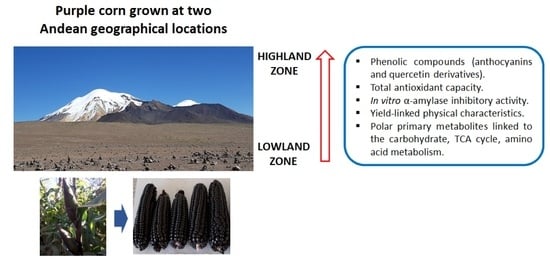
Primary and Phenolic Metabolites Analyses, In Vitro Health-Relevant Bioactivity and Physical Characteristics of Purple Corn (Zea mays L.) Grown at Two Andean Geographical Locations
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physical Characteristics of Purple Corn from the Two Andean Geographical Locations
2.2. Proximal Composition in Purple Corn Kernels
2.3. Purple Corn Phenolic Composition
2.4. In Vitro Antioxidant and Anti-Hyperglycemic Activity of Purple Corn
2.5. Untargeted GC-MS Metabolomic Analysis of Polar Metabolite Compounds in Purple Corn and Principal Component Analysis (PCA)
3. Materials and Methods
3.1. Materials
3.2. Enzymes and Reagents
3.3. Measurement of Physical Characteristics
3.4. Chemical Proximal Composition of Purple Corn Kernels
3.5. Extraction of the Free and Bound Phenolic Fractions from Purple Corn Kernel and Cob Samples
3.6. Extraction of Anthocyanin Compounds from Purple Corn Kernel and Cob Samples
3.7. Total Phenolic and Total Monomeric Anthocyanin Contents
3.8. Analysis of Phenolic Profiles by Ultra-High Performance Liquid Chromatography (UHPLC)
3.9. 2,2-Diphenyl-1-picrylhydrazyl Radical (DPPH·) and 2-2′-Azino-bis(3-Ethylbenzothiazoline-6-Sulfonic Acid) Radical Cation (ABTS·+) Inhibition Antioxidant Capacity
3.10. Inhibitory Activity against α-Amylase and α-Glucosidase Enzymes
3.11. Gas Chromatography Mass Spectrometry (GC-MS) Untargeted Metabolome Analysis of Polar Compounds
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grobman, A.; Salhuana, W.; Sevilla, R. Races of Maize in Peru their Origins, Evolution, and Classification; National Academy of Sciences—National Research Council: Washington, DC, USA, 1961; pp. 154–157. [Google Scholar]
- Grobman, A.; Bonavia, D.; Dillehay, T.D.; Piperno, D.R.; Iriarte, J.; Holst, I. Preceramic maize from paredones and huaca prieta, Peru. Proc. Natl. Acad. Sci. USA 2012, 109, 1755–1759. [Google Scholar] [CrossRef] [Green Version]
- Lao, F.; Sigurdson, G.T.; Giusti, M.M. Health benefits of purple corn (Zea mays L.) phenolic compounds. Compr. Rev. Food Sci. Food Saf. 2017, 16, 234–246. [Google Scholar] [CrossRef] [Green Version]
- Ranilla, L.G.; Huamán-Alvino, C.; Flores-Báez, O.; Aquino-Méndez, E.M.; Chirinos, R.; Campos, D.; Sevilla, R.; Fuentealba, C.; Pedreschi, R.; Sarkar, D.; et al. Evaluation of phenolic antioxidant-linked in vitro bioactivity of Peruvian corn (Zea mays L.) diversity targeting for potential management of hyperglycemia and obesity. J. Food Sci. Technol. 2019, 56, 2909–2924. [Google Scholar] [CrossRef] [PubMed]
- Lao, F.; Giusti, M.M. Extraction of purple corn (Zea mays L.) cob pigments and phenolic compounds using food-friendly solvents. J. Cereal Sci. 2018, 80, 87–93. [Google Scholar] [CrossRef]
- Medina-Hoyos, A.; Narro-León, L.A.; Chávez-Cabrera, A. Cultivo de maíz morado (Zea mays L.) en zona altoandina de Perú: Adaptación e identificación de cultivares de alto rendimiento y contenido de antocianina. Sci. Agropecu. 2020, 11, 291–299. [Google Scholar] [CrossRef]
- Cevallos-Casals, B.A.; Cisneros-Zevallos, L. Stoichiometric and kinetic studies of phenolic antioxidants from Andean purple corn and red-fleshed sweetpotato. J. Agric. Food Chem. 2003, 51, 3313–3319. [Google Scholar] [CrossRef]
- Cuevas-Montilla, E.; Hillebrand, S.; Antezana, A.; Winterhalter, P. Soluble and bound phenolic compounds in different Bolivian purple corn (Zea mays L.) cultivars. J. Agric. Food Chem. 2011, 59, 7068–7074. [Google Scholar] [CrossRef]
- Ramos-Escudero, F.; Muñoz, A.M.; Alvarado-Ortíz, C.; Alvarado, A.; Yánez, J.A. Purple corn (Zea mays L.) phenolic compounds profile and its assessment as an agent against oxidative stress in isolated mouse organs. J. Med. Food 2012, 15, 206–215. [Google Scholar] [CrossRef] [Green Version]
- Ranilla, L.G.; Christopher, A.; Sarkar, D.; Shetty, K.; Chirinos, R.; Campos, D. Phenolic composition and evaluation of the antimicrobial activity of free and bound phenolic fractions from a Peruvian purple corn (Zea mays L.) accession. J. Food Sci. 2017, 82, 2968–2976. [Google Scholar] [CrossRef]
- Magaña-Cerino, J.M.; Peniche-Pavía, H.A.; Tiessen, A.; Gurrola-Díaz, C.M. Pigmented maize (Zea mays L.) contains anthocyanins with potential therapeutic action against oxidative stress—A review. Pol. J. Food Nutr. Sci. 2020, 70, 85–99. [Google Scholar] [CrossRef]
- Tomay, F.; Marinelli, A.; Leoni, V.; Caccia, C.; Matros, A.; Mock, H.P.; Tonelli, C.; Petroni, K. Purple corn extract induces long-lasting reprogramming and M2 phenotypic switch of adipose tissue macrophages in obese mice. J. Transl. Med. 2019, 17, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; de Mejia, E.G.; Luna-Vital, D.; Tao, T.; Chandrasekaran, S.; Chatham, L.; Juvik, J.; Singh, V.; Kumar, D. Relationship of phenolic composition of selected purple maize (Zea mays L.) genotypes with their anti-inflammatory, anti-adipogenic and anti-diabetic potential. Food Chem. 2019, 289, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Mazewski, C.; Liang, K.; de Mejia, E.G. Inhibitory potential of anthocyanin-rich purple and red corn extracts on human colorectal cancer cell proliferation in vitro. J. Funct. Foods 2017, 34, 254–265. [Google Scholar] [CrossRef]
- Finkel, M.L.; Sanchez, S.; Mak, T.; Granstein, J.; Lefkowitz, A. Anthocyanin-rich purple corn extract and its effects on the blood pressure of adults. J. Evid.-Based Complement. Altern. Med. 2013, 18, 237–242. [Google Scholar] [CrossRef]
- Paulsmeyer, M.; Chatham, L.; Becker, T.; West, M.; West, L.; Juvik, J. Survey of anthocyanin composition and concentration in diverse maize germplasms. J. Agric. Food Chem. 2017, 65, 4341–4350. [Google Scholar] [CrossRef]
- Hong, H.T.; Netzel, M.E.; O’Hare, T.J. Anthocyanin composition and changes during kernel development in purple-pericarp supersweet sweetcorn. Food Chem. 2020, 315, 126284. [Google Scholar] [CrossRef]
- Jing, P.U.; Noriega, V.; Schwartz, S.J. Giusti, M.M. Effects of growing conditions on purple corncob (Zea mays L.) anthocyanins. J. Agric. Food Chem. 2007, 55, 8625–8629. [Google Scholar] [CrossRef] [PubMed]
- Peruvian Ministry of Foreigh Trade and Tourism. Superfoods Peru. Available online: https://issuu.com/promperu/docs/catalogo_superfoods_espa_ol__1_ (accessed on 11 June 2021).
- Peruvian Ministry of Foreigh Trade and Tourism. Estadísticas de Biocomercio 2014–2018. Available online: https://www.siicex.gob.pe/siicex/portal5ES.asp?_page_=912.91100 (accessed on 11 June 2021).
- Peruvian Ministry of Agriculture and Irrigation. Manejo Agronómico del Maíz Morado en los Valles Interandinos del Perú. Available online: http://repositorio.minagri.gob.pe/xmlui/bitstream/handle/MIDAGRI/851/Maiz%20morado%20inia.pdf?sequence=1&isAllowed=y (accessed on 11 June 2021).
- Chuma, B.A.; Cotter, M.; Kalisa, A.; Rajaona, A.; Senthilkumar, K.; Stuerz, S.; Vincent, I.; Asch, F. Altitude, temperature, and N management effects on yield and yield components of contrasting lowland rice cultivars. J. Agron. Crop Sci. 2020, 206, 456–465. [Google Scholar] [CrossRef]
- Hirai, Y.; Hamagami, K. Effects of air temperature, nitrogen concentration in irrigation water, and cultivation practices on rice production in the rice terrace region. J. Fac. Agric. Kyushu Univ. 2009, 54, 305–311. [Google Scholar] [CrossRef]
- SENAMHI (National Meteorology and Hydrology Service of Peru). Available online: https://www.senamhi.gob.pe/main.php?dp=arequipa&p=estaciones (accessed on 5 April 2021).
- Fürtauer, L.; Weiszmann, J.; Weckwerth, W.; Nägele, T. Dynamics of plant metabolism during cold acclimation. Int. J. Mol. Sci. 2019, 20, 5411. [Google Scholar] [CrossRef] [Green Version]
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and drought stresses in crop plants: Implications, cross talk, and potential management opportunities. Front. Plant Sci. 2018, 9, 393. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.S.; Farooq, M.; Asch, F.; Krishna, J.S.; Prasad, P.V.; Siddique, K.H. Thermal stress impacts reproductive development and grain yield in rice. Plant Physiol. Biochem. 2017, 115, 57–72. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, H.; Flores-Magdaleno, H.; Ascencio-Hernández, R.; Khalil-Gardezi, A.; Tijerina-Chávez, L.; Mancilla-Villa, O.R.; Vázquez-Peña, M.A. Corn grain yield estimation from vegetation indices, canopy cover, plant density, and a neural network using multispectral and RGB images acquired with unmanned aerial vehicles. Agriculture 2020, 10, 277. [Google Scholar] [CrossRef]
- Jordan-Meille, L.; Holland, J.E.; McGrath, S.P.; Glendining, M.J.; Thomas, C.L.; Haefele, S.M. The grain mineral composition of barley, oat and wheat on soils with pH and soil phosphorus gradients. Eur. J. Agron. 2021, 126, 126281. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J. Insoluble-bound phenolics in food. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef]
- Cömert, E.D.; Gökmen, V. Antioxidants bound to an insoluble food matrix: Their analysis, regeneration behavior, and physiological importance. Compr. Rev. Food Sci. Food Saf. 2017, 16, 382–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peruvian Ministry of Health. Available online: https://repositorio.ins.gob.pe/xmlui/bitstream/handle/INS/1034/tablas-peruanas-QR.pdf?sequence=3&isAllowed=y (accessed on 5 April 2021).
- USDA (United States Department of Agriculture). Food Data Central. Available online: https://fdc.nal.usda.gov/ (accessed on 5 April 2021).
- González-Muñoz, A.; Quesille-Villalobos, A.M.; Fuentealba, C.; Shetty, K.; Ranilla, L.G. Potential of Chilean native corn (Zea mays L.) accessions as natural sources of phenolic antioxidants and in vitro bioactivity for hyperglycemia and hypertension management. J. Agric. Food Chem. 2013, 61, 10995–11007. [Google Scholar] [CrossRef] [PubMed]
- Lao, F.; Giusti, M.M. Quantification of purple corn (Zea mays L.) anthocyanins using spectrophotometric and HPLC approaches: Method comparison and correlation. Food Anal. Methods 2016, 9, 1367–1380. [Google Scholar] [CrossRef]
- Quispe Jacobo, F.; Arroyo Condorena, K.; Gorriti Gutiérrez, A. Características morfológicas y químicas de 3 cultivares de maíz morado (Zea mays L.) en Arequipa-Perú. Rev. Soc. Quim. Peru 2011, 77, 205–217. [Google Scholar]
- Gorriti, A.; Quispe, F.; Arroyo, J.L.; Córdova, A.; Jurado, B.; Santiago, I.; Taype, E. Extracción de antocianinas de las corontas de Zea mays L. maíz morado. Cienc. Investig. 2009, 12, 64–74. [Google Scholar] [CrossRef]
- Khamphasan, P.; Lomthaisong, K.; Harakotr, B.; Ketthaisong, D.; Scott, M.P.; Lertrat, K.; Suriharn, B. Genotypic variation in anthocyanins, phenolic compounds, and antioxidant activity in cob and husk of purple field corn. Agronomy 2018, 8, 271. [Google Scholar] [CrossRef] [Green Version]
- Mendoza-Mendoza, C.G.; Mendoza-Castillo, M.D.C.; Delgado-Alvarado, A.; Castillo-Gonzalez, F.; Kato-Yamakake, T.Á.; Cruz-Izquierdo, S. Total anthocyanins and color parameters in purple corn inbred lines. Rev. Fitotec. Mex. 2017, 40, 471–479. [Google Scholar]
- Yang, Z.; Zhai, W. Optimization of microwave-assisted extraction of anthocyanins from purple corn (Zea mays L.) cob and identification with HPLC–MS. Innovative Food Sci. Emerg. Technol. 2010, 11, 470–476. [Google Scholar] [CrossRef]
- Harakotr, B.; Suriharn, B.; Scott, M.P.; Lertrat, K. Genotypic variability in anthocyanins, total phenolics, and antioxidant activity among diverse waxy corn germplasm. Euphytica 2015, 203, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Blumthaler, M.; Ambach, W.; Ellinger, R. Increase in solar UV radiation with altitude. J. Photochem. Photobiol. B Biol. 1997, 39, 130–134. [Google Scholar] [CrossRef]
- Pandey, A.; Agrawal, S.B. Ultraviolet-B radiation: A potent regulator of flavonoids biosynthesis, accumulation and functions in plants. Curr. Sci. 2020, 119, 176–185. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Hu, W.; Sun, B.; Chen, Q.; Tang, H. Anthocyanin accumulation and related gene expression affected by low temperature during strawberry coloration. Acta Physiol. Plant. 2018, 40, 1–8. [Google Scholar] [CrossRef]
- He, Q.; Ren, Y.; Zhao, W.; Li, R.; Zhang, L. Low temperature promotes anthocyanin biosynthesis and related gene expression in the seedlings of purple head Chinese cabbage (Brassica rapa L.). Genes 2020, 11, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santin, M.; Castagna, A.; Miras-Moreno, B.; Rocchetti, G.; Lucini, L.; Hauser, M.T.; Ranieri, A. Beyond the visible and below the peel: How UV-B radiation influences the phenolic profile in the pulp of peach fruit. A biochemical and molecular study. Front. Plant Sci. 2020, 11, 1–17. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- KEGG (Kyoto Encyclopedia of Genes and Genomes). Available online: https://www.genome.jp/kegg/ (accessed on 1 June 2021).
- Du, S.; Huang, X.; Cai, Y.; Hao, Y.; Qiu, S.; Liu, L.; Cui, M.; Luo, L. Differential antioxidant compounds and activities in seedlings of two rice cultivars under chilling treatment. Front. Plant Sci. 2021, 12, 257. [Google Scholar] [CrossRef] [PubMed]
- Usman, B.; Sharma, N.; Satija, S.; Mehta, M.; Vyas, M.; Khatik, G.L.; Khurana, N.; Hansbro, P.M.; Williams, K.; Dua, K. Recent developments in alpha-glucosidase inhibitors for management of type-2 diabetes: An update. Curr. Pharm. Des. 2019, 25, 2510–2525. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guo, Y.; Li, X.; Gong, K.; Liu, K. Phenolics and related in vitro functional activities of different varieties of fresh waxy corn: A whole grain. BMC Chem. 2021, 15, 1–9. [Google Scholar] [CrossRef]
- Xiong, Y.; Ng, K.; Zhang, P.; Warner, R.D.; Shen, S.; Tang, H.Y.; Liang, Z.; Fang, Z. In vitro α-glucosidase and α-amylase inhibitory activities of free and bound phenolic extracts from the bran and kernel fractions of five sorghum grain genotypes. Foods 2020, 9, 1301. [Google Scholar] [CrossRef]
- Han, Y.; Chi, J.; Zhang, M.; Zhang, R.; Fan, S.; Huang, F.; Xue, K.; Liu, L. Characterization of saponins and phenolic compounds: Antioxidant activity and inhibitory effects on α-glucosidase in different varieties of colored quinoa (Chenopodium quinoa Willd). Biosci. Biotechnol. Biochem. 2019, 83, 2128–2139. [Google Scholar] [CrossRef]
- Kumar Singla, R.; Singh, R.; Kumar Dubey, A. Important aspects of post-prandial antidiabetic drug, acarbose. Curr. Top. Med. Chem. 2016, 16, 2625–2633. [Google Scholar] [CrossRef]
- Kaeswurm, J.A.; Könighofer, L.; Hogg, M.; Scharinger, A.; Buchweitz, M. Impact of B-ring substitution and acylation with hydroxy cinnamic acids on the inhibition of porcine α-amylase by anthocyanin-3-glycosides. Foods 2020, 9, 367. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, H.; Fernandes, A.; F-Brás, N.; Mateus, N.; de Freitas, V.; Fernandes, I. Anthocyanins as antidiabetic agents—In Vitro and in silico approaches of preventive and therapeutic effects. Molecules 2020, 25, 3813. [Google Scholar] [CrossRef]
- Bilska-Kos, A.; Mytych, J.; Suski, S.; Magoń, J.; Ochodzki, P.; Zebrowski, J. Sucrose phosphate synthase (SPS), sucrose synthase (SUS) and their products in the leaves of Miscanthus × giganteus and Zea mays at low temperature. Planta 2020, 252, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of sugars under abiotic stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Kim, J.K.; Wu, Q.; Park, S.U. Effects of cold stress on transcripts and metabolites in tartary buckwheat (Fagopyrum tataricum). Environ. Exp. Bot. 2018, 155, 488–496. [Google Scholar] [CrossRef]
- Lloyd, J.C.; Zakhleniuk, O.V. Responses of primary and secondary metabolism to sugar accumulation revealed by microarray expression analysis of the Arabidopsis mutant, pho3. J. Exp. Bot. 2004, 55, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Quemener, B.; Lahaye, M.; Thibault, J.F. Studies on the simultaneous determination of acidic and neutral sugars of plant cell wall materials by HPLC of their methyl glycosides after combined methanolysis and enzymic prehydrolysis. Carbohydr. Polym. 1993, 20, 87–94. [Google Scholar] [CrossRef]
- Taylor, N.L.; Heazlewood, J.L.; Day, D.A.; Millar, A.H. Lipoic acid-dependent oxidative catabolism of α-keto acids in mitochondria provides evidence for branched-chain amino acid catabolism in Arabidopsis. Plant Physiol. 2004, 134, 838–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.X.; Gao, X.X.; Li, M.Q.; Fu, J.Q.; Zhang, Y.L. Plastic responses in the metabolome and functional traits of maize plants to temperature variations. Plant Biol. 2016, 18, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Urrutia, M.; Blein-Nicolas, M.; Prigent, S.; Bernillon, S.; Deborde, C.; Balliau, T.; Maucourt, M.; Jacob, D.; Ballias, P.; Bénard, C.; et al. Maize metabolome and proteome responses to controlled cold stress partly mimic early-sowing effects in the field and differ from those of Arabidopsis. Plant Cell Environ. 2021, 44, 1504–1521. [Google Scholar] [CrossRef]
- Ghatak, A.; Chaturvedi, P.; Weckwerth, W. Metabolomics in plant stress physiology. In Plant Genetics and Molecular Biology; Varshney, R., Pandey, M., Chitikineni, A., Eds.; Springer: Cham, Switzerland, 2018; Volume 164, pp. 187–236. [Google Scholar]
- Zhao, X.; Wei, Y.; Zhang, J.; Yang, L.; Liu, X.; Zhang, H.; Shao, W.; He, L.; Zhang, Y.; Xu, J. Membrane lipids’ metabolism and transcriptional regulation in maize roots under cold stress. Front. Plant Sci. 2021, 12, 596. [Google Scholar] [CrossRef]
- IBPGR-International Board for Plant Genetic Resources. Descriptors for Maize; International Maize and Wheat Improvement Center: Mexico City, Mexico; International Board for Plant Genetic Resources: Rome, Italy, 1991; pp. 11–22. [Google Scholar]
- AOAC International. Official Methods of Analysis of AOAC International, 20th ed.; Association of Official Analytical Chemists: Rockville, MD, USA, 2016. [Google Scholar]
- Jing, P.U.; Giusti, M.M. Effects of extraction conditions on improving the yield and quality of an anthocyanin-rich purple corn (Zea mays L.) color extract. J. Food Sci. 2007, 72, C363–C368. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F1-2. [Google Scholar] [CrossRef]
- Rodriguez-Saona, L.E.; Wrolstad, R.E. Extraction, isolation, and purification of anthocyanins. Curr. Protoc. Food Anal. Chem. 2001, 1, F1-1. [Google Scholar] [CrossRef]
- Vargas-Yana, D.; Aguilar-Morón, B.; Pezo-Torres, N.; Shetty, K.; Ranilla, L.G. Ancestral Peruvian ethnic fermented beverage “Chicha” based on purple corn (Zea mays L.): Unraveling the health-relevant functional benefits. J. Ethnic Foods 2020, 7, 1–12. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Saura-Calixto, F. Effect of solvent and certain food constituents on different antioxidant capacity assays. Food Res. Int. 2006, 39, 791–800. [Google Scholar] [CrossRef]
- Fuentealba, C.; Hernández, I.; Saa, S.; Toledo, L.; Burdiles, P.; Chirinos, R.; Campos, D.; Brown, P.; Pedreschi, R. Colour and in vitro quality attributes of walnuts from different growing conditions correlate with key precursors of primary and secondary metabolism. Food Chem. 2017, 232, 664–672. [Google Scholar] [CrossRef] [PubMed]


| Corn Part | Characteristics | Lowland | Highland |
|---|---|---|---|
| Ear | Length (cm) | 14.1 ± 1.0 a | 14.2 ± 0.5 a |
| Diameter (cm) | 4.4 ± 0.2 a | 3.9 ± 0.1 b | |
| Weight (g) | 124.7 ± 14.3 a | 84.9 ± 9.2 b | |
| Number of rows | 11.0 ± 0.4 a | 10 ± 0.2 a | |
| Number of kernels per row | 25.0 ± 0.5 a | 25.0 ± 1.5 a | |
| kernel weight per ear (g) | 92.2 ± 6.9 a | 67.2 ± 7.3 b | |
| % kernel weight per ear | 75.7 ± 0.0 a | 78.4 ± 0.0 a | |
| Cob | Diameter (cm) | 2.4 ± 0.2 a | 2.4 ± 0.1 a |
| Weight (g) | 16.2 ± 0.5 a | 17.8 ± 2.5 a | |
| Kernel | 100-kernel weight (g) | 42.7 ± 4.7 a | 28.7 ± 4.8 b |
| Length (cm) | 1.2 ± 0.1 a | 1.0 ± 0.1 a | |
| Width (cm) | 1.0 ± 0.1 a | 0.9 ± 0.0 a |
| Analyses- | Kernel | Cob | ||
|---|---|---|---|---|
| Lowland | Highland | Lowland | Highland | |
| Proximal Composition | ||||
| (g/100 g) | ||||
| Moisture | 10.5 ±1.4 a | 9.3 ± 0.6 a | -- | -- |
| Lipids | 4.6 ± 0.6 a | 4.1 ± 0.4 a | -- | -- |
| Protein | 8.2 ± 0.4 a | 8.9 ± 0.8 a | -- | -- |
| Ash | 1.4 ± 0.1 b | 1.8 ± 0.1 a | -- | -- |
| Crude fiber | 1.5 ± 0.6 b | 2.6 ± 0.2 a | -- | -- |
| Total carbohydrates | 75.3 ± 1.9 a | 75.9 ± 1.4 a | -- | -- |
| Phenolic Compounds | ||||
| (mg GAE/100 g DW) | ||||
| Free | 586 ± 35 b | 889 ± 84 a | 2962 ± 1108 a | 4425 ± 427 a |
| Bound | 166 ± 34 a | 201 ± 9 a | 880 ± 50 a | 694 ± 32 b |
| Total | 752 ± 30 b | 1090 ± 92 a | 3842 ± 1090 a | 5118 ± 428 a |
| Total Monomeric Anthocyanins (mg C3G/100 g DW) | 328 ± 53 a | 415 ± 59 a | 1935 ± 692 a | 2894 ± 350 a |
| UHPLC Phenolic Profile | ||||
| (mg/100 g DW) | ||||
| Total anthocyanins 2 | 53.5 ± 5.8 a | 64.4 ± 10.9 a | 466 ± 127 b | 983 ± 68 a |
| Quercetin derivatives 3 | 1.7 ± 1.5 a | 3.9 ± 1.3 a | 25.3 ± 1.8 b | 177 ± 109 a |
| Total flavonoids | 55.2 ± 6.8 a | 68.3 ± 12.3 a | 492 ± 127 b | 1160 ± 41 a |
| Bound p-coumaric acid | 23.4 ± 5.7 a | 19.5 ± 1.0 a | 476 ± 8 a | 391 ± 65 a |
| Bound p-coumaric acid derivatives 4 | ND 1 | ND 1 | 11.8 ± 1.3 a | 11.6 ± 1.0 a |
| Bound ferulic acid | 108 ± 21 b | 153 ± 9 a | 380 ± 20 a | 322 ± 43 a |
| Bound ferulic acid derivatives 5 | 17.4 ± 2.4 a | 19.5 ± 1.7 a | 14.1 ± 2.9 a | 13.1 ± 1.8 a |
| Total bound phenolic acids | 149 ± 29 a | 192 ± 10 a | 882 ± 15 a | 737 ± 56 b |
| Total phenolic compounds (flavonoids + phenolic acids) | 204 ± 29 b | 261 ± 7 a | 1374 ± 115 b | 1897 ± 96 a |
| Analyses- | Kernel | Cob | ||
|---|---|---|---|---|
| Lowland | Highland | Lowland | Highland | |
| DPPH Antioxidant Assay | ||||
| (µmol TE/100 g DW) | ||||
| Free | 957 ± 21 b | 1759 ± 36 a | 5271 ± 1731 a | 7380 ± 595 a |
| Bound | 290 ± 48 a | 322 ± 9 a | 2003 ± 374 a | 2390 ± 56 a |
| Total | 1247 ± 69 b | 2080 ± 41 a | 7274 ± 1561 a | 9770 ± 610 a |
| ABTS Antioxidant Assay | ||||
| (µmol TE/100 g DW) | ||||
| Free | 3746 ± 188 a | 4273 ± 540 a | 21,718 ± 3113 b | 28,391 ± 1771 a |
| Bound | 1448 ± 19 b | 1532 ± 26 a | 9354 ± 548 a | 10,033 ± 606 a |
| Total | 5194 ± 183 a | 5805 ± 516 a | 31,072 ± 3190 b | 38,424 ± 1263 a |
| α-Amylase Inhibition (%) | ||||
| 50 mg (kernel), 10 mg (cob) | 5.9 ± 2.4 b | 11.0 ± 2.0 a | 8.2 ± 1.5 a | 15.6 ± 5.2 a |
| 62 mg (kernel), 12.5 mg (cob) | 9.2 ± 4.4 a | 14.2 ± 2.5 a | 9.8 ± 2.6 b | 19.9 ± 3.9 a |
| 125 mg (kernel), 25 mg (cob) | 14.9 ± 1.7 b | 20.8 ± 2.5 a | 17.7 ± 5.1 a | 28.9 ± 11.5 a |
| α-Glucosidase Inhibition (%) | ||||
| 2.5 mg (kernel), 0.5 mg (cob) | 49.9 ± 13.2 a | 45.8 ± 1.6 a | 89.4 ± 5.5 a | 83.4 ± 15.9 a |
| 3.6 mg (kernel), 0.7 mg (cob) | 58.9 ± 12.8 a | 54.9 ± 3.9 a | 91.8 ± 4.7 a | 88.5 ± 11.1 a |
| 6.25 mg (kernel), 1.25 mg (cob) | 70.7 ± 11.9 a | 67.1 ± 2.8 a | 95.0 ± 4.0 a | 92.5 ± 7.9 a |
| District * | Name of the Land | Altitude (m) | Latitude and Longitude |
|---|---|---|---|
| Iray (Lowland) | Huichara | 2249 | 15°50′34.2″ S 72°38′9.3″ W |
| Puyara | 2337 | 15°51′54.7″ S 72°37′7.4″ W | |
| Pacaichacra | 1712 | 15°53′43.2″ S 72°34′10″ W | |
| Chuquibamba (Highland) | Chiringay | 2896 | 15°48′59.6″ S 72°38′33.3″ W |
| Huayra A | 2768 | 15°50′20.7″ S 72°38′33.4″ W | |
| Huayra B | 2759 | 15°50′53.5″ S 72°38′33.6″ W |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranilla, L.G.; Rios-Gonzales, B.A.; Ramírez-Pinto, M.F.; Fuentealba, C.; Pedreschi, R.; Shetty, K. Primary and Phenolic Metabolites Analyses, In Vitro Health-Relevant Bioactivity and Physical Characteristics of Purple Corn (Zea mays L.) Grown at Two Andean Geographical Locations. Metabolites 2021, 11, 722. https://doi.org/10.3390/metabo11110722
Ranilla LG, Rios-Gonzales BA, Ramírez-Pinto MF, Fuentealba C, Pedreschi R, Shetty K. Primary and Phenolic Metabolites Analyses, In Vitro Health-Relevant Bioactivity and Physical Characteristics of Purple Corn (Zea mays L.) Grown at Two Andean Geographical Locations. Metabolites. 2021; 11(11):722. https://doi.org/10.3390/metabo11110722
Chicago/Turabian StyleRanilla, Lena Gálvez, Briggite Anyela Rios-Gonzales, María Fernanda Ramírez-Pinto, Claudia Fuentealba, Romina Pedreschi, and Kalidas Shetty. 2021. "Primary and Phenolic Metabolites Analyses, In Vitro Health-Relevant Bioactivity and Physical Characteristics of Purple Corn (Zea mays L.) Grown at Two Andean Geographical Locations" Metabolites 11, no. 11: 722. https://doi.org/10.3390/metabo11110722
APA StyleRanilla, L. G., Rios-Gonzales, B. A., Ramírez-Pinto, M. F., Fuentealba, C., Pedreschi, R., & Shetty, K. (2021). Primary and Phenolic Metabolites Analyses, In Vitro Health-Relevant Bioactivity and Physical Characteristics of Purple Corn (Zea mays L.) Grown at Two Andean Geographical Locations. Metabolites, 11(11), 722. https://doi.org/10.3390/metabo11110722