29–42. [Google Scholar] [CrossRef]
- Dong, J.; Park, J.-S.; Lee, S.-H. In vitro analysis of the zinc-finger motif in human replication protein A. Biochem. J. 1999, 337, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Pavletich, N.P.; Chambers, K.A.; Pabo, C.O. The DNA-binding domain of p53 contains the four conserved regions and the major mutation hot spots. Genes Dev. 1993, 7, 2556–2564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tezvergil-Mutluay, A.; Agee, K.A.; Hoshika, T.; Carrilho, M.; Breschi, L.; Tjäderhane, L.; Nishitani, Y.; Carvalho, R.M.; Looney, S.; Tay, F.R. The requirement of zinc and calcium ions for functional MMP activity in demineralized dentin matrices. Dent. Mater. 2010, 26, 1059–1067. [Google Scholar] [CrossRef] [Green Version]
- Evanics, F.; Maurmann, L.; Yang, W.W.; Bose, R.N. Nuclear magnetic resonance structures of the zinc finger domain of human DNA polymerase-α. Biochim. Biophys. Acta 2003, 1651, 163–171. [Google Scholar] [CrossRef]
- Hossain, M.A.; Barrow, J.J.; Shen, Y.; Haq, I.; Bungert, J. Artificial zinc finger DNA binding domains: Versatile tools for genome engineering and modulation of gene expression. J. Cell. Biochem. 2015, 116, 2435–2444. [Google Scholar] [CrossRef] [Green Version]
- Brunmeir, R.; Xu, F. Functional regulation of PPARs through post-translational modifications. Int. J. Mol. Sci. 2018, 19, 1738. [Google Scholar] [CrossRef] [Green Version]
- Glunde, K.; Bhujwalla, Z.M.; Ronen, S.M. Choline metabolism in malignant transformation. Nat. Rev. Cancer 2011, 11, 835–848. [Google Scholar] [CrossRef] [Green Version]
- Milkevitch, M.; Shim, H.; Pilatus, U.; Pickup, S.; Wehrle, J.P.; Samid, D.; Poptani, H.; Glickson, J.D.; Delikatny, E.J. Increases in NMR-visible lipid and glycerophosphocholine during phenylbutyrate-induced apoptosis in human prostate cancer cells. Biochim. Biophys. Acta 2005, 1734, 1–12. [Google Scholar] [CrossRef]
- Kettunen, M.; Brindle, K. Apoptosis detection using magnetic resonance imaging and spectroscopy. Prog. Nucl. Magn. Reson. Spectrosc. 2005, 47, 175–185. [Google Scholar] [CrossRef]
- Boyles, M.S.P.; Ranninger, C.; Reischl, R.; Rurik, M.; Tessadri, R.; Kohlbacher, O.; Duschl, A.; Huber, C.G. Copper oxide nanoparticle toxicity profiling using untargeted metabolomics. Part. Fibre Toxicol. 2016, 13, 49. [Google Scholar] [CrossRef] [Green Version]
- Gijón, M.A.; Leslie, C.C. Regulation of arachidonic acid release and cytosolic phospholipase A2 activation. J. Leukoc. Biol. 1999, 65, 330–336. [Google Scholar] [CrossRef]
- Cao, Y.; Pearman, A.T.; Zimmerman, G.A.; McIntyre, T.M.; Prescott, S.M. Intracellular unesterified arachidonic acid signals apoptosis. Proc. Natl. Acad. Sci. USA 2000, 97, 11280–11285. [Google Scholar] [CrossRef] [Green Version]
- Scorrano, L.; Penzo, D.; Petronilli, V.; Pagano, F.; Bernardi, P. Arachidonic acid causes cell death through the mitochondrial permeability transition. J. Biol. Chem. 2001, 276, 12035–12040. [Google Scholar] [CrossRef] [Green Version]
- Wolf, L.A.; Laster, S.M. Characterization of arachidonic acid-induced apoptosis. Cell Biophys. 1999, 30, 353–368. [Google Scholar] [CrossRef]
- Pompeia, C.; Freitas, J.J.; Kim, J.S.; Zyngier, S.B.; Curi, R. Arachidonic acid cytotoxicity in leukocytes: Implications of oxidative stress and eicosanoid synthesis. Biol. Cell 2002, 94, 251–265. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, Y.; Jiang, H.; Nie, D. The role of short-chain fatty acids in orchestrating two types of programmed cell death in colon cancer. Autophagy 2011, 7, 235–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vento, R.; D’Alessandro, N.; Giuliano, M.; Lauricella, M.; Carabillò, M.; Tesoriere, G. Induction of apoptosis by arachidonic acid in human retinoblastoma Y79 cells: Involvement of oxidative stress. Exp. Eye Res. 2000, 70, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Kim, M.-K.; Kim, H.S.; Moon, Y.-A. Arachidonic acid induces ER stress and apoptosis in HT-29 human colon cancer cells. Anim. Cells Syst. 2020, 24, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, M.; Sturlan, S.; Roth, E.; Wessner, B.; Bachleitner-Hofmann, T. Enhancement of arsenic trioxide-mediated apoptosis using docosahexaenoic acid in arsenic trioxide-resistant solid tumor cells. Int. J. Cancer 2004, 112, 707–712. [Google Scholar] [CrossRef]
- Sturlan, S.; Baumgärtner, M.; Roth, E.; Bachleitner-Hofmann, T. Docosahexaenoic acid enhances arsenic trioxide–mediated apoptosis in arsenic trioxide–resistant HL-60 cells. Blood 2003, 101, 4990–4997. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Lee, J.-H.; Kim, S.Y.; Yun, H.-Y.; Baek, K.J.; Kwon, N.S.; Yoon, Y.; Jeong, J.H.; Kim, D.-S. Phosphatidylcholine induces apoptosis of 3T3-L1 adipocytes. J. Biomed. Sci. 2011, 18, 91. [Google Scholar] [CrossRef] [Green Version]
- Khutornenko, A.; Roudko, V.V.; Chernyak, B.; Vartapetian, A.B.; Chumakov, P.; Evstafieva, A.G. Pyrimidine biosynthesis links mitochondrial respiration to the p53 pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 12828–12833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Yang, J.; Chen, M.; Li, L.; Huan, F.; Li, A.; Liu, Y.; Xia, Y.; Duan, J.-A.; Ma, S. Metabolomics profiles delineate uridine deficiency contributes to mitochondria-mediated apoptosis induced by celastrol in human acute promyelocytic leukemia cells. Oncotarget 2016, 7, 46557–46572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.; Liu, J.; Ren, L.; Mao, N.; Tan, F.; Ding, N.; Yang, J.; Li, M. Nutlin-3 downregulates p53 phosphorylation on serine392 and induces apoptosis in hepatocellular carcinoma cells. BMB Rep. 2014, 47, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Lambert, M.H.; Montana, V.G.; Parks, D.J.; Blanchard, S.G.; Brown, P.; Sternbach, D.D.; Lehmann, J.M.; Wisely, G.; Willson, T.M.; et al. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol. Cell 1999, 3, 397–403. [Google Scholar] [CrossRef]
- Edwards, I.J.; O’Flaherty, J.T. Omega-3 fatty acids and PPARgamma in cancer. PPAR Res. 2008, 2008, 358052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, K.; Yang, Y.; Liu, D.; Qi, Y.; Zhang, C.; Zhao, J.; Zhao, S. Activation of PPARgamma suppresses proliferation and induces apoptosis of esophageal cancer cells by inhibiting TLR4-dependent MAPK pathway. Oncotarget 2016, 7, 44572–44582. [Google Scholar] [CrossRef]
- Yang, W.-L.; Frucht, H. Activation of the PPAR pathway induces apoptosis and COX-2 inhibition in HT-29 human colon cancer cells. Carcinogenesis 2001, 22, 1379–1383. [Google Scholar] [CrossRef] [Green Version]
- Hershfinkel, M.; Moran, A.; Grossman, N.; Sekler, I. A zinc-sensing receptor triggers the release of intracellular Ca2+ and regulates ion transport. Proc. Natl. Acad. Sci. USA 2001, 98, 11749–11754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piomelli, D. Arachidonic acid in cell signaling. Curr. Opin. Cell Biol. 1993, 5, 274–280. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, J.; Cady, E.; Edwards, R.; Mccready, V.; Wilkie, D.; Wiltshaw, E. 31P-NMR studies of a human tumour in situ. Lancet 1983, 1, 1435–1436. [Google Scholar] [CrossRef]
- Griffiths, J.R.; Stevens, A.N.; Iles, R.A.; Gordon, R.E.; Shaw, D. 31P-NMR investigation of solid tumours in the living rat. Biosci. Rep. 1981, 1, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kang, S.C.; Yoon, N.E.; Kim, Y.; Choi, J.; Park, N.; Jung, H.; Jung, B.H.; Ju, J.H. Metabolomic profiles of induced pluripotent stem cells derived from patients with rheumatoid arthritis and osteoarthritis. Stem Cell Res. Ther. 2019, 10, 319. [Google Scholar] [CrossRef]
- Broadhurst, D.; Goodacre, R.; Reinke, S.; Kuligowski, J.; Wilson, I.; Lewis, M.R.; Dunn, W.B. Guidelines and considerations for the use of system suitability and quality control samples in mass spectrometry assays applied in untargeted clinical metabolomic studies. Metabolomics 2018, 14, 72. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Choi, J.M.; Cho, J.Y.; Kim, T.E.; Lee, H.J.; Jung, B.H. Regulation of endogenic metabolites by rosuvastatin in hyperlipidemia patients: An integration of metabolomics and lipidomics. Chem. Phys. Lipids 2018, 214, 69–83. [Google Scholar] [CrossRef]
- Pulito, C.; Mori, F.; Sacconi, A.; Casadei, L.; Ferraiuolo, M.; Valerio, M.; Santoro, R.; Goeman, F.; Maidecchi, A.; Mattoli, L.; et al. Cynara scolymus affects malignant pleural mesothelioma by promoting apoptosis and restraining invasion. Oncotarget 2015, 6, 18134–18150. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; Yang, X.; Zhou, J.; Chen, S.; Li, X.; Jian, F.; Deng, P.; Li, W. Analysis of plasma metabolic biomarkers in the development of 4-nitroquinoline-1-oxide-induced oral carcinogenesis in rats. Oncol. Lett. 2015, 9, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Sansone, S.A.; Fan, T.; Goodacre, R.; Griffin, J.L.; Hardy, N.W.; Kaddurah-Daouk, R.; Kristal, B.S.; Lindon, J.; Mendes, P.; Morrison, N.; et al. The metabolomics standards initiative. Nat. Biotechnol. 2007, 25, 846–848. [Google Scholar]
- Pang, Z.; Chong, J.; Li, S.; Xia, J. MetaboAnalystR 3.0: Toward an optimized workflow for global metabolomics. Metabolites 2020, 10, 186. [Google Scholar] [CrossRef] [PubMed]



| Diseases and Biological Functions | Activation z-Score | |
|---|---|---|
| Zn Ion 12 h | Zn Ion 18 h | |
| Growth of organism | −2.88 | −1.61 |
| Cell death of tumor cell lines | 2.63 | 1.83 |
| Activation of microglia | 1.98 | 1.98 |
| Apoptosis of tumor cell lines | 2.08 | 1.85 |
| Killing of cells | 1.96 | 1.94 |
| Cell death of epithelial cells | 2.40 | 1.43 |
| Cell viability | −2.31 | −1.47 |
| Quantity of reactive oxygen species | 2.12 | 1.63 |
| Cell death of carcinoma cell lines | 1.99 | 1.73 |
| Cell viability of tumor cell lines | −2.22 | −1.47 |
| Cancer | −1.04 | −2.58 |
| Uptake of amino acids | 1.57 | −1.93 |
| Cell survival | −2.18 | −1.18 |
| Pathway Name | Zn Ion Treatment Time (h) | ||||
|---|---|---|---|---|---|
| 0.16 | 1 | 6 | 12 | 24 | |
| Canonical Pathway | |||||
| Glioma Signaling | 3.09 | 3.43 | 2.40 | 4.12 | −4.46 |
| Endothelin−1 Signaling | 1.58 | 3.48 | 2.85 | 3.79 | −5.06 |
| Fc Epsilon RI Signaling | 3.68 | 2.72 | 3.36 | 4.00 | −4.00 |
| Phospholipase C Signaling | 2.99 | 1.91 | 4.63 | 3.81 | −2.18 |
| F−κB Signaling | 3.64 | 2.83 | 1.75 | 4.18 | −3.10 |
| Leukocyte Extravasation Signaling | 2.31 | 3.18 | 1.44 | 2.31 | −4.91 |
| CCR3 Signaling in Eosinophils | 2.35 | 2.75 | 1.18 | 3.53 | −4.71 |
| GNRH Signaling | 2.06 | 2.95 | 2.36 | 3.24 | −4.72 |
| NRF2−mediated Oxidative Stress Response | 2.89 | 2.12 | 2.89 | 2.89 | −3.66 |
| Gαq Signaling | 1.72 | 2.97 | 2.34 | 3.90 | −3.90 |
| fMLP Signaling in Neutrophils | 2.12 | 3.18 | 2.12 | 4.24 | −3.54 |
| α−Adrenergic Signaling | 2.50 | 2.05 | 3.00 | 3.00 | −3.50 |
| VEGF Family Ligand-Receptor Interactions | 2.27 | 2.65 | 1.89 | 3.40 | −3.78 |
| ErbB Signaling | 1.85 | 2.78 | 0.93 | 2.47 | −4.94 |
| Macropinocytosis Signaling | 2.84 | 2.40 | 1.96 | 3.27 | −3.27 |
| Fcγ Receptor-mediated Phagocytosis in Macrophages and Monocytes | 2.00 | 3.33 | 3.33 | 2.67 | −2.00 |
| Thrombin Signaling | 1.64 | 2.53 | 2.83 | 3.13 | −3.73 |
| Growth Hormone Signaling | 2.61 | 2.26 | 1.91 | 1.57 | −3.66 |
| CREB Signaling in Neurons | 2.53 | 2.21 | 2.85 | 3.16 | −3.16 |
| Tec Kinase Signaling | 2.18 | 2.18 | 1.91 | 2.99 | −3.27 |
| HGF Signaling | 1.94 | 1.94 | 1.94 | 2.53 | −4.92 |
| AMPK Signaling | 1.67 | 1.67 | 2.67 | 3.33 | −3.33 |
| Diseases and Functions | |||||
| Organismal death | −4.45 | −3.91 | −3.8 | −4.8 | 5.41 |
| Cell movement | 3.97 | 4.66 | 3.21 | 4.57 | −4.62 |
| Cell survival | 4.31 | 5.35 | 2.80 | 4.22 | −4.63 |
| Migration of cells | 3.69 | 4.88 | 3.13 | 4.19 | −4.22 |
| Cell viability | 4.23 | 5.12 | 2.73 | 4.15 | −4.17 |
| Body size | 3.41 | 3.00 | 4.23 | 3.74 | −3.79 |
| Expression of RNA | 2.32 | 2.68 | 4.14 | 3.29 | −4.52 |
| Cell proliferation of tumor cell lines | 1.57 | 4.25 | 2.18 | 3.50 | −3.47 |
| Cell viability of tumor cell lines | 3.45 | 4.34 | 1.80 | 3.07 | −3.59 |
| Transcription | 2.25 | 2.25 | 3.95 | 2.89 | −4.54 |
| Cell movement of tumor cell lines | 2.62 | 3.42 | 2.03 | 3.63 | −3.25 |
| Transcription of RNA | 2.30 | 1.86 | 3.97 | 2.75 | −4.30 |
| Cell viability of blood cells | 1.96 | 3.39 | 3.07 | 3.69 | −2.46 |
| Metabolism of protein | 1.96 | 2.50 | 4.52 | 3.00 | −1.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, N.; Lee, H.; Lee, G.; Kim, E.H.; Kim, S.H.; Lee, J.-O.; Song, Y.; Park, J.; Kim, S.-D.; Kim, Y.; et al. A Metabolomics Investigation of the Metabolic Changes of Raji B Lymphoma Cells Undergoing Apoptosis Induced by Zinc Ions. Metabolites 2021, 11, 689. https://doi.org/10.3390/metabo11100689
Yoon N, Lee H, Lee G, Kim EH, Kim SH, Lee J-O, Song Y, Park J, Kim S-D, Kim Y, et al. A Metabolomics Investigation of the Metabolic Changes of Raji B Lymphoma Cells Undergoing Apoptosis Induced by Zinc Ions. Metabolites. 2021; 11(10):689. https://doi.org/10.3390/metabo11100689
Chicago/Turabian StyleYoon, Naeun, Hyunbeom Lee, Geonhee Lee, Eun Hye Kim, Seong Hwan Kim, Jeong-O Lee, Yunseon Song, Jinyoung Park, So-Dam Kim, Yeojin Kim, and et al. 2021. "A Metabolomics Investigation of the Metabolic Changes of Raji B Lymphoma Cells Undergoing Apoptosis Induced by Zinc Ions" Metabolites 11, no. 10: 689. https://doi.org/10.3390/metabo11100689
APA StyleYoon, N., Lee, H., Lee, G., Kim, E. H., Kim, S. H., Lee, J.-O., Song, Y., Park, J., Kim, S.-D., Kim, Y., & Jung, B. H. (2021). A Metabolomics Investigation of the Metabolic Changes of Raji B Lymphoma Cells Undergoing Apoptosis Induced by Zinc Ions. Metabolites, 11(10), 689. https://doi.org/10.3390/metabo11100689






