The Application of Metabolomics in Forensic Science with Focus on Forensic Toxicology and Time-of-Death Estimation
Abstract
:1. Introduction
2. Metabolomics and Its Branch: Thanatometabolomics
2.1. Different Metabolomics’ Strategies
2.2. Thanatometabolomics
3. Goals of Metabolomics’ Assays
3.1. Postmortem Interval
3.2. Toxicology
3.2.1. Drugs
3.2.2. Ethyl Alcohol and Ethanol Substitutes
4. Biological Materials Commonly Used in Forensic Medicine
4.1. Types of Biological Materials in Forensic Samples
4.1.1. Peripheral Blood
4.1.2. Urine
4.1.3. Hair
4.1.4. Nails
4.1.5. Saliva
4.1.6. Sweat
4.1.7. Internal Organ Tissue
Liver
Brain
Kidney
4.2. Importance of Sample Preparation
5. Methods Used in Forensic Instrumental Analysis
6. Method Development
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kosmides, A.K.; Kamisoglu, K.; Calvano, S.E.; Corbett, S.A.; Androulakis, I.P. Metabolomic Fingerprinting: Challenges and Opportunities. Crit. Rev. Biomed. Eng. 2013, 41, 205–221. [Google Scholar] [CrossRef]
- Akçan, R.; Taştekin, B.; Yildirim, M.; Aydogan, H.C.; Sağlam, N. Omics era in forensic medicine: Towards a new age. Turk. J. Med. Sci. 2020, 50, 1480–1490. [Google Scholar] [CrossRef]
- Szeremeta, M.; Pietrowska, K.; Niemcunowicz-Janica, A.; Kretowski, A.; Ciborowski, M. Applications of Metabolomics in Forensic Toxicology and Forensic Medicine. Int. J. Mol. Sci. 2021, 22, 3010. [Google Scholar] [CrossRef] [PubMed]
- Dinis-Oliveira, R.J. Metabolism and metabolomics of opiates: A long way of forensic implications to unravel. J. Forensic Leg. Med. 2018, 61, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Mamas, M.; Dunn, W.; Neyses, L.; Goodacre, R. The role of metabolites and metabolomics in clinically applicable biomarkers of disease. Arch. Toxicol. 2010, 85, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, W.J.; Koal, T.; Wang, Y.; Kohl, M.; Enot, D.P.; Deigner, H.-P. Targeted Metabolomics for Biomarker Discovery. Angew. Chem. Int. Ed. 2010, 49, 5426–5445. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Wilson, I.D. Understanding ‘Global’ Systems Biology: Metabonomics and the Continuum of Metabolism. Nat. Rev. Drug Discov. 2003, 2, 668–676. [Google Scholar] [CrossRef]
- Harrigan, G.G.; Maguire, G.; Boros, L. Metabolomics in alcohol research and drug development. Alcohol Res. Health 2008, 31, 26–35. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics—The link between genotypes and phenotypes. In Functional Genomics; Town, C., Ed.; Springer: Dordrecht, The Netherlands, 2002; Volume 48, pp. 155–171. [Google Scholar] [CrossRef]
- Castillo-Peinado, L.; de Castro, M.L. Present and foreseeable future of metabolomics in forensic analysis. Anal. Chim. Acta 2016, 925, 1–15. [Google Scholar] [CrossRef]
- Myint, K.T.; Aoshima, K.; Tanaka, S.; Nakamura, T.; Oda, Y. Quantitative Profiling of Polar Cationic Metabolites in Human Cerebrospinal Fluid by Reversed-Phase Nanoliquid Chromatography/Mass Spectrometry. Anal. Chem. 2009, 81, 1121–1129. [Google Scholar] [CrossRef]
- Theodoridis, G.; Gika, H.G.; Wilson, I.D. LC-MS-based methodology for global metabolite profiling in metabonomics/metabolomics. TrAC Trends Anal. Chem. 2008, 27, 251–260. [Google Scholar] [CrossRef]
- Gika, H.G.; Theodoridis, G.A.; Wilson, I.D. Liquid chromatography and ultra-performance liquid chromatography–mass spectrometry fingerprinting of human urine: Sample stability under different handling and storage conditions for metabonomics studies. J. Chromatogr. A 2008, 1189, 314–322. [Google Scholar] [CrossRef]
- Stefanuto, P.-H.; Perrault, K.; Stadler, S.; Pesesse, R.; Leblanc, H.N.; Forbes, S.L.; Focant, J.-F. GC × GC–TOFMS and supervised multivariate approaches to study human cadaveric decomposition olfactive signatures. Anal. Bioanal. Chem. 2015, 407, 4767–4778. [Google Scholar] [CrossRef]
- Meyer, M.R.; Maurer, H.H. Toxicokinetics and Toxicogenetics. In Handbook of Forensic Medicine; John Wiley & Sons, Ltd.: Oxford, UK, 2014; pp. 889–899. [Google Scholar] [CrossRef]
- Locci, E.; Bazzano, G.; Chighine, A.; Locco, F.; Ferraro, E.; Demontis, R.; D’Aloja, E. Forensic NMR metabolomics: One more arrow in the quiver. Metabolomics 2020, 16, 1–16. [Google Scholar] [CrossRef]
- Macdonald, K.; Krishnan, A.; Cervenka, E.; Hu, G.; Guadagno, E.; Trakadis, Y. Biomarkers for major depressive and bipolar disorders using metabolomics: A systematic review. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2019, 180, 122–137. [Google Scholar] [CrossRef]
- Pang, H.; Jia, W.; Hu, Z. Emerging Applications of Metabolomics in Clinical Pharmacology. Clin. Pharmacol. Ther. 2019, 106, 544–556. [Google Scholar] [CrossRef]
- Locci, E.; Stocchero, M.; Noto, A.; Chighine, A.; Natali, L.; Napoli, P.E.; Caria, R.; De-Giorgio, F.; Nioi, M.; D’Aloja, E. A 1H NMR metabolomic approach for the estimation of the time since death using aqueous humour: An animal model. Metabolomics 2019, 15, 76. [Google Scholar] [CrossRef]
- Vinayavekhin, N.; Saghatelian, A. Untargeted Metabolomics. Curr. Protoc. Mol. Biol. 2010, 90, 30.1.1–30.1.24. [Google Scholar] [CrossRef]
- Chighine, A.; Locci, E.; Nioi, M.; D’Aloja, E. Looking for Post-Mortem Metabolomic Standardization: Waiting for Godot—The Importance of Post-Mortem Interval in Forensic Metabolomics. Chem. Res. Toxicol. 2021, 34, 1946–1947. [Google Scholar] [CrossRef]
- DiMaio, V.; DiMaio, D. Forensic Pathology. 2001. Available online: https://content.taylorfrancis.com/books/download?dac=C2009-0-01761-8&isbn=9781420042412&format=googlePreviewPdf (accessed on 20 November 2021).
- Kaliszan, M.; Hauser, R.; Kernbach-Wighton, G. Estimation of the time of death based on the assessment of post mortem processes with emphasis on body cooling. Leg. Med. 2009, 11, 111–117. [Google Scholar] [CrossRef]
- Henssge, C.; Madea, B. Estimation of the time since death. Forensic Sci. Int. 2007, 165, 182–184. [Google Scholar] [CrossRef] [PubMed]
- Buchan, M.; Anderson, G. Time Since Death: A Review of the Current Status of Methods used in the Later Postmortem Interval. Can. Soc. Forensic Sci. J. 2001, 34, 1–22. [Google Scholar] [CrossRef]
- Kugelberg, F.C.; Jones, A.W. Interpreting results of ethanol analysis in postmortem specimens: A review of the literature. Forensic Sci. Int. 2007, 165, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.; Loo, R.L.; Cloarec, O.; Coen, M.; Tang, H.; Maibaum, E.; Bruce, S.; Chan, Q.; Elliott, P.; Stamler, J.; et al. Detection of Urinary Drug Metabolite (Xenometabolome) Signatures in Molecular Epidemiology Studies via Statistical Total Correlation (NMR) Spectroscopy. Anal. Chem. 2007, 79, 2629–2640. [Google Scholar] [CrossRef] [Green Version]
- Strimbu, K.; Tavel, J.A. What are biomarkers? Curr. Opin. HIV AIDS 2010, 5, 463–466. [Google Scholar] [CrossRef]
- Pesko, B.K.; Weidt, S.; McLaughlin, M.; Wescott, D.J.; Torrance, H.; Burgess, K.; Burchmore, R. Postmortomics: The Potential of Untargeted Metabolomics to Highlight Markers for Time Since Death. OMICS 2020, 24, 649–659. [Google Scholar] [CrossRef]
- Jawor, P.; Ząbek, A.; Wojtowicz, W.; Król, D.; Stefaniak, T.; Młynarz, P. Metabolomic studies as a tool for determining the post-mortem interval (PMI) in stillborn calves. BMC Vet. Res. 2019, 15, 189. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Fan, F.; Ye, Y.; Lu, X.; Chen, F.; Wu, Z.; Liao, L. An experimental study on investigating the postmortem interval in dichlorvos poisoned rats by GC/MS-based metabolomics. Leg. Med. 2019, 36, 28–36. [Google Scholar] [CrossRef]
- Aronson, J.K. Biomarkers and surrogate endpoints. Br. J. Clin. Pharmacol. 2005, 59, 491–494. [Google Scholar] [CrossRef] [Green Version]
- Steuer, A.E.; Brockbals, L.; Kraemer, T. Metabolomic Strategies in Biomarker Research–New Approach for Indirect Identification of Drug Consumption and Sample Manipulation in Clinical and Forensic Toxicology? Front. Chem. 2019, 7, 319. [Google Scholar] [CrossRef]
- Steuer, A.E.; Raeber, J.; Steuer, C.; Boxler, M.I.; Dornbierer, D.A.; Bosch, O.G.; Quednow, B.B.; Seifritz, E.; Kraemer, T. Identification of new urinary gamma-hydroxybutyric acid markers applying untargeted metabolomics analysis following placebo-controlled administration to humans. Drug Test. Anal. 2018, 11, 813–823. [Google Scholar] [CrossRef]
- Dinis-Oliveira, R.J.; Carvalho, F.; Moreira, R.F.; Duarte, J.A.; Proença, J.; Santos, A.; Magalhães, T. Clinical and Forensic Signs Related to Opioids Abuse. Curr. Drug Abus. Rev. 2012, 5, 273–290. [Google Scholar] [CrossRef]
- Inturrisi, C.; Schultz, M.; Shin, S.; Umans, J.; Angel, L.; Simon, E. Evidence from opiate binding studies that heroin acts through its metabolites. Life Sci. 1983, 33, 773–776. [Google Scholar] [CrossRef]
- Way, E.L.; Kemp, J.W.; Young, J.M.; Grassetti, D.R. The pharmacologic effects of heroin in relationship to its rate of biotransformation. J. Pharmacol. Exp. Ther. 1960, 129, 144–154. Available online: http://www.ncbi.nlm.nih.gov/pubmed/13843200 (accessed on 21 January 2020).
- Andersen, J.M.; Ripel, Å.; Boix, F.; Normann, P.T.; Mørland, J. Increased Locomotor Activity Induced by Heroin in Mice: Pharmacokinetic Demonstration of Heroin Acting as a Prodrug for the Mediator 6-Monoacetylmorphine in Vivo. J. Pharmacol. Exp. Ther. 2009, 331, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Gottas, A.; Øiestad, E.L.; Boix, F.; Vindenes, V.; Ripel, A.; Thaulow, C.H.; Mørland, J. Levels of heroin and its metabolites in blood and brain extracellular fluid after i.v. heroin administration to freely moving rats. Br. J. Pharmacol. 2013, 170, 546–556. [Google Scholar] [CrossRef] [Green Version]
- Dinis-Oliveira, R.J.; Vieira, D.N.; Magalhães, T. Guidelines for Collection of Biological Samples for Clinical and Forensic Toxicological Analysis. Forensic Sci. Res. 2016, 1, 42–51. [Google Scholar] [CrossRef] [Green Version]
- Thaulow, C.H.; Øiestad, Å.M.L.; Rogde, S.; Andersen, J.M.; Høiseth, G.; Handal, M.; Mørland, J.; Vindenes, V. Can measurements of heroin metabolites in post-mortem matrices other than peripheral blood indicate if death was rapid or delayed? Forensic Sci. Int. 2018, 290, 121–128. [Google Scholar] [CrossRef]
- Rees, K.A.; Pounder, D.J.; Osselton, M.D. Distribution of opiates in femoral blood and vitreous humour in heroin/morphine-related deaths. Forensic Sci. Int. 2013, 226, 152–159. [Google Scholar] [CrossRef]
- Srinivasan, V.; Wielbo, D.; Tebbett, I. Analgesic effects of codeine-6-glucuronide after intravenous administration. Eur. J. Pain 1997, 1, 185–190. [Google Scholar] [CrossRef]
- Haavik, P.E. Kodein er prodrug—Virkestoffet er morfin. Tidsskr. Den Nor. Laegeforening 2000, 120, 1080. [Google Scholar]
- Paul, D.; Standifer, K.M.; Inturrisi, C.E.; Pasternak, G.W. Pharmacological characterization of morphine-6 beta-glucuronide, a very potent morphine metabolite. J. Pharmacol. Exp. Ther. 1989, 251, 477–483. Available online: http://www.ncbi.nlm.nih.gov/pubmed/2810109 (accessed on 30 January 2020). [PubMed]
- Oliveira, A.; Carvalho, F.; de Pinho, P.G.; Remião, F.; Medeiros, R.; Dinis-Oliveira, R.J. Quantification of morphine and its major metabolites M3G and M6G in antemortem and postmortem samples. Biomed. Chromatogr. 2014, 28, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Citti, C.; Palazzoli, F.; Licata, M.; Vilella, A.; Leo, G.; Zoli, M.; Vandelli, M.A.; Forni, F.; Pacchetti, B.; Cannazza, G. Untargeted rat brain metabolomics after oral administration of a single high dose of cannabidiol. J. Pharm. Biomed. Anal. 2018, 161, 1–11. [Google Scholar] [CrossRef]
- Piotrowski, P.; Bocian, S.; Śliwka, K.; Buszewski, B. Simultaneous analysis of zolpidem and its metabolite in whole blood and oral fluid samples by SPE-LC/MS for clinical and forensic purposes. Adv. Med. Sci. 2015, 60, 167–172. [Google Scholar] [CrossRef]
- Shima, N.; Miyawaki, I.; Bando, K.; Horie, H.; Zaitsu, K.; Katagi, M.; Bamba, T.; Tsuchihashi, H.; Fukusaki, E. Influences of methamphetamine-induced acute intoxication on urinary and plasma metabolic profiles in the rat. Toxicology 2011, 287, 29–37. [Google Scholar] [CrossRef]
- Zheng, T.; Liu, L.; Aa, J.; Wang, G.; Cao, B.; Li, M.; Shi, J.; Wang, X.; Zhao, C.; Gu, R.; et al. Metabolic phenotype of rats exposed to heroin and potential markers of heroin abuse. Drug Alcohol Depend. 2013, 127, 177–186. [Google Scholar] [CrossRef]
- Maenhout, T.M.; De Buyzere, M.L.; Delanghe, J.R. Non-oxidative ethanol metabolites as a measure of alcohol intake. Clin. Chim. Acta 2013, 415, 322–329. [Google Scholar] [CrossRef]
- Zakhari, S. Overview: How Is Alcohol Metabolized by the Body? Alcohol Res. Health 2006, 29, 245–254. [Google Scholar]
- Dinis-Oliveira, R.J.; Magalhães, T.; Moreira, R.F.; Proença, J.B.; Pontes, H.; Santos, A.; Duarte, J.A.; Carvalho, F. Clinical and forensic signs related to ethanol abuse: A mechanistic approach. Toxicol. Mech. Methods 2014, 24, 81–110. [Google Scholar] [CrossRef]
- Crunelle, C.L.; Cappelle, D.; Flamand, E.; Cox, J.; Covaci, A.; De Doncker, M.; Van Nuijs, A.L.N.; Michielsen, P.; Yegles, M.; Neels, H. Ethyl glucuronide in hair of non-excessive alcohol consumers: Correlations and gender influence. Forensic Toxicol. 2016, 34, 186–190. [Google Scholar] [CrossRef]
- Cappelle, D.; Neels, H.; Yegles, M.; Fransen, E.; Dueffels, K.; Bremenfeld, S.; Maudens, K.E.; Van Nuijs, A.L.N.; Covaci, A.; Crunelle, C.L. Ethyl glucuronide in nails: Method validation, influence of decontamination and pulverization, and particle size evaluation. Forensic Toxicol. 2016, 34, 158–165. [Google Scholar] [CrossRef]
- Aradottir, S.; Seidl, S.; Wurst, F.M.; Jönsson, B.; Alling, C. Phosphatidylethanol in Human Organs and Blood: A Study on Autopsy Material and Influences by Storage Conditions. Alcohol. Clin. Exp. Res. 2004, 28, 1718–1723. [Google Scholar] [CrossRef]
- Zheng, Y.; Beck, O.; Helander, A. Method development for routine liquid chromatography–mass spectrometry measurement of the alcohol biomarker phosphatidylethanol (PEth) in blood. Clin. Chim. Acta 2011, 412, 1428–1435. [Google Scholar] [CrossRef] [Green Version]
- Badawy, A.A.-B.; Doughrty, D.M.; Marsh-Richard, D.M.; Steptoe, A. Activation of Liver Tryptophan Pyrrolase Mediates the Decrease in Tryptophan Availability to the Brain after Acute Alcohol Consumption by Normal Subjects. Alcohol Alcohol. 2009, 44, 267–271. [Google Scholar] [CrossRef] [Green Version]
- Giménez-Gómez, P.; Pérez-Hernández, M.; López, M.D.G.; Vidal, R.; Abuin-Martínez, C.; O’Shea, E.; Colado, M.I. Increasing kynurenine brain levels reduces ethanol consumption in mice by inhibiting dopamine release in nucleus accumbens. Neuropharmacology 2018, 135, 581–591. [Google Scholar] [CrossRef]
- Álvarez-Sánchez, B.; Priego-Capote, F.; de Castro, M.D.L. Metabolomics analysis I. Selection of biological samples and practical aspects preceding sample preparation. TrAC Trends Anal. Chem. 2010, 29, 111–119. [Google Scholar] [CrossRef]
- Maher, A.D.; Zirah, S.F.M.; Holmes, A.E.; Nicholson, J.K. Experimental and Analytical Variation in Human Urine in 1H NMR Spectroscopy-Based Metabolic Phenotyping Studies. Anal. Chem. 2007, 79, 5204–5211. [Google Scholar] [CrossRef]
- Coe, J.I. Postmortem Chemistry Update Emphasis on Forensic Application. Am. J. Forensic Med. Pathol. 1993, 14, 91–117. [Google Scholar] [CrossRef]
- Donaldson, A.E.; Lamont, I.L. Estimation of post-mortem interval using biochemical markers. Aust. J. Forensic Sci. 2014, 46, 8–26. [Google Scholar] [CrossRef]
- Álvarez Sánchez, B.; Priego-Capote, F.; Jiménez, J.R.; Luque de Castro, M.D. Automated solid-phase extraction for concentration and clean-up of female steroid hormones prior to liquid chromatography–electrospray ionization–tandem mass spectrometry: An approach to lipidomics. J. Chromatogr. A 2008, 1207, 46–54. [Google Scholar] [CrossRef] [PubMed]
- León, Z.; Chisvert, A.; Tarazona, I.; Salvador, A. Solid-phase extraction liquid chromatography–tandem mass spectrometry analytical method for the determination of 2-hydroxy-4-methoxybenzophenone and its metabolites in both human urine and semen. Anal. Bioanal. Chem. 2010, 398, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Tsai, I.-L.; Weng, T.-I.; Tseng, Y.J.; Tan, H.K.-L.; Sun, H.-J.; Kuo, C.-H. Screening and Confirmation of 62 Drugs of Abuse and Metabolites in Urine by Ultra-High-Performance Liquid Chromatography-Quadrupole Time-of-Flight Mass Spectrometry. J. Anal. Toxicol. 2013, 37, 642–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tridico, S. Hair: Animal. In Wiley Encyclopedia of Forensic Science; John Wiley & Sons, Ltd.: Chichester, UK, 2014; pp. 1–15. [Google Scholar] [CrossRef]
- Kintz, P.; Villain, M.; Cirimele, V. Hair Analysis for Drug Detection. Ther. Drug Monit. 2006, 28, 442–446. [Google Scholar] [CrossRef]
- Kintz, P. Value of hair analysis in postmortem toxicology. Forensic Sci. Int. 2004, 142, 127–134. [Google Scholar] [CrossRef]
- Opel, K.L.; Fleishaker, E.L.; Nicklas, J.A.; Buel, E.; Mccord, B.R. Evaluation and Quantification of Nuclear DNA from Human Telogen Hairs. J. Forensic Sci. 2008, 53, 853–857. [Google Scholar] [CrossRef]
- Kintz, P.; Nicholson, D. Interpretation of a highly positive ethyl glucuronide result together with negative fatty acid ethyl esters result in hair and negative blood results. Forensic Toxicol. 2013, 32, 176–179. [Google Scholar] [CrossRef]
- Namera, A.; Urabe, S.; Saito, T.; Torikoshi-Hatano, A.; Shiraishi, H.; Arima, Y.; Nagao, M. A fatal case of 3,4-methylenedioxypyrovalerone poisoning: Coexistence of α-pyrrolidinobutiophenone and α-pyrrolidinovalerophenone in blood and/or hair. Forensic Toxicol. 2013, 31, 338–343. [Google Scholar] [CrossRef]
- Shima, N.; Sasaki, K.; Kamata, T.; Matsuta, S.; Katagi, M.; Miki, A.; Zaitsu, K.; Sato, T.; Nakanishi, T.; Tsuchihashi, H.; et al. Single-hair analysis of zolpidem on the supposition of its single administration in drug-facilitated crimes. Forensic Toxicol. 2015, 33, 122–130. [Google Scholar] [CrossRef]
- Kintz, P.; Villain, M.; Dumestre-Toulet, V.; Ludes, B. Drug-facilitated sexual assault and analytical toxicology: The role of LC-MS/MS. A case involving zolpidem. J. Clin. Forensic Med. 2005, 12, 36–41. [Google Scholar] [CrossRef]
- Baswan, S.; Kasting, G.B.; Li, S.K.; Wickett, R.; Adams, B.; Eurich, S.; Schamper, R. Understanding the formidable nail barrier: A review of the nail microstructure, composition and diseases. Mycoses 2017, 60, 284–295. [Google Scholar] [CrossRef] [Green Version]
- Cappelle, D.; Yegles, M.; Neels, H.; van Nuijs, A.; De Doncker, M.; Maudens, K.; Covaci, A.; Crunelle, C.L. Nail analysis for the detection of drugs of abuse and pharmaceuticals: A review. Forensic Toxicol. 2015, 33, 12–36. [Google Scholar] [CrossRef]
- Green, S.J.; Wilson, J.F. The Effect of Hair Color on the Incorporation of Methadone into Hair in the Rat. J. Anal. Toxicol. 1996, 20, 121–123. [Google Scholar] [CrossRef] [Green Version]
- Reid, R.W.; O’Connor, F.L.; Deakin, A.G.; Ivery, D.M.; Crayton, J.W. Cocaine and Metabolites in Human Graying Hair: Pigmentary Relationship. J. Toxicol. Clin. Toxicol. 1996, 34, 685–690. [Google Scholar] [CrossRef]
- Palmeri, A.; Pichini, S.; Pacifici, R.; Zuccaro, P.; Lopez, A. Drugs in Nails. Physiology, pharmacokinetics and forensic toxicology. Clin. Pharmacokinet. 2000, 38, 95–110. [Google Scholar] [CrossRef]
- Alexiou, D.; Koutselinis, A.; Manolidis, C.; Boukis, D.; Papadatos, J.; Papadatos, C. The Content of Trace Elements (Cu, Zn, Fe, Mg) in Fingernails of Children. Dermatology 1980, 160, 380–382. [Google Scholar] [CrossRef]
- Krumbiegel, F.; Hastedt, M.; Tsokos, M. Nails are a potential alternative matrix to hair for drug analysis in general unknown screenings by liquid-chromatography quadrupole time-of-flight mass spectrometry. Forensic Sci. Med. Pathol. 2014, 10, 496–503. [Google Scholar] [CrossRef]
- Castillo-Peinado, L.S.; Luque de Castro, M.D. An overview on forensic analysis devoted to analytical chemists. Talanta 2017, 167, 181–192. [Google Scholar] [CrossRef]
- Malathi, N.; Mythili, S.; Vasanthi, H.R. Salivary Diagnostics: A Brief Review. ISRN Dent. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Humphrey, S.P.; Williamson, R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef]
- Larsen, M.; Jensen, A.; Madsen, D.; Pearce, E. Individual variations of pH, buffer capacity, and concentrations of calcium and phosphate in unstimulated whole saliva. Arch. Oral Biol. 1999, 44, 111–117. [Google Scholar] [CrossRef]
- Álvarez-Sánchez, B.; Priego-Capote, F.; Luque de Castro, M.D. Study of sample preparation for metabolomic profiling of human saliva by liquid chromatography–time of flight/mass spectrometry. J. Chromatogr. A 2012, 1248, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Sun, H.; Wang, X. Saliva Metabolomics Opens Door to Biomarker Discovery, Disease Diagnosis, and Treatment. Appl. Biochem. Biotechnol. 2012, 168, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Mena-Bravo, A.; Luque de Castro, M.D. Sweat: A sample with limited present applications and promising future in metabolomics. J. Pharm. Biomed. Anal. 2014, 90, 139–147. [Google Scholar] [CrossRef]
- Calderón-Santiago, M.; Priego-Capote, F.; Jurado-Gámez, B.; Luque de Castro, M. Optimization study for metabolomics analysis of human sweat by liquid chromatography-tandem mass spectrometry in high resolution mode. J. Chromatogr. A 2014, 1333, 70–78. [Google Scholar] [CrossRef]
- Dinis-Oliveira, R.J. Metabolomics of drugs of abuse: A more realistic view of the toxicological complexity. Bioanalysis 2014, 6, 3155–3159. [Google Scholar] [CrossRef]
- Zhu, S.-S.; Long, R.; Song, T.; Zhang, L.; Dai, Y.-L.; Liu, S.-W.; Zhang, P. UPLC-Q-TOF/MS Based Metabolomics Approach to Study the Hepatotoxicity of Cantharidin on Mice. Chem. Res. Toxicol. 2019, 32, 2204–2213. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Wu, H.; Tjeerdema, R.S.; Viant, M.R. Evaluation of metabolite extraction strategies from tissue samples using NMR metabolomics. Metabolomics 2007, 3, 55–67. [Google Scholar] [CrossRef]
- Harada, S.; Takebayashi, T.; Kurihara, A.; Akiyama, M.; Suzuki, A.; Hatakeyama, Y.; Sugiyama, D.; Kuwabara, K.; Takeuchi, A.; Okamura, T.; et al. Metabolomic profiling reveals novel biomarkers of alcohol intake and alcohol-induced liver injury in community-dwelling men. Environ. Health Prev. Med. 2015, 21, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Griffin, J.L.; Scott, J.; Nicholson, J.K. The Influence of Pharmacogenetics on Fatty Liver Disease in the Wistar and Kyoto Rats: A Combined Transcriptomic and Metabonomic Study. J. Proteome Res. 2007, 6, 54–61. [Google Scholar] [CrossRef]
- Griffin, J.L.; Salek, R.M. Metabolomic applications to neuroscience: More challenges than chances? Expert Rev. Proteom. 2007, 4, 435–437. [Google Scholar] [CrossRef] [Green Version]
- Naz, S.; Moreira Dos Santos, D.C.; García, A.; Barbas, C. Analytical protocols based on LC–MS, GC–MS and CE–MS for nontargeted metabolomics of biological tissues. Bioanalysis 2014, 6, 1657–1677. [Google Scholar] [CrossRef]
- Gonzalez-Riano, C.; Garcia, A.; Barbas, C. Metabolomics studies in brain tissue: A review. J. Pharm. Biomed. Anal. 2016, 130, 141–168. [Google Scholar] [CrossRef]
- Kashem, M.A.; Ahmed, S.; Sultana, N.; Ahmed, E.U.; Pickford, R.; Rae, C.; Šerý, O.; McGregor, I.S.; Balcar, V.J. Metabolomics of Neurotransmitters and Related Metabolites in Post-Mortem Tissue from the Dorsal and Ventral Striatum of Alcoholic Human Brain. Neurochem. Res. 2016, 41, 385–397. [Google Scholar] [CrossRef]
- Huo, Z.; Yu, L.; Yang, J.; Zhu, Y.; Bennett, D.A.; Zhao, J. Brain and blood metabolome for Alzheimer’s dementia: Findings from a targeted metabolomics analysis. Neurobiol. Aging 2020, 86, 123–133. [Google Scholar] [CrossRef]
- Thierauf-Emberger, A.; Echle, J.; Dacko, M.; Lange, T. Comparison of ethanol concentrations in the human brain determined by magnetic resonance spectroscopy and serum ethanol concentrations. Int. J. Leg. Med. 2020, 134, 1713–1718. [Google Scholar] [CrossRef]
- Mora-Ortiz, M.; Trichard, M.; Oregioni, A.; Claus, S.P. Thanatometabolomics: Introducing NMR-based metabolomics to identify metabolic biomarkers of the time of death. Metabolomics 2019, 15, 37. [Google Scholar] [CrossRef] [Green Version]
- Weljie, A.M.; Newton, J.; Mercier, P.; Carlson, E.; Slupsky, C.M. Targeted Profiling: Quantitative Analysis of 1H NMR Metabolomics Data. Anal. Chem. 2006, 78, 4430–4442. [Google Scholar] [CrossRef]
- Xiao, J.F.; Zhou, B.; Ressom, H.W. Metabolite identification and quantitation in LC-MS/MS-based metabolomics. TrAC Trends Anal. Chem. 2012, 32, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Týčová, A.; Ledvina, V.; Klepárník, K. Recent advances in CE-MS coupling: Instrumentation, methodology, and applications. Electrophoresis 2017, 38, 115–134. [Google Scholar] [CrossRef]
- Snowden, S.; Dahlén, S.-E.; Wheelock, C.E. Application of metabolomics approaches to the study of respiratory diseases. Bioanalysis 2012, 4, 2265–2290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Zhu, S.; Zhao, M.; Dai, Y.; Zhang, L.; Ding, S.; Zhao, P.; Li, J. Integration of 1H NMR- and UPLC-Q-TOF/MS-based plasma metabonomics study to identify diffuse axonal injury biomarkers in rat. Brain Res. Bull. 2018, 140, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Worley, B. Multivariate Analysis in Metabolomics. Curr. Metab. 2012, 1, 92–107. [Google Scholar] [CrossRef]
- Riaño, C.G.; González, S.T.; García, A.; Muñoz, A.; DeFelipe, J.; Barbas, C. Metabolomics and neuroanatomical evaluation of post-mortem changes in the hippocampus. Brain Struct. Funct. 2017, 222, 2831–2853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, Y.-R.; Park, Y.S.; Park, Y.C.; Yoon, S.M.; JongAhn, H.; Kim, G.; Kwon, S.W. UPLC/Q-TOF MS based metabolomics approach to post-mortem-interval discrimination: Mass spectrometry based metabolomics approach. J. Pharm. Investig. 2012, 42, 41–46. [Google Scholar] [CrossRef]
- Isbell, T.A.; Strickland, E.C.; Hitchcock, J.; McIntire, G.; Colyer, C.L. Capillary electrophoresis-mass spectrometry determination of morphine and its isobaric glucuronide metabolites. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 980, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.L.; Telving, R.; Andreasen, M.F.; Hasselstrøm, J.B.; Johannsen, M. A Metabolomics Study of Retrospective Forensic Data from Whole Blood Samples of Humans Exposed to 3,4-Methylenedioxymethamphetamine: A New Approach for Identifying Drug Metabolites and Changes in Metabolism Related to Drug Consumption. J. Proteome Res. 2016, 15, 619–627. [Google Scholar] [CrossRef]
- Ellis, D.I.; Dunn, W.B.; Griffin, J.L.; Allwood, J.W.; Goodacre, R. Metabolic fingerprinting as a diagnostic tool. Pharmacogenomics 2007, 8, 1243–1266. [Google Scholar] [CrossRef] [Green Version]
- Peterson, D.S. Matrix-free methods for laser desorption/ionization mass spectrometry. Mass Spectrom. Rev. 2007, 26, 19–34. [Google Scholar] [CrossRef]
- Morelato, M.; Beavis, A.; Kirkbride, P.; Roux, C. Forensic applications of desorption electrospray ionisation mass spectrometry (DESI-MS). Forensic Sci. Int. 2013, 226, 10–21. [Google Scholar] [CrossRef]
- Deimler, R.E.; Razunguzwa, T.T.; Reschke, B.R.; Walsh, C.M.; Powell, M.J.; Jackson, G.P. Direct analysis of drugs in forensic applications using laser ablation electrospray ionization-tandem mass spectrometry (LAESI-MS/MS). Anal. Methods 2014, 6, 4810–4817. [Google Scholar] [CrossRef]

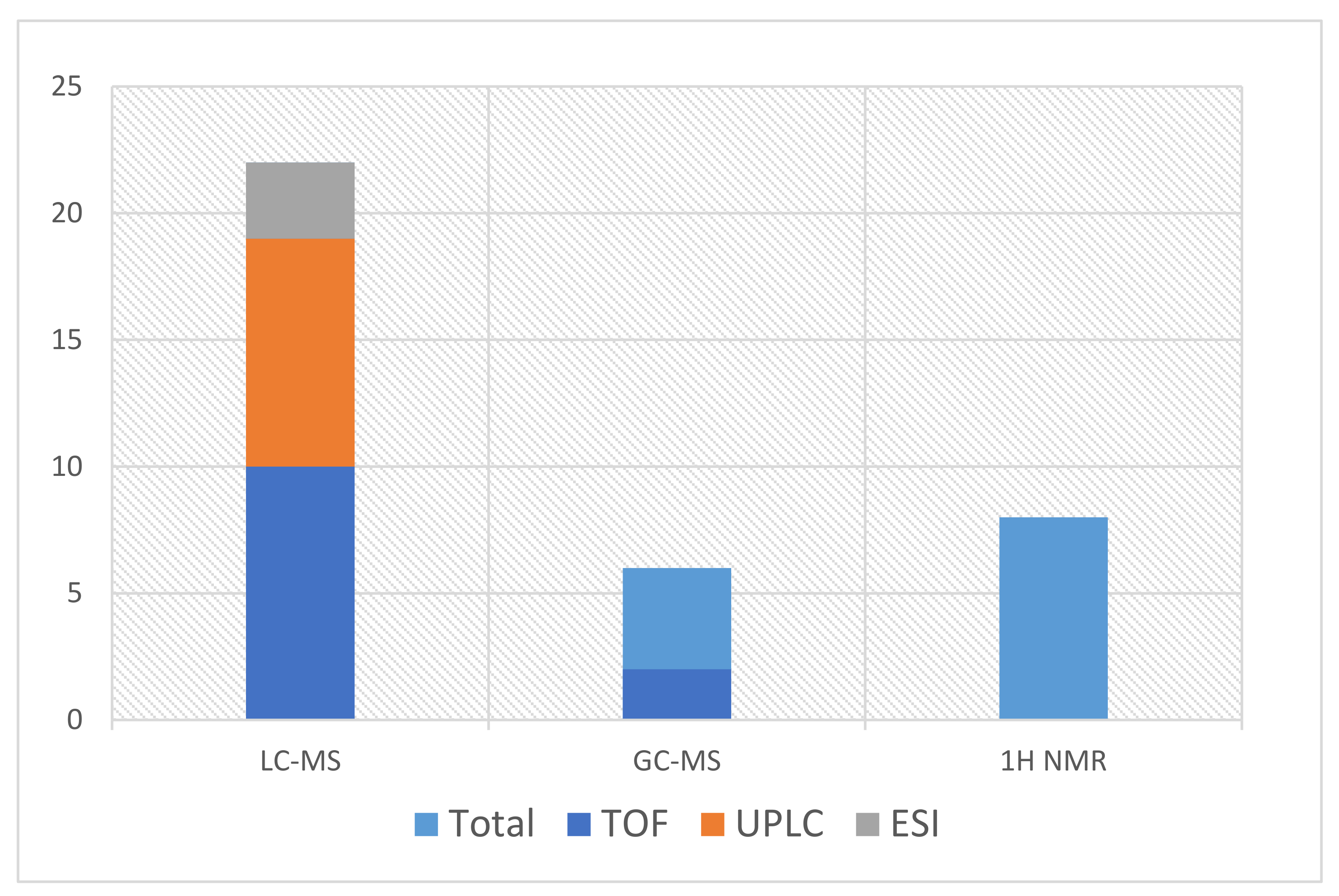
| Reference | Method | Type of Sample | Sample Preparation | Analitycal Problem | Analytical/Validation Conditions |
|---|---|---|---|---|---|
| Griffin et al. [94] | 1H NMR | Liver | Frozen tissue stored at −80 °C, homogenization | Steatosis caused by orotic acid | 600.2 MHz Lvl. of significacnce = 0.005 |
| Holmes et al. [27] | Urine | Samples buffered and internally calibrated | Detection of drug metabolome | 600.29 MHz in flow-injection mode | |
| Lin et al. [92] | Muscle | Tissue homogenization and extraction | Evaluation of different extraction methods/Tissue metabolome | 500.11 MHz | |
| Maher et al. [61] | Urine | - | The effect of long-term storage conditions on human urine metabolome | 600 MHz | |
| Mora-Ortiz et al. [101] | Heart, kidney, liver, spleen | Freezing and homogenization | Identification of metabolic biomarkers of the time of death | 700 MHz | |
| Welije et al. [102] | Urine | Simplified synthetic urine | The use of targeted profiling for mixture analysis | 600 MHz | |
| Huo et al. [99] | UPLC-MS/MS | Brain, plasma | Plasma sample derivatized with phenyl isothiocyanate Homogenized brain tissue sample | Determination of acylcarnitines, sphingolipids, glycerophospholipids | Biocrates AbsoluteIDQ® p180 Kit |
| Gottas et al. [39] | Brain, blood | Brain tissue homogenate and blood samples mixed with reagents | Determination of heroin levels | LOD = 0.07 ng/mL for heroine, 0.26 ng/mL for morphine, 0.36 ng/mL for 6-MAM, 0.23 ng/mL for M3G; LLOQ = 3 ng/mL for heroine, 1.7 ng/mL for morphine, 2 ng/mL for 6-MAM, 2.2 ng/mL for M3G | |
| Kang et al. [109] | UPLC-QTOF-MS | Liver | Homogenization and extraction | Metabolite changes related to postmortem interval | Column: ACQUITY UPLC column (BEH C18) 2.1 × 100 Column, (Waters, Milford, MA, USA) |
| Tsai et al. [66] | Urine | Urine diluted with water and centrifugated | Screening and confirmation of 62 drugs of abuse and their metabolites | LOD = 2.8 and 187.5 ng/mL for 62 metabolites | |
| Tuhalow et al. [41] | UHPLC-MS/MS | Blood, urine, vitreous humour | - | Postmortem heroin levels’ determination | LOQ = 0.0033 mg/L and 0.0086 mg/L for 6-AM and morphine, 0.0090 mg/L for codeine, and 0.014 mg/L for M3G and M6G in blood, pericardial fluid, and vitreous humor |
| Harada et al. [93] | CE-MS | Plasma | Centrifugation and extraction | Novel biomarkers of alcohol intake | - |
| Gimenez-Gomez et al. [59] | HPLC, GC-MS | Plasma, brain | Samples’ deproteinization and homogenization (brain tissue) | Reduction of EtOH consumption induced by KYN and KYNA increments | Column: HR-80; 80 mm × 4.6 mm, 3 µm |
| Aradottir et al. [56] | HPLC | Liver, lung, spleen, heart, muscle, blood | Tissue homogenization and extration; blood extraction | Postmortem concentration of PEth in blood and organs influenced by storage conditions | Column: Licrosphere 100 DIOL, 5-m particle size |
| Gonzalez Riano et al. [108] | LC-MS | Brain | Tissue homogenization and extraction | Postmortem changes in hippocampus | - |
| Myint et al. [11] | Cereprospinal fluid | Protein-free samples were passed through an Oasis MCX 96-well plate cartridge; etuates were evaporated | Cationic metabolome analysis | LOD = 0.3–9.9 pmol | |
| Andersen et al. [38] | LC-MS/MS | Plasma | Organic phase evaporation | Importance of heroin and its metabolites in eliciting a behavioral response in mice | LOD = 0.0065 mg/L for M3G, 0.00060 mg/L for M6G, 0.00049 mg/L for morphine, 0.00033 mg/L for 6MAM, and 0.00096 mg/L for heroin |
| Leon et al. [65] | Urine, semen | Addition of β-glucuronidase, incubation, SPE procedure | HMB determination | LOD = 0.027 and 0.103 ng/mL (urine) LOD = 1–3 ng/mL (semen) | |
| Kashem et al. [98] | Brain | Tissue homogenization | Neurotransmitter metabolome and protein expression changes of humans exposed to heavy, long-term ethanol consumption | Column: BEH C18 (150 mm 9 2.1 mm; Waters), with 1.7-m particle size | |
| Kintz et al. [71] | Blood, urine | - | Alcohol-related EtG and FAEE presence | - | |
| Kintz et al. [69] | Hair | Decontamination, pulverization, incubation, and extraction of the sample | Hair analysis in postmortem toxicology | - | |
| Shima et al. [73] | Hair | Sample decontamination, pulverization, and extraction | Zolpidem incorporated into hair | LOD = 50 fg/2-cm hair Recovery = 48% Precision RSD = 2.7% Intraday accuracy = 3.8% | |
| Alvarez-Sanchez et al. [64] | LC-ESI-MS/MS | Urine | Enzymatic hydrolysis followed by mini-SPE procedure | Determination of free and glucuronide-conjugated female steroid hormones | Column: Agilent Zorbax Eclipse XDB-C18 (4.6 mm × 150 mm, 5 m particle size) LOD = 1.8–18 pg LOQ = 6–61 pg |
| Zheng et al. [57] | Blood | Lipid extracts of whole blood samples | Alcohol biomarker PEth in blood | R2= 0.994 LOD = 0.01 μmol/L | |
| Zheng et al. [50] | GC-MS | Serum, urine | Serum and urine samples were pretreated, extracted, and derivatised | Identification of potential biomarkers of heroin abuse | Column: 10 m × 0.18 mm i.d. fused-silica capillary column chemically bonded with a 0.18-m DB5-MS stationary phase |
| Crunelle et al. [54] | Hair | Samples were mechanically pulverized | Correlations and gender influence on ethyl glucuronide concentration | LOQ = 2.10 pg/mg LOD = 0.70 pg/mg | |
| Cappelle et al. [55] | GC-MS/MS | Nails | Sample decontamination, pulverization, and derivatization | Determination of EtG | Linearity range: 2–100 pg/mg LLOQ = 2 pg/mg |
| Stefanuto et al. [14] | GC-TOF-MS | VOC | Thermal desorption | VOC profile of human remains during early stages of decomposition | Columns: Restek Rxi-5Sil (5% phenyl–95% dimethyl polysiloxane) (Bellefonte, PA, USA) (30 m × 0.25 mm id × 0.25-μm df); Restek Rxi-17 (50% phenyl–50% dimethyl polysiloxane) (Bellefonte, PA, USA) (1.0 m × 0.15 mm id × 0.15-μm df) |
| Shima et al. [49] | Urine, plasma | Serum and urine samples were pretreated, extracted, and derivatised | Methamphetamine-induced acute intoxication influence on metabolome | Column: CP-SIL 8 (30 m × 0.25 mm i.d., 0.25-m film thickness, GL sciences) | |
| Alvarez-Sanchez et al. [86] | LC-TOF/MS | Saliva | Hydrolysis (both basic and acidic) of saliva + ultrasound energy | Metabolomic profiling of human saliva | Column: Zorbax Eclipse XDB-C18 column (4.6 mm × 150 mm, 5-m particle size) Flow: 1 mL/min |
| Calderon-Santiago et al. [89] | LC-QTOF-MS/MS | Sweat |
| Method development for analysis of human sweat | Column: C18 reverse-phase (Mediterranean, 50 mm × 0.46 mm i.d., 3 m particle size) Flow: 0.8 mL/min |
| Column: Luna hydrophilic interaction chromatography column (HILIC) (100 mm × 0.46 mm i.d., 3-m particle size) Flow: 0.6 mL/min | |||||
| Krumbiegel et al. [81] | Nails | Ground by a ball mill and extracted twice | Usefulness of nail samples instead of hair for a general unknown screening (GUS) fordrugs | Column: Poroshell 120 EC-C18 column (2.1 × 100 mm, 2.7 µm, Agilent Technologies, Santa Clara, CA, USA) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dawidowska, J.; Krzyżanowska, M.; Markuszewski, M.J.; Kaliszan, M. The Application of Metabolomics in Forensic Science with Focus on Forensic Toxicology and Time-of-Death Estimation. Metabolites 2021, 11, 801. https://doi.org/10.3390/metabo11120801
Dawidowska J, Krzyżanowska M, Markuszewski MJ, Kaliszan M. The Application of Metabolomics in Forensic Science with Focus on Forensic Toxicology and Time-of-Death Estimation. Metabolites. 2021; 11(12):801. https://doi.org/10.3390/metabo11120801
Chicago/Turabian StyleDawidowska, Joanna, Marta Krzyżanowska, Michał Jan Markuszewski, and Michał Kaliszan. 2021. "The Application of Metabolomics in Forensic Science with Focus on Forensic Toxicology and Time-of-Death Estimation" Metabolites 11, no. 12: 801. https://doi.org/10.3390/metabo11120801
APA StyleDawidowska, J., Krzyżanowska, M., Markuszewski, M. J., & Kaliszan, M. (2021). The Application of Metabolomics in Forensic Science with Focus on Forensic Toxicology and Time-of-Death Estimation. Metabolites, 11(12), 801. https://doi.org/10.3390/metabo11120801






