Predicting Hair Cortisol and Cortisone Concentration in Postpartum Women through Repeated Measurements of Perceived Stress
Abstract
:1. Introduction
2. Results
2.1. Sample Description
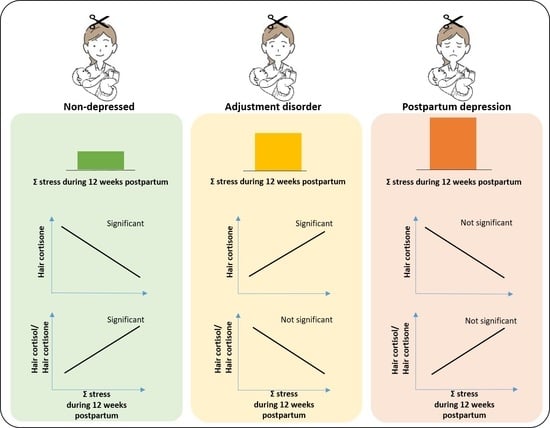
2.2. Stress and the Association with Glucocorticoid Concentration
3. Discussion
4. Materials and Methods
4.1. Procedure
4.2. Participants
4.3. Patient Involvement Statement
4.4. Questionnaires
4.5. Sample Collection and Preparation
4.6. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holmes, T.H.; Rahe, R.H. The Social Readjustment Rating Scale. J. Psychosom. Res. 1967, 11, 213–218. [Google Scholar] [CrossRef]
- Bosquet Enlow, M.; Devick, K.L.; Brunst, K.J.; Lipton, L.R.; Coull, B.A.; Wright, R.J. Maternal Lifetime Trauma Exposure, Prenatal Cortisol, and Infant Negative Affectivity. Infancy 2017, 22, 492–513. [Google Scholar] [CrossRef]
- Scharlau, F.; Pietzner, D.; Vogel, M.; Gaudl, A.; Ceglarek, U.; Thiery, J.; Kratzsch, J.; Hiemisch, A.; Kiess, W. Evaluation of Hair Cortisol and Cortisone Change during Pregnancy and the Association with Self-Reported Depression, Somatization, and Stress Symptoms. Stress 2018, 21, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Stalder, T.; Steudte-Schmiedgen, S.; Alexander, N.; Klucken, T.; Vater, A.; Wichmann, S.; Kirschbaum, C.; Miller, R. Stress-Related and Basic Determinants of Hair Cortisol in Humans: A Meta-Analysis. Psychoneuroendocrinology 2017, 77, 261–274. [Google Scholar] [CrossRef]
- Kalra, S.; Einarson, A.; Karaskov, T.; Van Uum, S.; Koren, G. The Relationship between Stress and Hair Cortisol in Healthy Pregnant Women. Clin. Investig. Med. 2007, 30, E103–E107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braig, S.; Grabher, F.; Ntomchukwu, C.; Reister, F.; Stalder, T.; Kirschbaum, C.; Rothenbacher, D.; Genuneit, J. The Association of Hair Cortisol with Self-Reported Chronic Psychosocial Stress and Symptoms of Anxiety and Depression in Women Shortly after Delivery. Paediatr. Perinat. Epidemiol. 2016, 30, 97–104. [Google Scholar] [CrossRef]
- Kramer, M.S.; Lydon, J.; Séguin, L.; Goulet, L.; Kahn, S.R.; McNamara, H.; Genest, J.; Dassa, C.; Chen, M.F.; Sharma, S.; et al. Stress Pathways to Spontaneous Preterm Birth: The Role of Stressors, Psychological Distress, and Stress Hormones. Am. J. Epidemiol. 2009, 169, 1319–1326. [Google Scholar] [CrossRef]
- Schlotz, W.; Kumsta, R.; Layes, I.; Entringer, S.; Jones, A.; Wüst, S. Covariance between Psychological and Endocrine Responses to Pharmacological Challenge and Psychosocial Stress: A Question of Timing. Psychosom. Med. 2008, 70, 787–796. [Google Scholar] [CrossRef]
- Bauer, A.; Parsonage, M.; Knapp, M.; Iemmi, V.; Adelaja, B. Costs of Perinatal Mental Health Problems; Personal Social Services Research Unit: Canterbury, UK, 2014. [Google Scholar] [CrossRef]
- APA Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013; ISBN 9780890425541.
- D’Anna-Hernandez, K.L.; Ross, R.G.; Natvig, C.L.; Laudenslager, M.L. Hair Cortisol Levels as a Retrospective Marker of Hypothalamic–Pituitary Axis Activity throughout Pregnancy: Comparison to Salivary Cortisol. Physiol. Behav. 2011, 104, 348–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cacioppo, J.T.; Berntson, G.G.; Malarkey, W.B.; Kiecolt-Glaser, J.K.; Sheridan, J.F.; Poehlmann, K.M.; Burleson, M.H.; Ernst, J.M.; Hawkley, L.C.; Glaser, R. Autonomic, Neuroendocrine, and Immune Responses to Psychological Stress: The Reactivity Hypothesis A. Ann. N. Y. Acad. Sci. 2006, 840, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Schommer, N.C.; Hellhammer, D.H.; Kirschbaum, C. Dissociation between Reactivity of the Hypothalamus-Pituitary-Adrenal Axis and the Sympathetic-Adrenal-Medullary System to Repeated Psychosocial Stress. Psychosom. Med. 2003, 65, 450–460. [Google Scholar] [CrossRef]
- Miller, G.E.; Chen, E.; Zhou, E.S. If It Goes up, Must It Come down? Chronic Stress and the Hypothalamic-Pituitary-Adrenocortical Axis in Humans. Psychol. Bull. 2007, 133, 25–45. [Google Scholar] [CrossRef] [Green Version]
- Schnakenberg, P.; Hahn, L.; Stickel, S.; Stickeler, E.; Habel, U.; Eickhoff, S.B.; Chechko, N.; Dukart, J. Examining Early Structural and Functional Brain Alterations in Postpartum Depression through Multimodal Neuroimaging. Sci. Rep. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Bergdahl, J.; Bergdahl, M. Perceived Stress in Adults: Prevalence and Association of Depression, Anxiety and Medication in a Swedish Population. Stress Health 2002, 18, 235–241. [Google Scholar] [CrossRef]
- Dettenborn, L.; Muhtz, C.; Skoluda, N.; Stalder, T.; Steudte, S.; Hinkelmann, K.; Kirschbaum, C.; Otte, C. Introducing a Novel Method to Assess Cumulative Steroid Concentrations: Increased Hair Cortisol Concentrations over 6 Months in Medicated Patients with Depression. Stress 2012, 15, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Staufenbiel, S.M.; Penninx, B.W.J.H.; Spijker, A.T.; Elzinga, B.M.; van Rossum, E.F.C. Hair Cortisol, Stress Exposure, and Mental Health in Humans: A Systematic Review. Psychoneuroendocrinology 2013, 38, 1220–1235. [Google Scholar] [CrossRef]
- Stalder, T.; Kirschbaum, C. Analysis of Cortisol in Hair–State of the Art and Future Directions. Brain Behav. Immun. 2012, 26, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Weckesser, L.J.; Dietz, F.; Schmidt, K.; Grass, J.; Kirschbaum, C.; Miller, R. The Psychometric Properties and Temporal Dynamics of Subjective Stress, Retrospectively Assessed by Different Informants and Questionnaires, and Hair Cortisol Concentrations. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Römer, B.; Lewicka, S.; Kopf, D.; Lederbogen, F.; Hamann, B.; Gilles, M.; Schilling, C.; Onken, V.; Frankhauser, P.; Deuschle, M. Cortisol Metabolism in Depressed Patients and Healthy Controls. Neuroendocrinology 2009, 90, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Gidlow, C.J.; Randall, J.; Gillman, J.; Silk, S.; Jones, M.V. Hair Cortisol and Self-Reported Stress in Healthy, Working Adults. Psychoneuroendocrinology 2016, 63, 163–169. [Google Scholar] [CrossRef]
- Russell, E.; Koren, G.; Rieder, M.; Van Uum, S. Hair Cortisol as a Biological Marker of Chronic Stress: Current Status, Future Directions and Unanswered Questions. Psychoneuroendocrinology 2012, 37, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Kirschbaum, C.; Grass, J.; Stalder, T. LC–MS Based Analysis of Endogenous Steroid Hormones in Human Hair. J. Steroid Biochem. Mol. Biol. 2016, 162, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum, C.; Tietze, A.; Skoluda, N.; Dettenborn, L. Hair as a Retrospective Calendar of Cortisol Production—Increased Cortisol Incorporation into Hair in the Third Trimester of Pregnancy. Psychoneuroendocrinology 2009, 34, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Abell, J.G.; Stalder, T.; Ferrie, J.E.; Shipley, M.J.; Kirschbaum, C.; Kivimäki, M.; Kumari, M. Assessing Cortisol from Hair Samples in a Large Observational Cohort: The Whitehall II Study. Psychoneuroendocrinology 2016, 73, 148. [Google Scholar] [CrossRef] [Green Version]
- Cox, J.; Holden, J.; Psychiatry, R.S.-T.B. journal of; 1987, undefined Detection of Postnatal Depression: Development of the 10-Item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry 1987, 150, 782–786. [Google Scholar]
- Beesdo-Baum, K.; Zaudig, M.; Wittchen, H.-U. Strukturiertes Klinisches Interview Für DSM-5 Störungen—Klinische Version; Hogrefe: Göttingen, Germany, 2019. [Google Scholar]
- Cooper, G.A.A.; Kronstrand, R.; Kintz, P. Society of Hair Testing Guidelines for Drug Testing in Hair. Forensic. Sci. Int. 2012, 218, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Quinete, N.; Bertram, J.; Reska, M.; Lang, J.; Kraus, T. Highly Selective and Automated Online SPE LC–MS3 Method for Determination of Cortisol and Cortisone in Human Hair as Biomarker for Stress Related Diseases. Talanta 2015, 134, 310–316. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows; IBM Corp.: Endicott, NY, USA, 2017. [Google Scholar]
- Cohen, J. A Power Primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]


| Non-Depressed | Adjustment Disorder | Postpartum Depression | Statistics | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age | 32.01 (4.45) | 32.25 (4.91) | 30.69 (5.22) | F (2, 239) = 1.41, p = 0.247 |
| Perceived stress sum | 152.19 (62.39) | 184.65 (55.19) | 234.64 (50.87) | F (2, 239) = 29.66, p < 0.001 a, b, c |
| 0–3 weeks | 42.98 (17.69) | 55.63 (19.56) | 57.53 (15.89) | F (2, 239) = 16.17, p < 0.001 a, b |
| 3–6 weeks | 40.02 (17.23) | 51.06 (16.47) | 61.33 (17.19) | F (2, 239) = 26.04, p < 0.001 a, b, c |
| 6–9 weeks | 37.65 (19.27) | 43.47 (17.03) | 58.19 (17.99) | F (2, 239) = 17.82, p < 0.001 b, c |
| 9–12 weeks | 31.52 (17.87) | 34.48 (15.93) | 57.58 (19.57) | F (2, 240) = 32.52, p < 0.001 b, c |
| EPDS at birth | 3.48 (2.34) | 9.70 (4.39) | 8.36 (4.16) | Welch (2, 70.16) = 75.52, p < 0.001 a, b |
| EPDS 3 weeks | 4.48 (2.79) | 10.71 (3.59) | 11.97 (5.74) | Welch (2, 72.61) = 94.04, p < 0.001 a, b |
| EPDS 6 weeks | 3.33 (2.53) | 8.60 (3.94) | 11.72 (4.78) | Welch (2, 71.22) = 89.29, p < 0.001 a, b, c |
| EPDS 9 weeks | 2.71 (2.44) | 6.56 (3.67) | 11.56 (5.53) | Welch (2, 70.11) = 66.39, p < 0.001 a, b, c |
| EPDS 12 weeks | 2.30 (2.08) | 6.11 (2.94) | 12.61 (3.99) | Welch (2, 72.09) = 140.54, p < 0.001 a, b, c |
| HCC T0 (pg/mg) | 10.59 (15.20) | 8.63 (8.46) | 7.47 (5.21) | * F (2, 239) = 0.58, p = 0.943 |
| HCC T1 (pg/mg) | 5.54 (4.61) | 4.99 (3.57) | 6.21(5.32) | * Welch (2, 93.07) = 0.82, p = 0.444 |
| HCNC T0 (pg/mg) | 30.67 (30.07) | 37.47 (33.99) | 32.21 (33.54) | * F (2, 239) = 1.09, p = 0.337 |
| HCNC T1 (pg/mg) | 19.07 (13.24) | 23.202 (15.13) | 22.72 (16.79) | * F (2, 239) = 3.12, p = 0.046 |
| HCC/HCNC ratio T0 (pg/mg) | 0.60 (2.95) | 0.40 (1.12) | 1.30 (6.36) | * F (2, 239) = 2.04, p = 0.132 |
| HCC/HCNC ratio T1 (pg/mg) | 0.32 (0.19) | 0.26 (0.19) | 0.29 (0.13) | * F (2, 239) = 4.58, p = 0.011 a |
| Sum of Perceived Stress | |||
|---|---|---|---|
| ND n = 141 | AD n = 63 | PPD n = 36 | |
| HCC T1 | 0.018 (0.417) | 0.135 (0.291) | –0.139 (0.419) |
| HCNC T1 | –0.153 (0.035) | 0.318 (0.011) | –0.208 (0.225) |
| HCC/HCNC ratio T1 | 0.304 (0.000) | –0.126 (0.324) | 0.104 (0.548) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lang, J.; Stickel, S.; Gaum, P.M.; Habel, U.; Bertram, J.; Eickhoff, S.B.; Chechko, N. Predicting Hair Cortisol and Cortisone Concentration in Postpartum Women through Repeated Measurements of Perceived Stress. Metabolites 2021, 11, 815. https://doi.org/10.3390/metabo11120815
Lang J, Stickel S, Gaum PM, Habel U, Bertram J, Eickhoff SB, Chechko N. Predicting Hair Cortisol and Cortisone Concentration in Postpartum Women through Repeated Measurements of Perceived Stress. Metabolites. 2021; 11(12):815. https://doi.org/10.3390/metabo11120815
Chicago/Turabian StyleLang, Jessica, Susanne Stickel, Petra M. Gaum, Ute Habel, Jens Bertram, Simon B. Eickhoff, and Natalia Chechko. 2021. "Predicting Hair Cortisol and Cortisone Concentration in Postpartum Women through Repeated Measurements of Perceived Stress" Metabolites 11, no. 12: 815. https://doi.org/10.3390/metabo11120815
APA StyleLang, J., Stickel, S., Gaum, P. M., Habel, U., Bertram, J., Eickhoff, S. B., & Chechko, N. (2021). Predicting Hair Cortisol and Cortisone Concentration in Postpartum Women through Repeated Measurements of Perceived Stress. Metabolites, 11(12), 815. https://doi.org/10.3390/metabo11120815