The Bark of the Spruce Picea jezoensis Is a Rich Source of Stilbenes
Abstract
:1. Introduction
2. Results
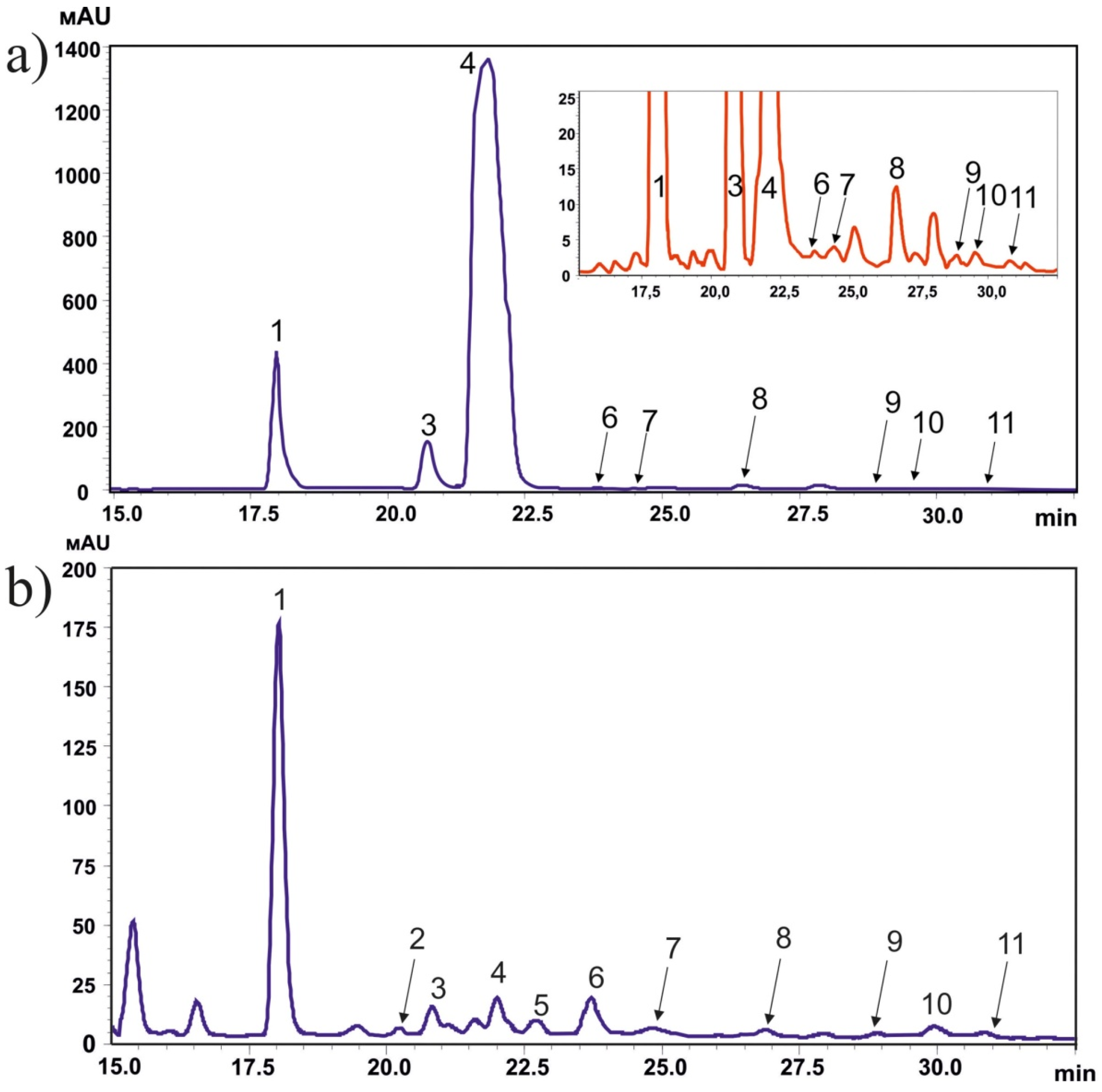
2.1. Stilbene Identification and Quantification in the Bark and Wood of P. jezoensis by HPLC-MS-UV
2.2. The Effect of Different Solvents on Stilbene Extraction from the Bark of P. jezoensis
2.3. The Effect of Different Extraction Temperatures and Extraction Time on Stilbene Yields from the P. jezoensis Bark
2.4. Seasonal Variation in Stilbene Content in the Bark of P. jezoensis
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Optimization of Stilbene Extraction
4.3. High-Performance Liquid Chromatography and Mass-Spectrometry
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seca, A.M.L.; Pinto, D.C.G.A. Biological potential and medical use of secondary metabolites. Medicines 2019, 6, 66. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.; Fareed, S.; Ansari, S.; Rahman, M.; Ahmad, I.Z.; Saeed, M. Current approaches toward production of secondary plant metabolites. J. Pharm. Bioallied. Sci. 2012, 4, 10–20. [Google Scholar] [CrossRef]
- Jeandet, P.; Douillt-Breuil, A.C.; Bessis, R.; Debord, S.; Sbaghi, M.; Adrian, M. Phytoalexins from the Vitaceae: Biosynthesis, phytoalexin gene expression in transgenic plants, antifungal activity, and metabolism. J. Agric. Food Chem. 2002, 50, 2731–2741. [Google Scholar] [CrossRef]
- Kiselev, K.V. Perspectives for production and application of resveratrol. Appl. Microbiol. Biotechnol. 2011, 90, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Suwalsky, M.; Villena, F.; Gallardo, M.J. In vitro protective effects of resveratrol against oxidative damage in human erythrocytes. Biochim. Biophys. Acta-Biomembr. 2015, 1848, 76–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubrovina, A.S.; Kiselev, K.V. Regulation of stilbene biosynthesis in plants. Planta 2017, 346, 597–623. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Tiwari, R.; Tiwari, G.; Ramachandran, V. Resveratrol: A vital therapeutic agent with multiple health benefits. Drug Res. 2021. [Google Scholar] [CrossRef]
- Shayganfard, M. Molecular and biological functions of resveratrol in psychiatric disorders: A review of recent evidence. Cell Biosci. 2020, 10, 128. [Google Scholar] [CrossRef]
- Chong, J.; Poutaraud, A.; Hugueney, P. Metabolism and roles of stilbenes in plants. Plant Sci. 2009, 177, 143–155. [Google Scholar] [CrossRef]
- Schmidlin, L.; Poutaraud, A.; Claudel, P.; Mestre, P.; Prado, E.; Santos-Rosa, M.; Wiedemann-Merdinoglu, S.; Karst, F.; Merdinoglu, D.; Hugueney, P. A stress-inducible resveratrol O-methyltransferase involved in the biosynthesis of pterostilbene in grapevine. Plant Physiol. 2008, 148, 1630–1639. [Google Scholar] [CrossRef] [Green Version]
- Hall, D.; De Luca, V. Mesocarp localization of a bi-functional resveratrol/hydroxycinnamic acid glucosyltransferase of Concord grape (Vitis labrusca). Plant J. 2007, 49, 579–591. [Google Scholar] [CrossRef]
- Pezet, R. Purification and characterization of a 32-kDa laccase-like stilbene oxidase produced by Botrytis cinerea Pers.:Fr. FEMS Microbiol. Lett. 1998, 167, 203–208. [Google Scholar] [CrossRef]
- Keylor, M.H.; Matsuura, B.S.; Stephenson, C.R.J. Chemistry and biology of resveratrol-derived natural products. Chem. Rev. 2015, 115, 8976–9027. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Sobarzo-Sánchez, E.; Sanches Silva, A.; Clément, C.; Nabavi, S.F.; Battino, M.; Rasekhian, M.; Belwal, T.; Habtemariam, S.; Koffas, M.; et al. Whole-cell biocatalytic, enzymatic and green chemistry methods for the production of resveratrol and its derivatives. Biotechnol. Adv. 2020, 38, 107461. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Vannozzi, A.; Sobarzo-Sanchez, E.; Uddin, M.S.; Bru, R.; Martinez-Marquez, A.; Clément, C.; Cordelier, S.; Manayi, A.; Nabavi, S.F.; et al. Phytostilbenes as agrochemicals: Biosynthesis, bioactivity, metabolic engineering and biotechnology. Nat. Prod. Rep. 2021, 28, 1282–1329. [Google Scholar] [CrossRef] [PubMed]
- Belchi-Navarro, S.; Almagro, L.; Sabater-Jara, A.B.; Fernandez-Perez, F.; Bru, R.; Pedreno, M.A. Induction of trans-resveratrol and extracellular pathogenesis-related proteins in elicited suspension cultured cells of Vitis vinifera cv. Monastrell. J. Plant Physiol. 2013, 170, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Ma, L.; Xi, H.F.; Wang, L.J.; Li, S.H. Resveratrol synthesis under natural conditions and after UV-C irradiation in berry skin is associated with berry development stages in ‘Beihong’ (V. vinifera × V. amurensis). Food Chem. 2015, 168, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.-F.; Ma, L.; Wang, L.-N.; Li, S.-H.; Wang, L.-J. Differential response of the biosynthesis of resveratrols and flavonoids to UV-C irradiation in grape leaves. N. Z. J. Crop Hortic. Sci. 2015, 43, 163–172. [Google Scholar] [CrossRef]
- Xu, A.; Zhan, J.C.; Huang, W.D. Effects of ultraviolet C, methyl jasmonate and salicylic acid, alone or in combination, on stilbene biosynthesis in cell suspension cultures of Vitis vinifera L. cv. Cabernet Sauvignon. Plant Cell Tissue Organ Cult. 2015, 122, 197–211. [Google Scholar] [CrossRef]
- Tyunin, A.P.; Kiselev, K.V. Alternations in VaSTS gene cytosine methylation and t-resveratrol production in response to UV-C irradiation in Vitis amurensis Rupr. cells. Plant Cell Tissue Organ Cult. 2016, 124, 33–45. [Google Scholar] [CrossRef]
- Kiselev, K.V.; Aleynova, O.A.; Grigorchuk, V.P.; Dubrovina, A.S. Stilbene accumulation and expression of stilbene biosynthesis pathway genes in wild grapevine Vitis amurensis Rupr. Planta 2017, 245, 151–159. [Google Scholar] [CrossRef]
- Kiselev, K.V.; Ogneva, Z.V.; Suprun, A.R.; Grigorchuk, V.P.; Dubrovina, A.S. Action of ultraviolet-C radiation and p-coumaric acid on stilbene accumulation and expression of stilbene biosynthesis-related genes in the grapevine Vitis amurensis Rupr. Acta Physiol. Plant. 2019, 41, 28. [Google Scholar] [CrossRef]
- Jeandet, P.; Delaunois, B.; Conreux, A.; Donnez, D.; Nuzzo, V.; Cordelier, S.; Clément, C.; Courot, E. Biosynthesis, metabolism, molecular engineering and biological functions of stilbene phytoalexins in plants. Biofactors 2010, 36, 331–341. [Google Scholar] [CrossRef]
- Pearce, R.B. Effects of exposure to high ozone concentrations on stilbenes in Sitka spruce (Picea sitchensis (Bong.) Carr.) bark and on its lignification response to infection with Heterobasidion annosum (Fr.) Bref. Physiol. Mol. Plant Pathol. 1996, 48, 117–129. [Google Scholar] [CrossRef]
- Kiselev, K.V.; Grigorchuk, V.P.; Ogneva, Z.V.; Suprun, A.R.; Dubrovina, A.S. Stilbene biosynthesis in the needles of spruce Picea jezoensis. Phytochemistry 2016, 131, 57–67. [Google Scholar] [CrossRef]
- Kiselev, K.V.; Grigorchuk, V.P.; Ogneva, Z.V.; Suprun, A.R.; Dubrovina, A.S. The effect of ultraviolet-C and precursor feeding on stilbene biosynthesis in spruce Picea jezoensis. J. Plant Physiol. 2019, 234–235, 133–137. [Google Scholar] [CrossRef]
- Latva-Mäenpää, H.; Laakso, T.; Sarjala, T.; Wähälä, K.; Saranpää, P. Variation of stilbene glucosides in bark extracts obtained from roots and stumps of Norway spruce (Picea abies [L.] Karst.). Trees-Struct. Funct. 2013, 27, 131–139. [Google Scholar] [CrossRef]
- Ioannidis, K.; Melliou, E.; Alizoti, P.; Magiatis, P. Identification of black pine (Pinus nigra Arn.) heartwood as a rich source of bioactive stilbenes by qNMR. J. Sci. Food Agric. 2017, 97, 1708–1716. [Google Scholar] [CrossRef]
- Francezon, N.; Meda, N.-S.-B.R.; Stevanovic, T. Optimization of bioactive polyphenols extraction from Picea mariana bark. Molecules 2017, 22, 2118. [Google Scholar] [CrossRef] [Green Version]
- Kosovic, E.; Topiař, M.; Cuřínová, P.; Sajfrtova, M. Stability testing of resveratrol and viniferin obtained from Vitis vinifera L. by various extraction methods considering the industrial viewpoint. Sci. Rep. 2020, 10, 5564. [Google Scholar] [CrossRef] [Green Version]
- Suprun, A.R.; Dubrovina, A.S.; Tyunin, A.P.; Kiselev, K.V. Profile of stilbenes and other phenolics in Fanagoria white and red Russian wines. Metabolites 2021, 11, 231. [Google Scholar] [CrossRef]
- Piao, S.J.; Chen, L.X.; Kang, N.; Qiu, F. Simultaneous determination of five characteristic stilbene glycosides in root bark of Morus albus L. (Cortex Mori) using High-performance liquid chromatography. Phytochem. Anal. 2011, 22, 230–235. [Google Scholar] [CrossRef]
- Benova, B.; Adam, M.; Onderkova, K.; Kralovsky, J.; Krajicek, M. Analysis of selected stilbenes in Polygonum cuspidatum by HPLC coupled with CoulArray detection. J. Sep. Sci. 2008, 31, 2404–2409. [Google Scholar] [CrossRef]
- Houilleé, B.; Besseau, S.; Delanoue, G.; Oudin, A.; Papon, N.; Clastre, M.; Simkin, A.J.; Gueérin, L.; Courdavault, V.; Giglioli-Guivarch, N.; et al. Composition and tissue-specific distribution of stilbenoids in grape canes are affected by downy mildew pressure in the vineyard. J. Agric. Food Chem. 2015, 63, 8472–8477. [Google Scholar] [CrossRef]
- Solhaug, K.A. Stilbene glucosides in bark and needles from picea species. Scand. J. For. Res. 1990, 5, 59–67. [Google Scholar] [CrossRef]
- Valletta, A.; Iozia, L.M.; Leonelli, F. Impact of environmental factors on stilbene biosynthesis. Plants 2021, 10, 90. [Google Scholar] [CrossRef]
- Plumed-Ferrer, C.; Vakevainen, K.; Komulainen, H.; Rautiainen, M.; Smeds, A.; Raitanen, J.E.; Eklund, P.; Willfor, S.; Alakomi, H.L.; Saarela, M.; et al. The antimicrobial effects of wood-associated polyphenols on food pathogens and spoilage organisms. Int. J. Food Microbiol. 2013, 164, 99–107. [Google Scholar] [CrossRef]
- Ferrentino, G.; Haman, N.; Morozova, K.; Tonon, G.; Scampicchio, M. Phenolic compounds extracted from spruce (Picea abies) by supercritical carbon dioxide as antimicrobial agents against gram-positive bacteria assessed by isothermal calorimetry. J. Therm. Anal. Calorim. 2021, 145, 3093–3103. [Google Scholar] [CrossRef]
- Gabaston, J.; Richard, T.; Biais, B.; Waffo-Téguo, P.; Pedrot, E.; Jourdes, M.; Corio-Costet, M.; Mérillon, J. Stilbenes from common spruce (Picea abies) bark as natural antifungal agent against downy mildew (Plasmopara viticola). Ind. Crops Prod. 2017, 103, 267–273. [Google Scholar] [CrossRef]
- Shibutani, S.; Samejima, M.; Saburi, Y. Antimicrobial activity of extractives from the bark of Japanese coniferous trees. Bull. Tokyo Univ For. 1989, 99, 219–233. [Google Scholar]
- Shibutani, S.; Samejima, M.; Doi, S. Effects of stilbenes from bark of Picea glehnii (Sieb. et Zucc) and their related compounds against feeding behaviour of Reticulitermes speratus (Kolbe). J. Wood Sci. 2004, 50, 439–444. [Google Scholar] [CrossRef]
- Soural, I.; Vrchotová, N.; Tříska, J.; Balík, J.; Horník, Š.; Cuřínová, P.; Sýkora, J. Various extraction methods for obtaining stilbenes from grape cane of Vitis vinifera L. Molecules 2015, 20, 6093–6112. [Google Scholar] [CrossRef] [Green Version]
- Aleynova, O.A.; Suprun, A.R.; Nityagovsky, N.N.; Dubrovina, A.S.; Kiselev, K.V. The influence of the grapevine bacterial and fungal endophytes on biomass accumulation and stilbene production by the in vitro cultivated cells of Vitis amurensis Rupr. Plants 2021, 10, 1276. [Google Scholar] [CrossRef]
- Dubrovina, A.S.; Aleynova, O.A.; Manyakhin, A.Y.; Kiselev, K.V. The role of calcium-dependent protein kinase genes CPK16, CPK25, CPK30, and CPK32 in stilbene biosynthesis and the stress resistance of grapevine Vitis amurensis Rupr. Appl. Biochem. Microbiol. 2018, 54, 410–417. [Google Scholar] [CrossRef]
- Huang, X.; Mazza, G. Simultaneous analysis of serotonin, melatonin, piceid and resveratrol in fruits using liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 3890–3899. [Google Scholar] [CrossRef]

| Stilbenes | Bark, mg/g DW | Needles, mg/g DW | Wood, mg/g DW |
|---|---|---|---|
| trans-astringin | 16.32 ± 2.20 a | 6.49 ± 1.01 b | 0.02 ± 0.01 c |
| cis-astringin | 0.02 ± 0.01 a | 0.02 ± 0.01 a | 0.01 ± 0.01 a |
| trans-piceid | 4.14 ± 0.30 a | 1.36 ± 0.62 b | 0.01 ± 0.01 c |
| trans-isorhapontin | 144.28 ± 11.38 a | 0 b | 0 b |
| trans-piceatannol | 0.01 ± 0.01 b | 0.04 ± 0.01 a | 0.02 ± 0.01 ab |
| cis-piceid | 0.01 ± 0.01 a | 0.02 ± 0.01 a | 0 a |
| cis-isorhapontin | 0.32 ± 0.09 a | 0 b | 0 b |
| trans-resveratrol | 0.14 ± 0.01 a | 0.24 ± 0.07 a | 0.01 ± 0.01 b |
| trans-isorhapontigenin | 0.02 ± 0.01 a | 0.01 ± 0.01 ab | 0 b |
| cis-resveratrol | 0.02 ± 0.01 a | 0.01 ± 0.01 ab | 0 b |
| cis-isorhapontigenin | 0.01 ± 0.01 a | 0 a | 0 a |
| Total | 165.29 ± 14.49 a | 8.19 ± 1.71 b | 0.07 ± 0.02 c |
| Stilbenes, mg/g DW | MeOH (100%) | MeOH (70%) | EtOH (96%) | EtOH (70%) | H2O | Hexane | Ethyl Acetate | Acetone |
|---|---|---|---|---|---|---|---|---|
| t-astringin | 26.46 ± 2.75 a | 20.78 ± 1.88 ab | 16.72 ± 0.94 b | 16.17 ± 1.43 bc | 7.11 ± 0.56 e | 0.87 ± 0.22 f | 5.91 ± 0.55 e | 10.09 ± 0.74 d |
| cis-astringin | 0.71 ± 0.22 a | 0.59 ± 0.18 ab | 0.14 ± 0.06 c | 0.17 ± 0.08 c | 0.38 ± 0.10 b | 0 d | 0 d | 0 d |
| t-piceid | 6.73 ± 0.78 a | 5.95 ± 0.79 ab | 4.91 ± 0.67 b | 4.08 ± 0.59 b | 2.14 ± 0.33 c | 0.21 ± 0.06 e | 1.47 ± 0.12 d | 2.40 ± 0.32 c |
| t-isorhapontin | 193.16 ± 2.21 a | 174.46 ± 1.98 b | 148.5 ± 1.77 c | 130.79 ± 5.04 d | 64.71 ± 2.89 g | 6.62 ± 1.16 i | 46.80 ± 1.32 h | 74.52 ± 1.16 f |
| t-piceatannol | 0 b | 0.01 ± 0.01 b | 0.01 ± 0.01 b | 0.01 ± 0.01 b | 0.27 ± 0.05 a | 0 b | 0.01 ± 0.01 b | 0 b |
| cis-piceid | 0.09 ± 0.04 b | 0.12 ± 0.05 ab | 0.02 ± 0.01 c | 0.06 ± 0.02 b | 0.26 ± 0.08 a | 0 c | 0.01 ± 0.01 c | 0 c |
| cis-isorhapontin | 1.58 ± 0.11 a | 1.59 ± 0.21 a | 0.41 ± 0.09 c | 0.59 ± 0.09 c | 1.04 ± 0.10 b | 0 e | 0.13 ± 0.07 d | 0.27 ± 0.08 cd |
| t-resveratrol | 0.16 ± 0.04 b | 0.16 ± 0.03 b | 0.16 ± 0.05 b | 0.20 ± 0.06 ab | 0.42 ± 0.12 a | 0.01 ± 0.01 c | 0.02 ± 0.01 c | 0.03 ± 0.02 c |
| t-isorhapontigenin | 0.13 ± 0.03 a | 0 b | 0.02 ± 0.01 b | 0.03 ± 0.02 b | 0.03 ± 0.02 b | 0 b | 0 b | 0 b |
| cis-resveratrol | 0.05 ± 0.03 a | 0.05 ± 0.02 a | 0.04 ± 0.02 ab | 0.02 ± 0.01 ab | 0.02 ± 0.01 ab | 0 b | 0.01 ± 0.01 ab | 0.01 ± 0.01 ab |
| cis-isorhapontigenin | 0.01 ± 0.01 a | 0.01 ± 0.01 a | 0.01 ± 0.01 a | 0 a | 0 a | 0 a | 0 a | 0 a |
| Total | 229.08 ± 6.57 a | 203.72 ± 4.12 b | 170.94 ± 6.68 c | 152.12 ± 7.54 c | 76.38 ± 3.70 f | 7.71 ± 0.92 h | 54.36 ± 2.44 g | 87.32 ± 2.87 e |
| Stilbenes, mg/g DW | 2 h, 20 °C | 2 h, 40 °C | 2 h, 60 °C | 4h, 20 °C | 4 h, 40 °C | 4 h, 60 °C | 6 h, 20 °C | 6 h, 40 °C | 6 h, 60 °C |
|---|---|---|---|---|---|---|---|---|---|
| t-astringin | 22.92 ± 1.20 b | 24.79 ± 0.69 ab | 27.46 ± 0.57 a | 25.54 ± 1.51 ab | 25.93 ± 0.59 ab | 28.76 ± 2.07 a | 24.94 ± 2.43 ab | 25.21 ± 0.96 ab | 25.54 ± 3.04 ab |
| cis-astringin | 0.61 ± 0.03 a | 0.60 ± 0.03 a | 0.71 ± 0.05 a | 0.64 ± 0.06 a | 0.65 ± 0.03 a | 0.69 ± 0.04 a | 0.68 ± 0.05 a | 0.67 ± 0.06 a | 0.73 ± 0.08 a |
| t-piceid | 5.87 ± 0.31 b | 6.09 ± 0.11 ab | 6.73 ± 0.32 ab | 6.48 ± 0.34 ab | 6.49 ± 0.07 ab | 7.14 ± 0.26 a | 6.13 ± 0.35 ab | 6.30 ± 0.18 ab | 6.59 ± 0.63 ab |
| t-isorhapontin | 175.78 ± 2.64 c | 183.31 ± 3.72 bc | 204.16 ± 1.27 ab | 196.53 ± 4.95 b | 199.35 ± 2.38 b | 204.14 ± 2.97 ab | 207.6 ± 3.92 b | 217.06 ± 3.57 ab | 214.78 ± 2.75 a |
| t-piceatannol | 0 a | 0.01 ± 0.01 a | 0.01 ± 0.01 a | 0.01 ± 0.01 a | 0.01 ± 0.01 a | 0.01 ± 0.01 a | 0.01 ± 0.01 a | 0.01 ± 0.01 a | 0.01 ± 0.01 a |
| cis-piceid | 0.04 ± 0.02 b | 0.07 ± 0.01 ab | 0.09 ± 0.01 a | 0.06 ± 0.02 ab | 0.08 ± 0.01 ab | 0.09 ± 0.02 a | 0.06 ± 0.01 ab | 0.08 ± 0.01 ab | 0.09 ± 0.01 a |
| cis-isorhapontin | 0.82 ± 0.07 b | 1.06 ± 0.14 b | 1.58 ± 0.11 ab | 0.91 ± 0.35 b | 1.33 ± 0.58 ab | 1.56 ± 0.12 ab | 0.91 ± 0.10 b | 1.4 ± 0.07 ab | 1.79 ± 0.25 a |
| t-resveratrol | 0.08 ± 0.02 b | 0.09 ± 0.01 b | 0.16 ± 0.02 a | 0.09 ± 0.04 b | 0.12 ± 0.01 ab | 0.15 ± 0.04 a | 0.10 ± 0.01 b | 0.13 ± 0.01 a | 0.17 ± 0.02 a |
| t-isorhapontigenin | 0.10 ± 0.03 ab | 0.08 ± 0.01 b | 0.13 ± 0.02 a | 0.08 ± 0.01 b | 0.10 ± 0.02 ab | 0.12 ± 0.02 a | 0.08 ± 0.02 ab | 0.09 ± 0.02 ab | 0.11 ± 0.02 ab |
| cis-resveratrol | 0.03 ± 0.01 a | 0.03 ± 0.01 a | 0.05 ± 0.01 a | 0.03 ± 0.01 a | 0.03 ± 0.01 | 0.04 ± 0.01 a | 0.03 ± 0.01 a | 0.04 ± 0.01 a | 0.04 ± 0.01 a |
| cis-isorhapontigenin | 0 a | 0 a | 0.01 ± 0.01 a | 0 a | 0 a | 0.01 ± 0.01 a | 0 a | 0 a | 0.01 ± 0.01 a |
| Total | 206.23 ± 4.14 d | 216.15 ± 3.99 c | 241.09 ± 3.22 ab | 230.38 ± 6.33 bc | 234.09 ± 3.94 b | 242.71 ± 5.29 ab | 240.54 ± 6.63 b | 250.99 ± 3.41 ab | 249.87 ± 4.85 a |
| Stilbenes | Collected in Spring | Collected in Summer | Collected in Autumn | Collected in Winter |
|---|---|---|---|---|
| t-astringin | 16.12 ± 1.74 b | 12.65 ± 1.19 c | 20.58 ± 1.12 a | 23.8 ± 0.69 a |
| cis-astringin | 0.54 ± 0.17 a | 0.16 ± 0.07 b | 0.32 ± 0.09 ab | 0 c |
| t-piceid | 2.73 ± 0.74 b | 3.67 ± 0.36 b | 4.28 ± 0.19 ab | 4.87 ± 0.12 a |
| t-isorhapontin | 130.9 ± 18.37 ab | 104.35 ± 11.7 b | 112.98 ± 5.13 b | 134.26 ± 5.54 a |
| t-piceatannol | 0.19 ± 0.08 a | 0.02 ± 0.02 b | 0.05 ± 0.01 b | 0 c |
| cis-piceid | 0.04 ± 0.03 b | 0.03 ± 0.01 b | 0.21 ± 0.02 a | 0 c |
| cis-isorhapontin | 0.19 ± 0.08 b | 6.8 ± 3.3 a | 0 c | 0 c |
| t-resveratrol | 0.18 ± 0.05 cd | 0.32 ± 0.09 bc | 0.56 ± 0.05 a | 0.07 ± 0.04 d |
| t-isorhapontigenin | 0.04 ± 0.03 ab | 0.22 ± 0.08 a | 0 b | 0 b |
| cis-resveratrol | 0 a | 0 a | 0.01 ± 0.01 a | 0.01 ± 0.01 a |
| cis-isorhapontigenin | 0.01 ± 0.01 ab | 0.03 ± 0.01 a | 0 b | 0 b |
| Total | 150.95 ± 19.93 ab | 126.54 ± 15.06 b | 138.97 ± 6.42 b | 163.02 ± 6.36 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suprun, A.R.; Dubrovina, A.S.; Aleynova, O.A.; Kiselev, K.V. The Bark of the Spruce Picea jezoensis Is a Rich Source of Stilbenes. Metabolites 2021, 11, 714. https://doi.org/10.3390/metabo11110714
Suprun AR, Dubrovina AS, Aleynova OA, Kiselev KV. The Bark of the Spruce Picea jezoensis Is a Rich Source of Stilbenes. Metabolites. 2021; 11(11):714. https://doi.org/10.3390/metabo11110714
Chicago/Turabian StyleSuprun, Andrey R., Alexandra S. Dubrovina, Olga A. Aleynova, and Konstantin V. Kiselev. 2021. "The Bark of the Spruce Picea jezoensis Is a Rich Source of Stilbenes" Metabolites 11, no. 11: 714. https://doi.org/10.3390/metabo11110714







