Metabolite and Bioactive Compounds Profiling of Meteora Sea Buckthorn Berries through High-Resolution NMR Analysis
Abstract
:1. Introduction
2. Results
2.1. Flavonoids
2.2. Saccharides
2.3. Organic Acids
2.4. Amino Acids
2.5. Vitamins
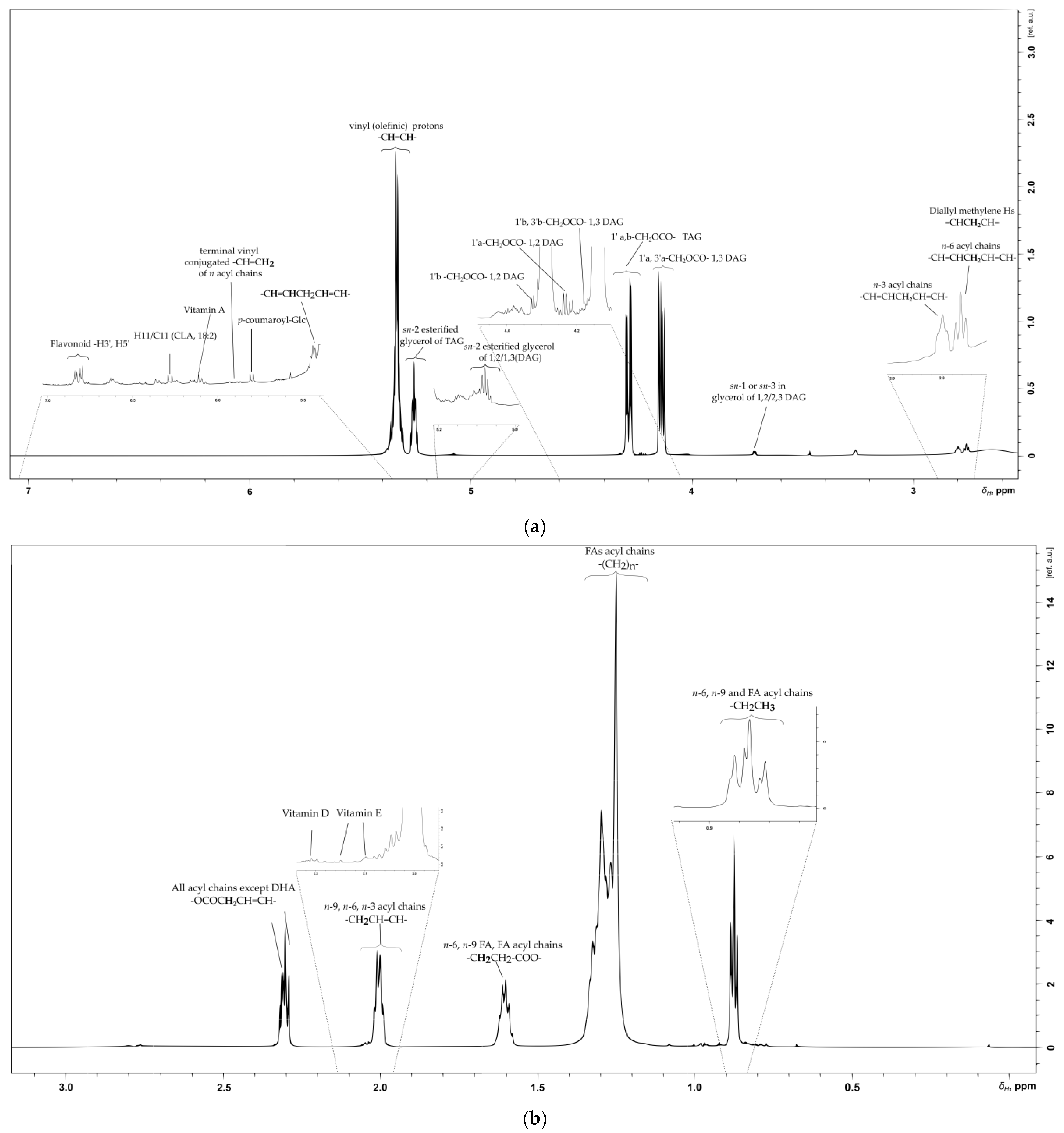
2.6. Lipid, Glycerolipid and Fatty Acid Content of SB Berries
2.6.1. Olefinic Region
2.6.2. Glyceryl Region
2.6.3. Aliphatic Region
3. Discussion
4. Materials and Methods
4.1. Solvents and Chemicals
4.1.1. Sea Buckthorn Berries Samples
4.1.2. Osmotic Dehydrated Berries Samples
4.2. NMR Sample Preparation
4.2.1. Sea Buckthorn Berries Polar Extract NMR Sample
4.2.2. Sea Buckthorn Berries Nonpolar Extract NMR Sample
4.2.3. NMR Experiments
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rousi, A. The genus Hippophae, L. A taxonomic study. In Proceedings of the Annales Botanici Fennici; Societas Biologica Fennica Vanamo: Helsinki, Finland, 1971; pp. 177–227. [Google Scholar]
- Li, T.S.; Beveridge, T.H. Sea Buckthorn (Hippophae rhamnoides L.): Production and Utilization; NRC Research Press: Ottawa, ON, Canada, 2003. [Google Scholar]
- Li, T.; Wang, L. Physiological components and health effects of ginseng, echinacea and seabuckthorn. Funct. Foods Biochem. Process. Asp. 1998, 1, 329. [Google Scholar]
- Chandra, S.; Zafar, R.; Dwivedi, P.; Shinde, L.; Prita, B. Pharmacological and nutritional importance of sea buckthorn (Hippophae). Pharma Innov. 2018, 7, 258. [Google Scholar]
- Sharma, U.K.; Sharma, K.; Sharma, N.; Sharma, A.; Singh, H.P.; Sinha, A.K. Microwave-assisted efficient extraction of different parts of Hippophae rhamnoides for the comparative evaluation of antioxidant activity and quantification of its phenolic constituents by reverse-phase high-performance liquid chromatography (RP-HPLC). J. Agric. Food Chem. 2008, 56, 374–379. [Google Scholar] [CrossRef]
- Guo, R.; Guo, X.; Li, T.; Fu, X.; Liu, R.H. Comparative assessment of phytochemical profiles, antioxidant and antiproliferative activities of Sea buckthorn (Hippophaë rhamnoides L.) berries. Food Chem. 2017, 221, 997–1003. [Google Scholar] [CrossRef]
- Jaśniewska, A.; Diowksz, A. Wide Spectrum of Active Compounds in Sea Buckthorn (Hippophae rhamnoides) for Disease Prevention and Food Production. Antioxidants 2021, 10, 1279. [Google Scholar] [CrossRef]
- Zielińska, A.; Nowak, I. Abundance of active ingredients in sea-buckthorn oil. Lipids Health Dis. 2017, 16, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ciesarová, Z.; Murkovic, M.; Cejpek, K.; Kreps, F.; Tobolková, B.; Koplík, R.; Belajová, E.; Kukurová, K.; Daško, Ľ.; Panovská, Z. Why is sea buckthorn (Hippophae rhamnoides L.) so exceptional? A review. Food Res. Int. 2020, 133, 109170. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, G.; Zhang, J.; Zhang, Y.; Li, J.; Xiong, C.; Zhang, Q.; Li, X.; Lai, X. Metabolic discrimination of sea buckthorn from different Hippophaë species by 1 H NMR based metabolomics. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Sytařová, I.; Orsavová, J.; Snopek, L.; Mlček, J.; Byczyński, Ł.; Mišurcová, L. Impact of phenolic compounds and vitamins C and E on antioxidant activity of sea buckthorn (Hippophaë rhamnoides L.) berries and leaves of diverse ripening times. Food Chem. 2020, 310, 125784. [Google Scholar] [CrossRef]
- Vilas-Franquesa, A.; Saldo, J.; Juan, B. Potential of sea buckthorn-based ingredients for the food and feed industry—A review. Food Prod. Process. Nutr. 2020, 2, 1–17. [Google Scholar] [CrossRef]
- Gutzeit, D.; Baleanu, G.; Winterhalter, P.; Jerz, G. Vitamin C content in sea buckthorn berries (Hippophae rhamnoides L. ssp. rhamnoides) and related products: A kinetic study on storage stability and the determination of processing effects. J. Food Sci. 2008, 73, C615–C620. [Google Scholar] [CrossRef]
- Suryakumar, G.; Gupta, A. Medicinal and therapeutic potential of Sea buckthorn (Hippophae rhamnoides L.). J. Ethnopharmacol. 2011, 138, 268–278. [Google Scholar] [CrossRef]
- Kallio, H.; Yang, B.; Peippo, P. Effects of different origins and harvesting time on vitamin C, tocopherols, and tocotrienols in sea buckthorn (Hippophaë rhamnoides) berries. J. Agric. Food Chem. 2002, 50, 6136–6142. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.-F.; Shang, X.; Zhang, Z.-J.; Zhang, Y.-N. Phytochemical composition and antibacterial activity of the essential oils from different parts of sea buckthorn (Hippophae rhamnoides L.). J. Food Drug Anal. 2017, 25, 327–332. [Google Scholar] [CrossRef] [Green Version]
- Olas, B. The beneficial health aspects of sea buckthorn (Elaeagnus rhamnoides (L.) A. Nelson) oil. J. Ethnopharmacol. 2018, 213, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Koskovac, M.; Cupara, S.; Kipic, M.; Barjaktarevic, A.; Milovanovic, O.; Kojicic, K.; Markovic, M. Sea buckthorn oil—A valuable source for cosmeceuticals. Cosmetics 2017, 4, 40. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Kälviäinen, N.; Tuorila, H. Sensory and hedonic characteristics of juice of sea buckthorn (Hippophae rhamnoides L.) origins and hybrids. LWT-Food Sci. Technol. 2001, 34, 102–110. [Google Scholar] [CrossRef]
- Tiitinen, K.M.; Hakala, M.A.; Kallio, H.P. Quality components of sea buckthorn (Hippophae rhamnoides) varieties. J. Agric. Food Chem. 2005, 53, 1692–1699. [Google Scholar] [CrossRef]
- Araya-Farias, M.; Makhlouf, J.; Ratti, C. Drying of seabuckthorn (Hippophae rhamnoides L.) berry: Impact of dehydration methods on kinetics and quality. Dry. Technol. 2011, 29, 351–359. [Google Scholar] [CrossRef]
- Araya-Farias, M.; Macaigne, O.; Ratti, C. On the development of osmotically dehydrated seabuckthorn fruits: Pretreatments, osmotic dehydration, postdrying techniques, and nutritional quality. Dry. Technol. 2014, 32, 813–819. [Google Scholar] [CrossRef]
- Panche, A.; Diwan, A.; Chandra, S. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, E47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.-S.; Kwon, Y.-S.; Sa, Y.-J.; Kim, M.-J. Isolation and identification of sea buckthorn (Hippophae rhamnoides) phenolics with antioxidant activity and α-glucosidase inhibitory effect. J. Agric. Food Chem. 2011, 59, 138–144. [Google Scholar] [CrossRef]
- Huang, H.-C.; Yang, C.-P.; Wang, S.-Y.; Chang, C.-I.; Sung, P.-J.; Huang, G.-J.; Chien, S.-C.; Kuo, Y.-H. Anti-inflammatory flavonol acylglycosides from the aerial part of Lindera akoensis Hayata. RSC Adv. 2017, 7, 50868–50874. [Google Scholar] [CrossRef] [Green Version]
- Rezende, F.M.; Ferreira, M.J.P.; Clausen, M.H.; Rossi, M.; Furlan, C.M. Acylated Flavonoid Glycosides are the Main Pigments that Determine the Flower Colour of the Brazilian Native Tree Tibouchina pulchra (Cham.) Cogn. Molecules 2019, 24, 718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krauze-Baranowska, M.; Sowiński, P.; Kawiak, A.; Sparzak, B. Flavonoids from Pseudotsuga menziesii. Z. Für Nat. C 2013, 68, 87–96. [Google Scholar] [CrossRef]
- Ahmadu, A.; Hassan, H.; Abubakar, M.; Akpulu, I. Flavonoid glycosides from Byrsocarpus coccineus leaves. Schum and Thonn (Connaraceae). Afr. J. Tradit. Complementary Altern. Med. 2007, 4, 257–260. [Google Scholar] [CrossRef] [Green Version]
- Akhov, L.; Barl, B. Isolation of quercetin glycosides from leaves of sea buckthorn (Hippophae rhamnoides ssp. mongolica). In In Proceedings of the XXVI International Horticultural Congress: Berry Crop Breeding, Production and Utilization for a New Century, Toronto, ON, Canada, 11–17 August 2002; pp. 389–395. [Google Scholar]
- Fiorentino, A.; Ricci, A.; D'Abrosca, B.; Golino, A.; Izzo, A.; Pascarella, M.T.; Piccolella, S.; Esposito, A. Kaempferol glycosides from Lobularia maritima and their potential role in plant interactions. Chem. Biodivers. 2009, 6, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Soliman, F.M.; Shehata, A.H.; Khaleel, A.E.; Ezzat, S.M. An acylated kaempferol glycoside from flowers of Foeniculum vulgare and F. dulce. Molecules 2002, 7, 245–251. [Google Scholar] [CrossRef]
- Wang, D.-M.; Pu, W.-J.; Wang, Y.-H.; Zhang, Y.-J.; Wang, S.-S. A new isorhamnetin glycoside and other phenolic compounds from Callianthemum taipaicum. Molecules 2012, 17, 4595–4603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganbaatar, C.; Gruner, M.; Mishig, D.; Duger, R.; Schmidt, A.W.; Knölker, H.-J. Flavonoid glycosides from the aerial parts of Polygonatum odoratum (Mill.) Druce growing in Mongolia. Open Nat. Prod. J. 2015, 8, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.H.; Jang, H.J.; Park, K.H.; Kim, S.-H.; Kim, J.K.; Kim, J.-C.; Jang, T.S.; Kim, K.H. Phytochemical Analysis of the Fruits of Sea Buckthorn (Hippophae rhamnoides): Identification of Organic Acid Derivatives. Plants 2021, 10, 860. [Google Scholar] [CrossRef] [PubMed]
- Manga, S.S.E.; Tih, A.E.; Abderamane, B.; Ghogomu, R.T.; Blond, A.; Bodo, B. Flavonoid glycosides and their p-coumaroyl esters from Campylospermum calanthum leaves. Z. Für Nat. C 2012, 67, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Usui, A.; Matsuo, Y.; Tanaka, T.; Ohshima, K.; Fukuda, S.; Mine, T.; Nakayama, H.; Ishimaru, K. Ferulic acid esters of oligo-glucose from Allium macrostemon. Nat. Prod. Commun. 2017, 12, 1934578X1701200125. [Google Scholar] [CrossRef] [Green Version]
- Salinero, C.; Feás, X.; Mansilla, J.P.; Seijas, J.A.; Vázquez-Tato, M.P.; Vela, P.; Sainz, M.J. 1H-nuclear magnetic resonance analysis of the triacylglyceride composition of cold-pressed oil from Camellia japonica. Molecules 2012, 17, 6716–6727. [Google Scholar] [CrossRef] [Green Version]
- Dais, P.; Misiak, M.; Hatzakis, E. Analysis of marine dietary supplements using NMR spectroscopy. Anal. Methods 2015, 7, 5226–5238. [Google Scholar] [CrossRef]
- Choi, Y.H.; Kim, H.K.; Wilson, E.G.; Erkelens, C.; Trijzelaar, B.; Verpoorte, R. Quantitative analysis of retinol and retinol palmitate in vitamin tablets using 1H-nuclear magnetic resonance spectroscopy. Anal. Chim. Acta 2004, 512, 141–147. [Google Scholar] [CrossRef]
- Ohnmacht, S.; West, R.; Simionescu, R.; Atkinson, J. Assignment of the 1H and 13C NMR of tocotrienols. Magn. Reson. Chem. 2008, 46, 287–294. [Google Scholar] [CrossRef]
- Ahsan, H.; Ahad, A.; Siddiqui, W.A. A review of characterization of tocotrienols from plant oils and foods. J. Chem. Biol. 2015, 8, 45–59. [Google Scholar] [CrossRef] [Green Version]
- Komba, S.; Kotake-Nara, E.; Tsuzuki, W. Simultaneous synthesis of vitamins D2, D4, D5, D6, and D7 from commercially available phytosterol, β-sitosterol, and identification of each vitamin D by HSQC NMR. Metabolites 2019, 9, 107. [Google Scholar] [CrossRef] [Green Version]
- Paukstelis, J.; Mueller, D.; Seib, P.; Lillard, D., Jr. NMR spectroscopy of ascorbic acid and its derivatives. In Ascorbic Acid: Chemistry, Metabolism, and Uses; Seib, P.A., Tolbert, B.M., Eds.; American Chemical Society: Washington, DC, USA, 1982; Volume 200. [Google Scholar]
- Vlahov, G.; Chepkwony, P.K.; Ndalut, P.K. 13C NMR characterization of triacylglycerols of Moringa oleifera seed oil: An “oleic-vaccenic acid” oil. J. Agric. Food Chem. 2002, 50, 970–975. [Google Scholar] [CrossRef]
- Venianakis, T.; Primikyri, A.; Alexandri, E.; Papamokos, G.; Gerothanassis, I.P. Molecular models of three ω-3 fatty acids based on NMR and DFT calculations of 1H NMR chemical shifts. J. Mol. Liq. 2021, 117460. [Google Scholar] [CrossRef]
- Alexandri, E.; Ahmed, R.; Siddiqui, H.; Choudhary, M.I.; Tsiafoulis, C.G.; Gerothanassis, I.P. High resolution NMR spectroscopy as a structural and analytical tool for unsaturated lipids in solution. Molecules 2017, 22, 1663. [Google Scholar] [CrossRef] [PubMed]
- Yurawecz, M.P. Advances in Conjugated Linoleic Acid Research; The American Oil Chemists Society: Urbana, IL, USA, 2003; Volume 2. [Google Scholar]
- Yang, B.; Karlsson, R.M.; Oksman, P.H.; Kallio, H.P. Phytosterols in sea buckthorn (Hippophaë rhamnoides L.) berries: Identification and effects of different origins and harvesting times. J. Agric. Food Chem. 2001, 49, 5620–5629. [Google Scholar] [CrossRef] [PubMed]
- Seager, R.; Osborn, T.J.; Kushnir, Y.; Simpson, I.R.; Nakamura, J.; Liu, H. Climate variability and change of Mediterranean-type climates. J. Clim. 2019, 32, 2887–2915. [Google Scholar] [CrossRef] [Green Version]
- Christie, W. Fatty acids and lipids: Structures, extraction and fractionation into classes. Gas Chromatogr. Lipids 1989, 24, 116–120. [Google Scholar]

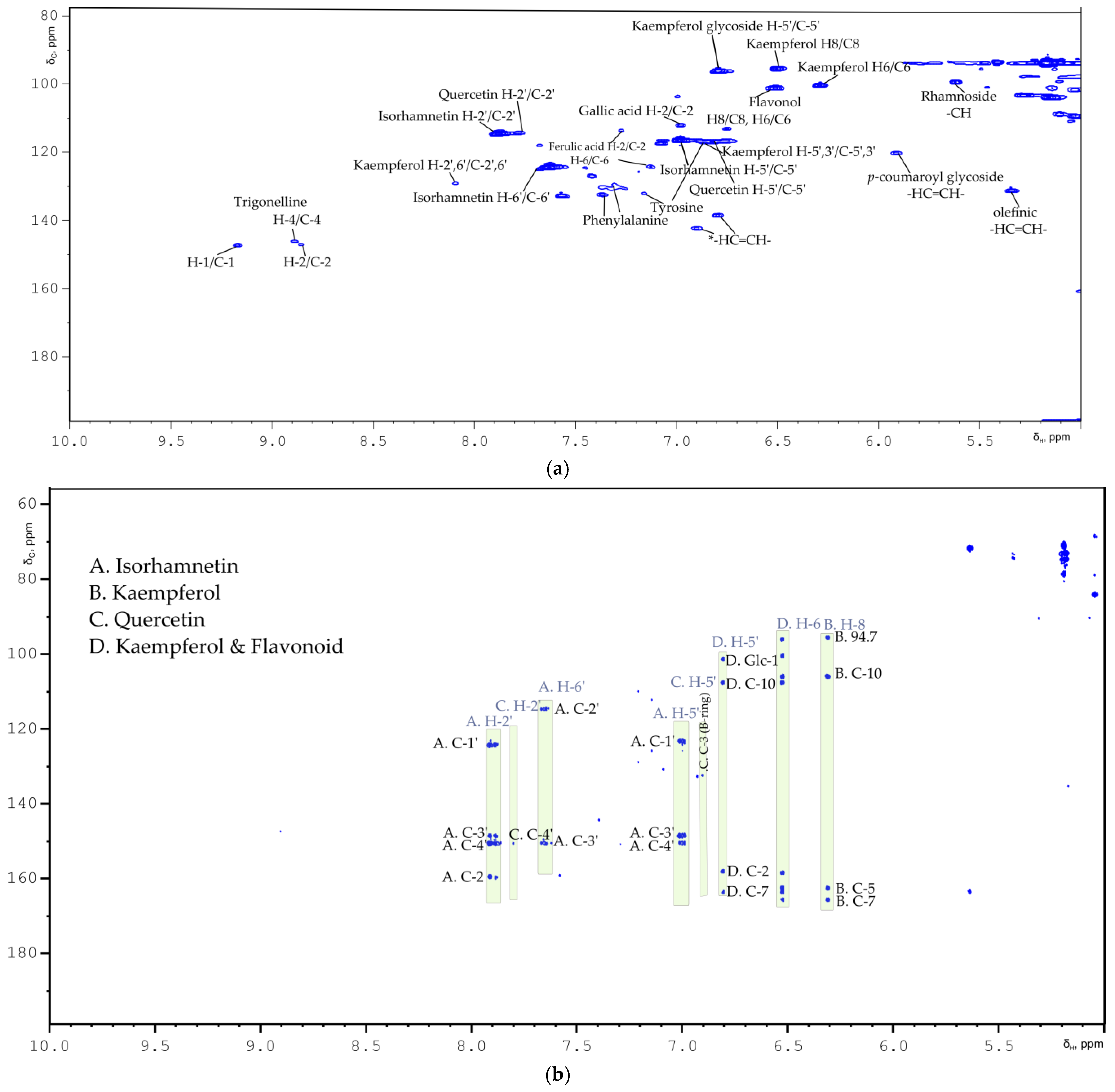
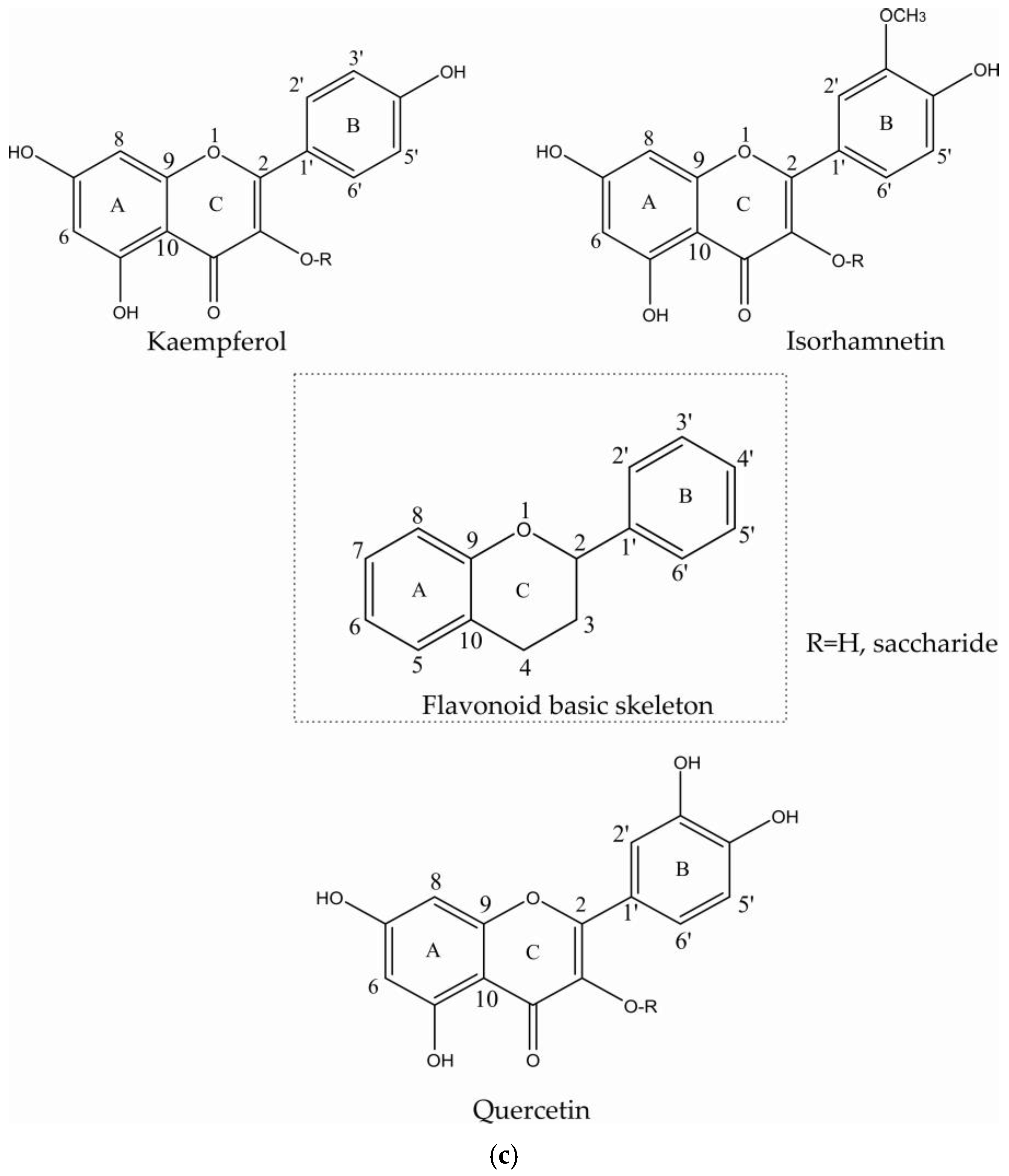
| Compound | Multiplicity/J Coupling (Hz) | 1H (ppm) | 13C (ppm) | Proton/Carbon Position | 1H-13C HMBC (ppm) |
|---|---|---|---|---|---|
| Kaempferol | (m) | 8.08 | 130.7 | H-2′, H-6′/C-2′, C-6′ | * |
| Kaempferol | (m) | 6.90 | 116.3 | H-5′, H-3′/C-5′, C-3′ | 132.8 (C-3 B ring) |
| Quercetin | * | 7.76 | 114.5 | H-2′/C-2′ | 151.0 (C-4′) |
| Quercetin | * | 6.88 | 116.9 | H-5′/C-5′ | 132.5 (C-3 B ring) |
| Isorhamnetin | (d)/J = 8.85 | 7.00 | 116.5 | C-5′ | 123.0 (C-1′), 147.2 (C-4′), 149.3 (C-4′) |
| Isorhamnetin | (d)/J = 1.82 | 7.64 | 124.5 | C-6′ | 113.2 (C-2′), 149.3 (C-3′), 158.3 (C-2 ring C) |
| Isorhamnetin | * | 7.90 | 113.2 | C-2′ | 123.0 (C-1′), 147.2 (C-4′), 149.3 (C-3′), 158.3 (C-2 ring C) |
| Isorhamnetin | * | 3.96 | 52.97 | -OCH3 | 147.2 (C-3′) |
| Kaempferol | (d)/J = 2.05 | 6.29 | 100.4 | C-6 | 94.7, 104.7 (C-10) 160.8, 164.5 (C-7) |
| Kaempferol | (d)/J = 1.96 | 6.50 | 95.6 | C-8 | 94.7 (C-8), 104.7 (C-10), 157.0 (C-9), 161.2 (C-5), 164.5 (C-7) |
| Flavonol glucoside | (d)/J = 2.06 | 6.50 | 101.27 | C-8 or C-6 | 99.3, 106.6 (C-10) |
| Kaempferol glycoside | (d)/J = 1.95 | 6.79 | 96.4 | H-5′/C-5′ | 101.5 (Glc-1), 106.6 (C-10), 158.5 (C-2), 162.4 (C-7) |
| Flavonoids in lipophilic fragment for both fresh and osmotic SB berries | |||||
| Flavonoid p-coumaroyl glycoside | (d)/J = 12.3 | 6.36 | 112.4 | H-8‴/C-8‴ Coumaroyl phenyl ring | 121.8 (C-6′), 131.6 (C-5′ B ring), 145.7 (C-3′ B ring) |
| p-coumaroyl | (d)/J = 16.0 | 6.27 | 115.8 | C-8 -HC=CH- | 126.7 (C-1′ ring), 167.6 (CO) |
| Flavonoid i | (d)/J = 8.7 | 6.80 | 115.24 | B ring C-2′, C-5′ | 132.2 (C-3 ring C), 157.5 (C-2 ring C) |
| Flavonoid ii | (d)/J = 8.5 | 6.83 | 116.06 | B ring C-2′, C-5′ | 115.7, 127.1, |
| Flavonoid iv | * | 6.59 | 115.0 | B ring H-3′/C-3′, H-5′/C-5′ | 135.0 (C-3 ring C) |
| Flavonoid iii | (d)/J = 8.7 | 6.81 | 113.27 | B ring H-5′/C-5′ | 132.2 (C-3 ring C) |
| Flavonoid glycoside i | * | 7.60 | 128.0 | C-6′ | 127.4 (C-1′), 132.2 (C-3), 143.0 (C-3′), 157.2 (C-2) |
| Flavonoid ii | * | 7.63 | 131.0 | C-6′ | 127.4 (C-1′), 132.2 (C-3), 143.0 (C-3′), 157.2 (C-2) |
| Flavonoid iii | (d)/J = 8.6 | 7.63 | 132.6 | C-6′ | 127.4 (C-1′), 132.2 (C-3), 143.0 (C-3′), 157.2 (C-2) |
| Compound | Multiplicity/J Coupling (Hz) | 1H (ppm) | 13C (ppm) | Proton/Carbon Position | 1H-13C HMBC (ppm) |
|---|---|---|---|---|---|
| α-d-Glucose | (d)/J = 3.5 | 5.17 | 94.0 | anomeric –CH- | 71.0, 73.3, 74.8, 78.7 (Saccharide ring) |
| β-d-Glucose | (d)/J = 7.9 | 4.56 | 98.0 | anomeric –CH- | 76.3, 77.7 (Saccharide ring) |
| sucrose | (d)/J = 3.9 | 5.42 | 93.72 | anomeric –CH- | 74.1 (C-2), 105.3 (C-1′anomeric Glc-ferouloyl ester) |
| α-arabinose | (d)/J = 7.4 | 5.16 | 104.15 | anomeric –CH- | 71.0, 73.3, 74.8, 78.7 (Saccharide ring), 135.6 (C-3 ring C-flavonoid) |
| Rhamnoside | (s) br | 5.62 | 99.7 | anomeric -CH- | 71.8 (Saccharide ring), 163.5 (flavonolC-5-ring A) |
| Rhamnosyl -CH3 | (d)/J = 6.3 | 1.08 | 18.23 | -CH3 | * |
| N-acetyl glucosamine | (m) | 4.01 | 73.8 | -HC-NH- H-2/C-2 | 72.4 (C-3), 75.6 (C-5), 172.5 (CO) |
| d-Fructose (configurations α- & β-) | (td) | 4.00 | 68.66 | H-1/C-1 | 70.0 (C-3), 73.4, 77.0 (C-2), 80.5 |
| * | 3.83 | 83.39 | H-1/C-1 | 71.5 (C-3), 77.9 (C-2) | |
| l-quebrachitol | (s) | 3.45 | 58.32 | –OCH3 | * |
| p-coumaroyl glycoside | (d)/J = 12.8 | 5.90 | 120.2 | cis-olefinic HC=CH- | 130.0 (C-2′) 108.2 (furanose ring), |
| Kaempferol glycoside | (d)/J = 7.7 | 5.47 | 101.05 | H-1″/C-1″ anomeric sugar ring | * |
| Kaempferol dissacharide | * | 3.43 | 78.0 | H-5″/C-5″ sugar ring | 96.4 (H-5′/C-5′ B ring), 101.05 (C-1″) |
| Kaempferol glycoside | (d)/J = 7.9 | 4.56 | 100.3 | H-1‴C-1‴ anomeric | 76.0, 78.0 Glycoside backbone |
| Feruloyl ester | * | 6.41 | 113.5 | H-8/C-8 | 104.5 |
| Feruloyl ester | (d)/J = 7.8 | 4.25 | 105.3 | H-1′/C-1′anomeric Glc | 100.3 (Glc C-1″), 104.5 (Glc C-1‴) |
| Flavonol glycoside B | (d)/J = 2.0 | 6.49 | 95.28 | H-6/C-6 | 96.6 (C-8 A ring), 100.7 (C-6), 106.1 (C-10), 158.5 (C-2), 163.1 (C-4′), 165.6 (C-9), 179.8 (C-4) |
| Flavonol glycoside B | * | 6.80 | 96.6 | H-8/C-8 | 101.2 (glucoside C-1′), 158.2 (C-9/2), 163.6 (C-5) |
| Compound | Multiplicity/J Coupling (Hz) | 1H (ppm) | 13C (ppm) | Proton/Carbon Position | 1H -13C HMBC (ppm) |
|---|---|---|---|---|---|
| Vanillic acid | (s) | 3.94 | 57.22 | -OCH3 | 147.0 (C-1) |
| Citric acid | * | 2.83 | 48.4 | -CH2- | 176.0, 178.8 |
| Quinic acid | * | 2.10 | 42.0 | H2-C2/H6-C6 | 38.9 (C-5), 68.2 (C-2), 77.1 (C-3), 179.8 (CO) |
| Quinic acid | * | 1.88 | 42.0 | H2-C2/H6-C6 | 38.9, 68.2 (C-2), 77.1 (C-3), 179.8 (CO) |
| Malic acid | (m) | 4.43 | 69.0 | –CH-(C-2) | 41.0 (C-3), 171.4 (CO-4), 174.5 (CO-5) |
| Malic acid | (dd)/J = 4.65, 16.35 | 2.84 | 41.0 | -CH2-(C-3) | 69.0 (C-2), 171.4 (CO-4), 174.5 (CO-5) |
| Malic acid | (dd)/J = 7.52, 16.44 | 2.70 | 41.0 | -CH2-(C-3) | 69.0 (C-2), 171.4 (CO-4), 174.5 (CO-5) |
| Gallic acid | * | 6.99 | 112.2 | C-2 | 122.1 (C-3) |
| p-coumaroyl- | (d)/J = 12.8 | 5.92 | 120.0 | cis-olefinic HC=CH- | 130.0 (C-2′) |
| Ferulic acid | * | 7.26 | 113.5 | H-2/C-2 | 150.0 (C-4) |
| Ferulic acid | (d)/J = 8.7 | 7.11 | 124.0 | H-6/C-6 | * |
| Ferulic acid | (d)/J = 8.7 | 6.99 | 116.0 | H-5/C-5 | 122.1 (C-6), 147.3 (C-7), 149.2 (C-3) |
| Ascorbic Acid | * | 4.82 | 77.81 | H-4/C-4 | 72.3 (C-5), 66.2 (C-6), 179.3 (C-1) |
| Olefinic group | (m) | 5.36 | 131.0 | -HC=CH- | 89.9 |
| sn-2 esterified glycerol (1-MAG) | (m) | 4.19 | 69.2 | -CH-O-CO-R | 71.8, 81.0 |
| sn-2 esterified glycerol (1,3-DAG/1,2-Diaclyglycerols) | (m) | 4.12 | 71.98 | -CH-O-CO-R | 62.9 |
| terminal methyl of FAs | (t)/J = 7.24 | 1.23 | 15.78 | -CH3 | 30.7, 53.1, 67.0, 81.0 |
| Compound | Multiplicity/J Coupling (Hz) | 1H (ppm) | 13C (ppm) | Proton/Carbon Position | 1H-13C HMBC (ppm) |
|---|---|---|---|---|---|
| Trigonelline | (m) | 8.89 | 146.3 | H4/C4 | 147.7 (C-2) |
| (m) | 8.86 | 147.3 | H2/C2 | 147.5 (C-1) | |
| (s) | 9.17 | 147.5 | 1H/C1 | 146.3 (C-4) | |
| Phenylalanine | (m) | 7.42 | 127.1 | -CH- | * |
| 7.36 | 132.6 | -CH- | * | ||
| 7.32 | 130.2 | -CH- | * | ||
| Tyrosine | (m) | 7.16 | 132.2 | -CH- | * |
| 6.89 | 116.8 | -CH- | * | ||
| Histidine | (d) | 7.26 | 113.6 | -CH- | * |
| Asparagine | (m) | 2.97, 2.94 | 36.07 | -CH- | * |
| Alanine | (d)/J = 7.27 | 1.49 | 20.32 | -CH3 | 50.6, 174.9 |
| Leucine | (d) | 0.92 | 21.9 | -CH3 | 21.9, 24.6, 38.1 |
| (d) | 0.94 | 24.24 | -CH3 | * | |
| Isoleucine | (d) | 1.01 | 16.24 | -CH3 | * |
| (t) | 0.86 | 11.78 | -CH3 | * | |
| Valine | (d) | 0.92 | 16.15 | -CH3 | * |
| (d) | 0.87 | 14.97 | -CH3 | * |
| Vitamins | Multiplicity/J Coupling (Hz) | 1H (ppm) | 13C (ppm) | Proton/Carbon Position | 1H-13C HMBC (ppm) |
|---|---|---|---|---|---|
| Vitamin A (retinol) | * | 6.11 | 132.2 | 1st isoprenoid unit H-8, H-10/C-8, C-10-HC=CH- | 12.6 (9-CH3), 125.6 (C-1), 131.2 (C-5), 137.9 (C-6) |
| * | 6.35 | 138.0 | 2nd isoprenoid unit C-12-HC=CH- | 12.6, 127.5 (C-3), 131.2 (C-5), 132.2 (C-8/C-10), 145.4 (C-9) | |
| * | 6.61 | 125.2 | 2nd isoprenoid unit C-11-HC=CH- | 132.2 (C-2) | |
| Vitamin E (α & γ- Tocopherol) | * | 2.10 | 11.8 | Ortho methyl C8-CH3 | 117.0 (C-9), 118.4 (C-5), 122.7 (C-8), 144.3 (C-6) |
| * | 2.14 | 12.3 | aryl C7-CH3 | 29.3 (-CH3), 121.0 (C-7), 122.7 (C-8), 144.3 (C-6) | |
| Vitamin E tocopherol | * | 2.58 | 21.0 | -CH2, H4/C4 | 31.5 (C3), 74.7 (C2), 117.0 (C10), 145.7 (C9) |
| Vitamin E tocotrienol | * | 1.65 | * | 8′-CH3 | 125.9 (C7′), 133.3 (C8′) |
| Vitamin D | * | 2.20 | 52.9 | H-14/C-14 | * |
| Compound | Multiplicity /J Coupling (Hz) | 1H (ppm) | 13C (ppm) | Proton/Carbon Position | 1H-13C HMBC (ppm) |
|---|---|---|---|---|---|
| terminal vinyl, conjugated of n acyl chains | * | 5.88 | -CH=CH2 | 127.1 | |
| terminal vinyl, conjugated of n acyl chains | * | 5.81 | 130.8 | -CH=CH2 | * |
| p-coumaroyl-Glc | (d)/J = 12.8 | 5.81 | 117.2 | -CH=CH- | 127.2 |
| p-coumaroyl-Glc | (d)/J = 16 | 7.60 | 144.5 | C-8 -HC=CH- | 127, 132.7, 143.5, 157.0 |
| Olefinic (TAG) | * | 5.50 | * | -HC=CH- | 130.0 |
| Linoleic olefinic (C18:2, n-6) in TAG | * | 5.33 | 130.1 | -HC=CH- | 24.3, 27.2, 30.0, 127.8, 129.7 |
| olefinic | * | 5.34 | * | -HC=CH- | |
| * | 5.66 | * | -HC=CH- | 63.9, 128.4 | |
| * | 5.34 | * | -HC=CH- | 24.3, 27.2, 30.0, 127.8, 129.7 | |
| * | 5.32 | 128.23 | -HC=CH- | ||
| * | 5.32 | 125.0 | -HC=CH- | ||
| * | 5.32 | 122.1 | -HC=CH- | ||
| * | 5.29 | * | -HC=CH- | 27.2, 127.9 | |
| * | 5.25 | 126 | -HC=CH- | 33.8, 41.7, 59.5, 61.7, 63.9, 130.0 | |
| * | 5.10 | 124.4 | -HC=CH- | 12.5, 15.7, 26.3, 28.2, 39.4 | |
| sn-2 esterified glycerol of TAG | (m) | 5.26 | 69.10 | -CHOCO | 130.0 |
| Glycerol in sn-2 esterified glycerol of 1,2/1,3 DAG | (m) | 5.08 | 72.2 | -CHOCO | 61.5, 173.0 |
| 18:2 CLA | * | 6.27 | 125.9 | H11/C11 | 127.1 |
| sn-2 esterified glycerol of 1,3 of DAG | * | 4.82 | 75.0 | -CH-O-CO- | 68.6, 173.3 |
| 1,2 DAG | (dd)/J1′a,1′b overlapped/J1′a, 2′= 4.50 | 4.32 | * | 1′b-CH2-O-CO- | * |
| TAG | (dd)/J3′a,3′b 11.9/ J3′a,2′ 4.4 | 4.30 | 62.19 | 1′a,b-CH2–OCO– | 33.9, 62.0, 68.8, 173.2 |
| 1,3 DAG | (dd)/1′a,1′b 11.95/J1′a,2′ 4.14 | 4.18 | 1′b, 3′b-CH2-O-CO- | ||
| 1,3 DAG | (dd)/J1′a,1′b 11.4/ J1′a,2′ 5.9 | 4.13 | 62.19 | 1′a, 3′a-CH2–OCO– | 33,9, 62.0, 68.8, 173.2 |
| 1,2 DAG | (dd)/J1′a,1′b 11.9/ J1′a,2′ = 5.9 | 4.23 | 62.31 | 1′a-CH2–O-CO– | 33.9, 62.0, 68.8, 173.2 |
| sn-1 or sn-3 in glycerol of 1,2/2,3 DAG | (m) | 3.71 | 61.75 | -CH2-OH | 62.3, 65.8, 72.1 |
| All FAs | (m) | 2.30 | 34.3 | -OOC-CH2-CH2- | 62.1, 69.0, (24.4, 29.2) |
| Bisallylic Hs in acyl chains | 2.78 | 25.8 | -CH=CHCH2CH=CH- | 25.6, 127.0, 130.0, 132.2 | |
| n-9, n-6 Acyl chains | (m) | 2.00–2.03 | 27.5 | -CH2CH=CH | 29.4, 128.1, 130.0, 19.6, 21.8, 24.3, 39.5 |
| n-6, n-9, SFA, SDA acyl chains | (m) | 1.60 | 25.2 | -OCOCH2CH3 | 16.8, 28.8, 33.8, 41.1, 173.0 |
| Fatty acid (n-2) | * | 1.28 | 22.8 | -CH2CH3 | 13.9, 16.0, 24.7, 27.5, 29.1, 31.9, 33.8 |
| C4/H4 FAs | * | 1.27 | 29.5 | -CH2-(CH2)n-COOH | * |
| 18:2 CLA | C4 | 1.31 | 29.8 | -CH2- | 27.1, 29.1, 42.1, 130.0 |
| 18:2 CLA | C16 | 1.25 | 32.0 | -CH2- | 29.1, 31.9 |
| Conjugated linoleic acid | C:18 | 0.87 | 14.2 | -CH3 | * |
| SFA/Conjugated linoleic acid | * | 0.96 | 14.5 | -CH3 | 15.5, 38.6, 55.3, 79.4, 145.0 |
| SFA C:18 /Conjugated linoleic acid | * | 0.87 | 14.2 | -CH3 | 16.8, 22.8, 31.9 |
| SFA C:18 | * | 0.67 | 12.0 | -CH3 | * |
| n-9 FA | (t) | 0.89 | 33.0 | -CH3 | 22.8, 33.8, 36.4, 38.3, 47.1, 55.3 |
| terminal methylin n-3 FA | * | 0.99 | 19.5 | -CH3 | 50.3, 36.7 |
| terminal methylin n-3 FA | * | 0.91 | 19.0 | -CH3 | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zompra, A.A.; Chasapi, S.A.; Karagkouni, E.C.; Karamouzi, E.; Panopoulos, P.; Spyroulias, G.A. Metabolite and Bioactive Compounds Profiling of Meteora Sea Buckthorn Berries through High-Resolution NMR Analysis. Metabolites 2021, 11, 822. https://doi.org/10.3390/metabo11120822
Zompra AA, Chasapi SA, Karagkouni EC, Karamouzi E, Panopoulos P, Spyroulias GA. Metabolite and Bioactive Compounds Profiling of Meteora Sea Buckthorn Berries through High-Resolution NMR Analysis. Metabolites. 2021; 11(12):822. https://doi.org/10.3390/metabo11120822
Chicago/Turabian StyleZompra, Aikaterini A., Styliani A. Chasapi, Evdokia C. Karagkouni, Eugenia Karamouzi, Panagiotis Panopoulos, and Georgios A. Spyroulias. 2021. "Metabolite and Bioactive Compounds Profiling of Meteora Sea Buckthorn Berries through High-Resolution NMR Analysis" Metabolites 11, no. 12: 822. https://doi.org/10.3390/metabo11120822






