Profile of Bile Acid Metabolomics in the Follicular Fluid of PCOS Patients
Abstract
:1. Introduction
2. Results
2.1. Characteristics of the Participants
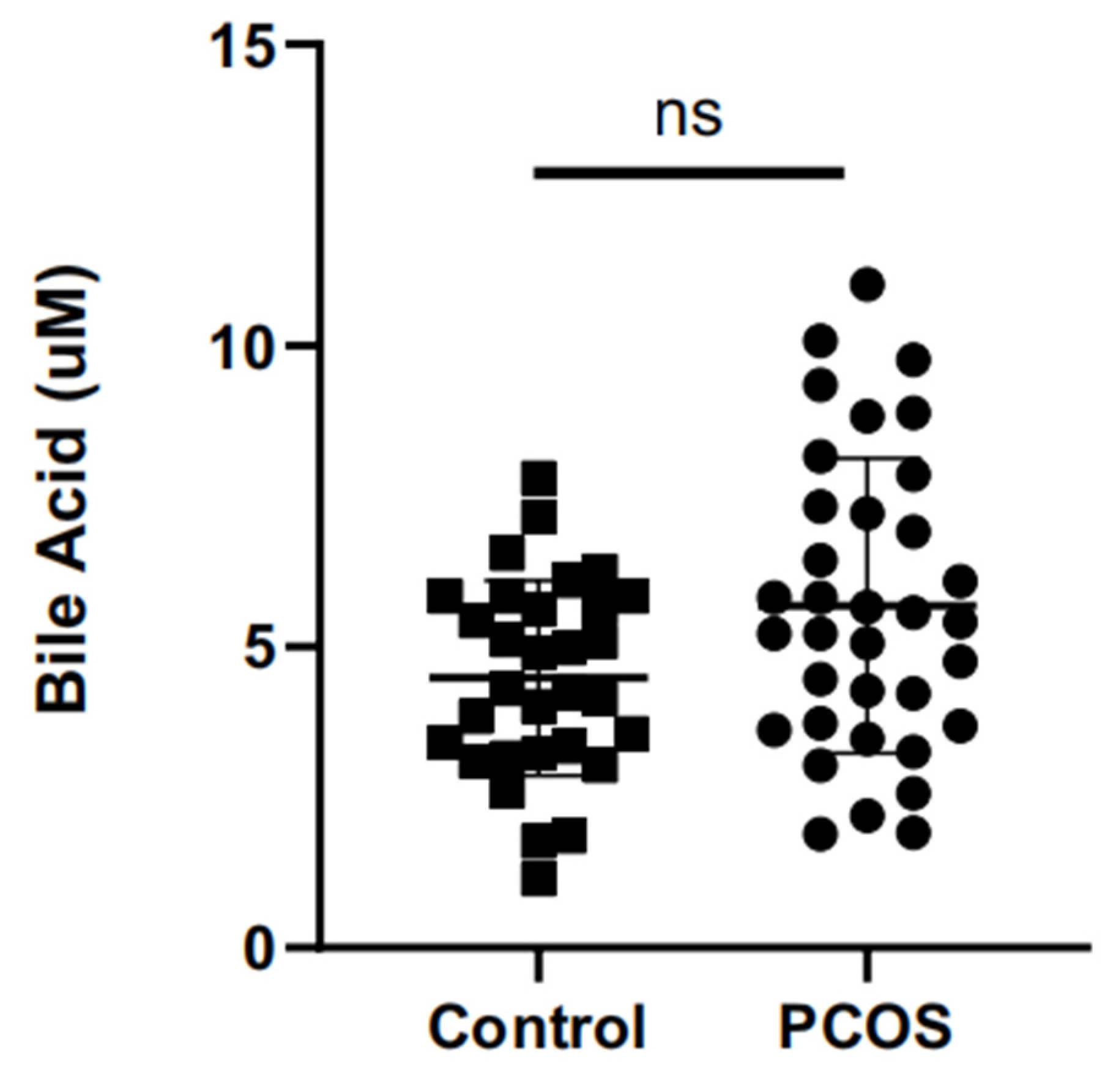
2.2. Differences of Total Bile Acids in Follicular Fluid
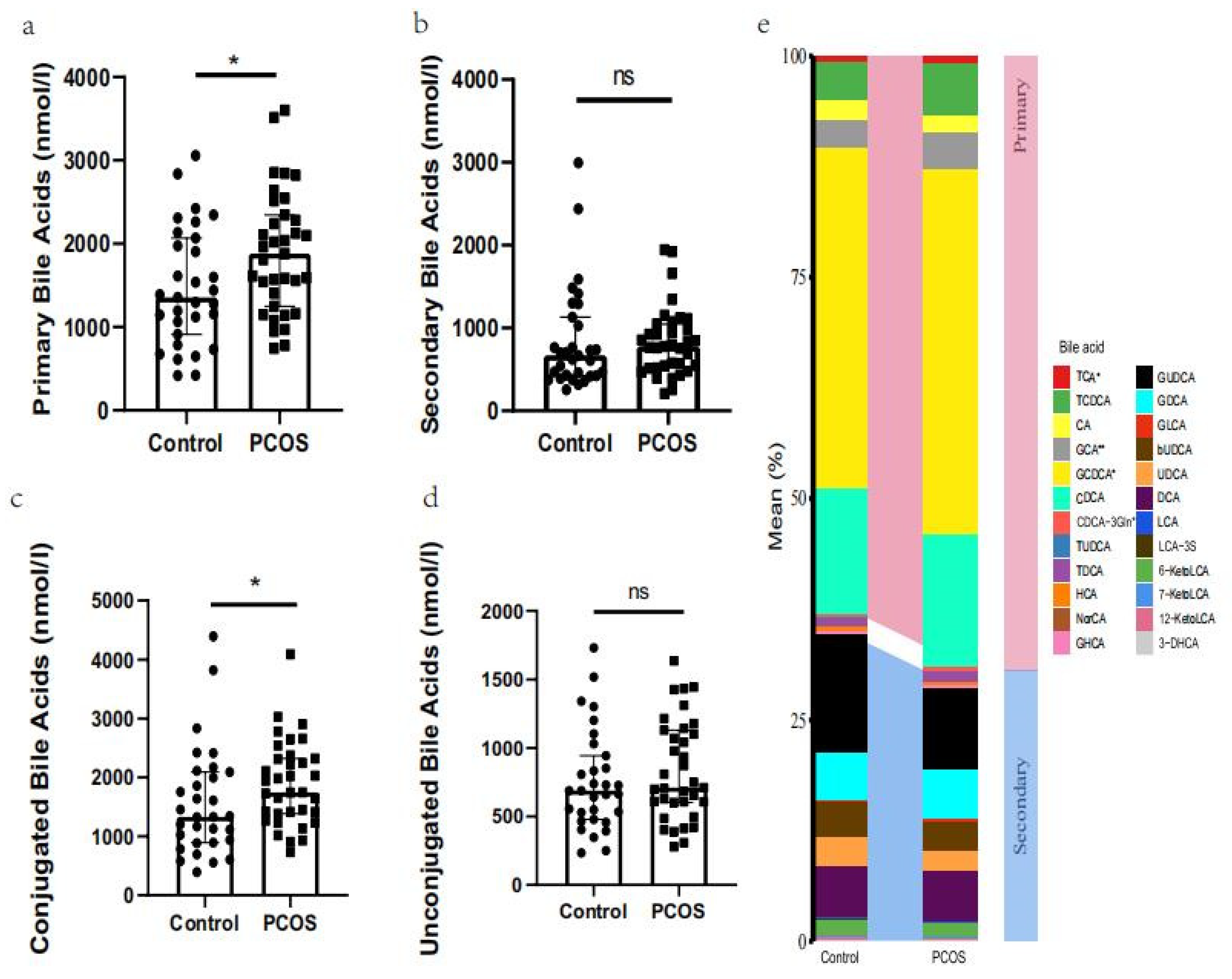
2.3. Expression Profile of Bile Acid Metabolites
2.4. Correlation between Bile Acid Metabolites and Clinical Characteristics
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. The Collection of FF
4.3. The Detection of Total Bile Acid
4.4. FF Bile Acid Profile Assessment
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Escobar-Morreale, H.F. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat. Rev. Endocrinol. 2018, 14, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Christian, R.C.; Dumesic, D.A.; Behrenbeck, T.; Oberg, A.L.; Sheedy, P.F., 2nd; Fitzpatrick, L.A. Prevalence and predictors of coronary artery calcification in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2003, 88, 2562–2568. [Google Scholar] [CrossRef]
- Persson, S.; Elenis, E.; Turkmen, S.; Kramer, M.S.; Yong, E.L.; Poromaa, I.S. Higher risk of type 2 diabetes in women with hyperandrogenic polycystic ovary syndrome. Fertil. Steril. 2021, 116, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Cui, J.; Goodarzi, M.O. Polycystic Ovary Syndrome and Risk of Type 2 Diabetes, Coronary Heart Disease, and Stroke. Diabetes 2021, 70, 627–637. [Google Scholar] [CrossRef]
- Paul, C.; Lagana, A.S.; Maniglio, P.; Triolo, O.; Brady, D.M. Inositol’s and other nutraceuticals’ synergistic actions counteract insulin resistance in polycystic ovarian syndrome and metabolic syndrome: State-of-the-art and future perspectives. Gynecol. Endocrinol. 2016, 32, 431–438. [Google Scholar] [CrossRef]
- Tu, J.; Chen, Y.; Li, Z.; Yang, H.; Chen, H.; Yu, Z. Long non-coding RNAs in ovarian granulosa cells. J. Ovarian Res. 2020, 13, 63. [Google Scholar] [CrossRef]
- Zhou, L.; Han, X.; Li, W.; Wang, N.; Yao, L.; Zhao, Y.; Zhang, L. N6-methyladenosine Demethylase FTO Induces the Dysfunctions of Ovarian Granulosa Cells by Upregulating Flotillin 2. Reprod. Sci. 2021. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Naessen, T.; Kushnir, M.M.; Chaika, A.; Nosenko, J.; Mogilevkina, I.; Rockwood, A.L.; Carlstrom, K.; Bergquist, J.; Kirilovas, D. Steroid profiles in ovarian follicular fluid in women with and without polycystic ovary syndrome, analyzed by liquid chromatography-tandem mass spectrometry. Fertil. Steril. 2010, 94, 2228–2233. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, H.; Li, Z.; Fan, H.; Yan, X.; Liu, X.; Xuan, J.; Feng, D.; Wei, X. The Release of Peripheral Immune Inflammatory Cytokines Promote an Inflammatory Cascade in PCOS Patients via Altering the Follicular Microenvironment. Front. Immunol. 2021, 12, 685724. [Google Scholar] [CrossRef]
- Houten, S.M.; Watanabe, M.; Auwerx, J. Endocrine functions of bile acids. EMBO J. 2006, 25, 1419–1425. [Google Scholar] [CrossRef]
- Qi, X.; Yun, C.; Sun, L.; Xia, J.; Wu, Q.; Wang, Y.; Wang, L.; Zhang, Y.; Liang, X.; Wang, L.; et al. Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat. Med. 2019, 25, 1225–1233. [Google Scholar] [CrossRef]
- Zhang, B.; Shen, S.; Gu, T.; Hong, T.; Liu, J.; Sun, J.; Wang, H.; Bi, Y.; Zhu, D. Increased circulating conjugated primary bile acids are associated with hyperandrogenism in women with polycystic ovary syndrome. J. Steroid. Biochem. Mol. Biol. 2019, 189, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Bianco, S.; Bellefleur, A.M.; Beaulieu, E.; Beauparlant, C.J.; Bertolin, K.; Droit, A.; Schoonjans, K.; Murphy, B.D.; Gevry, N. The Ovulatory Signal Precipitates LRH-1 Transcriptional Switching Mediated by Differential Chromatin Accessibility. Cell Rep. 2019, 28, 2443–2454.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Wang, S.; Li, M.; Gao, Z.; Xu, Y.; Zhao, X.; Hu, C.; Zhang, Y.; Liu, R.; Hu, R.; et al. Association of Serum Bile Acids Profile and Pathway Dysregulation With the Risk of Developing Diabetes Among Normoglycemic Chinese Adults: Findings from the 4C Study. Am. Diabetes Care 2021, 44, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xie, G.; Wang, X.; Fan, J.; Qiu, Y.; Zheng, X.; Qi, X.; Cao, Y.; Su, M.; Wang, X.; et al. Serum and urine metabolite profiling reveals potential biomarkers of human hepatocellular carcinoma. Mol. Cell Proteom. 2011, 10, M110.004945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, A.; Sun, H.; Yan, G.; Han, Y.; Ye, Y.; Wang, X. Urinary metabolic profiling identifies a key role for glycocholic acid in human liver cancer by ultra-performance liquid-chromatography coupled with high-definition mass spectrometry. Clin. Chim. Acta 2013, 418, 86–90. [Google Scholar] [CrossRef]
- Shi, C.; Yang, J.; Hu, L.; Liao, B.; Qiao, L.; Shen, W.; Xie, F.; Zhu, G. Glycochenodeoxycholic acid induces stemness and chemoresistance via the STAT3 signaling pathway in hepatocellular carcinoma cells. Aging 2020, 12, 15546–15555. [Google Scholar] [CrossRef]
- Warden, C.; Barnett, J.M.; Brantley, M.A., Jr. Taurocholic acid inhibits features of age-related macular degeneration in vitro. Exp. Eye Res. 2020, 193, 107974. [Google Scholar] [CrossRef]
- Gaikwad, N.W. Bileome: The bile acid metabolome of rat. Biochem. Biophys. Res. Commun. 2020, 533, 458–466. [Google Scholar] [CrossRef]
- Smith, L.P.; Nierstenhoefer, M.; Yoo, S.W.; Penzias, A.S.; Tobiasch, E.; Usheva, A. The bile acid synthesis pathway is present and functional in the human ovary. PLoS ONE 2009, 4, e7333. [Google Scholar] [CrossRef] [Green Version]
- Nagy, R.A.; van Montfoort, A.P.; Dikkers, A.; van Echten-Arends, J.; Homminga, I.; Land, J.A.; Hoek, A.; Tietge, U.J. Presence of bile acids in human follicular fluid and their relation with embryo development in modified natural cycle IVF. Hum. Reprod. 2015, 30, 1102–1109. [Google Scholar] [CrossRef] [Green Version]
- Nagy, R.A.; Hollema, H.; Andrei, D.; Jurdzinski, A.; Kuipers, F.; Hoek, A.; Tietge, U.J.F. The Origin of Follicular Bile Acids in the Human Ovary. Am. J. Pathol. 2019, 189, 2036–2045. [Google Scholar] [CrossRef]
- Ommati, M.M.; Farshad, O.; Niknahad, H.; Arabnezhad, M.R.; Azarpira, N.; Mohammadi, H.R.; Haghnegahdar, M.; Mousavi, K.; Akrami, S.; Jamshidzadeh, A.; et al. Cholestasis-associated reproductive toxicity in male and female rats: The fundamental role of mitochondrial impairment and oxidative stress. Toxicol. Lett. 2019, 316, 60–72. [Google Scholar] [CrossRef]
- Mo, J.; Sun, L.; Cheng, J.; Lu, Y.; Wei, Y.; Qin, G.; Liang, J.; Lan, G. Non-targeted Metabolomics Reveals Metabolic Characteristics of Porcine Atretic Follicles. Front. Vet. Sci. 2021, 8, 679947. [Google Scholar] [CrossRef]
- Takae, K.; Nakata, M.; Watanabe, T.; Sasada, H.; Fujii, H.; Tomioka, I. Evidence for the involvement of FXR signaling in ovarian granulosa cell function. J. Reprod. Dev. 2019, 65, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Duggavathi, R.; Volle, D.H.; Mataki, C.; Antal, M.C.; Messaddeq, N.; Auwerx, J.; Murphy, B.D.; Schoonjans, K. Liver receptor homolog 1 is essential for ovulation. Genes Dev. 2008, 22, 1871–1876. [Google Scholar] [CrossRef] [Green Version]
- Hinshelwood, M.M.; Shelton, J.M.; Richardson, J.A.; Mendelson, C.R. Temporal and spatial expression of liver receptor homologue-1 (LRH-1) during embryogenesis suggests a potential role in gonadal development. Dev. Dyn. 2005, 234, 159–168. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Q.; Wang, Y.; Song, T.; Zheng, Y.; Wang, W.; Shi, Y. LRH-1 high expression in the ovarian granulosa cells of PCOS patients. Endocrine 2021, 74, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Xu, H.; Xu, Y.; Xu, Y.; Shi, Q. Serum metabolomics analysis of patients with polycystic ovary syndrome by mass spectrometry. Mol. Reprod. Dev. 2019, 86, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative Insulin Sensitivity Check Index: A Simple, Accurate Method for Assessing Insulin Sensitivity In Humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xie, G.; Zhao, A.; Zheng, X.; Huang, F.; Wang, Y.; Yao, C.; Jia, W.; Liu, P. Serum Bile Acids Are Associated with Pathological Progression of Hepatitis B-Induced Cirrhosis. J. Proteome Res. 2016, 15, 1126–1134. [Google Scholar] [CrossRef] [Green Version]
- Xie, G.; Wang, Y.; Wang, X.; Zhao, A.; Chen, T.; Ni, Y.; Wong, L.; Zhang, H.; Zhang, J.; Liu, C.; et al. Profiling of serum bile acids in a healthy Chinese population using UPLC-MS/MS. J. Proteome Res. 2015, 14, 850–859. [Google Scholar] [CrossRef] [PubMed]
| Control (n = 31) | PCOS (n = 35) | p Value | |
|---|---|---|---|
| Age (years) | 31.03 ± 3.95 | 29.23 ± 3.84 | 0.064 |
| AFC (counts) | 14.77 ± 5.61 | 27.97 ± 11.02 | p < 0.001 |
| AMH (ng/mL) | 3.50 ± 1.60 | 8.40 ± 4.48 | p < 0.001 |
| LH (IU/L) | 5.98 ± 1.95 | 11.73 ± 6.89 | p < 0.001 |
| FSH (IU/L) | 6.67 ± 1.38 | 6.25 ± 1.63 | 0.267 |
| T (ng/dL) | 20.76 ± 9.99 | 38.13 ± 22.12 | p < 0.001 |
| BMI (kg/m2) | 23.14 ± 2.78 | 24.29 ± 3.00 | 0.113 |
| Glucose (mmol/L) | 5.26 ± 0.43 | 5.17 ± 0.34 | 0.345 |
| AST (U/L) | 16.96 ± 3.75 | 17.58 ± 3.20 | 0.470 |
| ALT (U/L) | 13.64 ± 5.29 | 16.45 ± 7.85 | 0.096 |
| GGT (U/L) | 13.20 ± 4.36 | 16.06 ± 8.05 | 0.083 |
| TG (mmol/L) | 0.92 ± 0.39 | 0.98 ± 0.43 | 0.497 |
| QUICKI | 0.56 ± 0.09 | 0.51 ± 0.11 | 0.112 |
| Bile Acid Metabolites (nmol/L) | Control (n = 31) | PCOS (n = 35) | p Value |
|---|---|---|---|
| GCA # | 69.3455 (35.0402–108.194) | 93.0293 (60.9071–139.1637) | 0.0088 |
| TCA # | 8.9051 (6.1289–18.8495) | 20.0173 (10.2183–32.6538) | 0.0302 |
| GCDCA # | 813.247 (567.252–1181.14) | 1045.532 (813.6052–1415.405) | 0.0380 |
| CDCA-3Gln # | 2.0029 (1.4314–4.7204) | 4.5151 (1.8605–16.4768) | 0.0489 |
| TUDCA | 1.1045 (0.4843–3.7344) | 1.7891 (0.4667–5.5518) | 0.7018 |
| TCDCA | 101.073 (58.0077–132.758) | 131.5096 (87.4559–197.0543) | 0.0586 |
| TDCA | 17.21 (6.3327–36.5325) | 19.6411 (4.5489–42.3435) | 0.6570 |
| HCA | 6.5258 (4.3054–11.0712) | 7.5380 (4.2251–12.5456) | 0.8183 |
| CA | 39.3982 (27.9622–56.5257) | 41.8243 (29.0361–67.7721) | 0.5742 |
| NorCA | 1.3382 (0.8645–2.2769) | 1.5677 (1.0934–2.4134) | 0.3376 |
| GHCA | 6.3761 (3.6203–11.9017) | 7.7032 (5.1268–12.2494) | 0.4282 |
| GUDCA | 137.575 (76.6138–343.196) | 181.9499 (77.9537–314.5836) | 0.9086 |
| GDCA | 88.5226 (54.237–145.261) | 92.7559 (36.1559–218.6125) | 0.5484 |
| GLCA | 3.9102 (0.8078–9.2406) | 3.3065 (0.6672–13.6760) | 0.7278 |
| bUDCA | 50.0141 (30.1774–96.4139) | 48.4582 (24.3200–105.7436) | 0.8985 |
| UDCA | 40.5876 (24.5589–65.4812) | 40.5971 (19.3277–75.6631) | 0.8382 |
| CDCA | 352.781 (177.59–457.672) | 375.8702 (218.3674–594.6697) | 0.3123 |
| DCA | 115.298 (55.4131–182.886) | 112.2262 (34.3780–209.7214) | 0.7497 |
| LCA | 2.7428 (1.2261–3.8918) | 1.9536 (0.8700–4.8796) | 0.5399 |
| LCA-3S | 1.5409 (0.3816–2.4319) | 0.9994 (0.1386–3.2181) | 0.6923 |
| 6-KetoLCA | 39.4591 (30.957–53.853) | 35.8543 (27.1566–47.3019) | 0.3311 |
| 7-KetoLCA | 4.1440 (2.8514–7.435) | 3.8017 (2.1842–6.8645) | 0.3376 |
| 12-KetoLCA | 3.1705 (1.1653–6.6363) | 1.9183 (0.5242–6.5477) | 0.7208 |
| 3-DHCA | 1.8208 (1.3506–3.4268) | 2.1905 (1.4482–3.4760) | 0.7400 |
| Bile Acid | Clinical Characteristic | Pearson’s r | p Value |
|---|---|---|---|
| TCA | AMH | 0.0922 | 0.4617 |
| FSH | 0.0400 | 0.7499 | |
| LH | 0.0898 | 0.4735 | |
| E2 | 0.0286 | 0.8197 | |
| T | 0.2348 | 0.0577 | |
| AFC | 0.1158 | 0.3546 | |
| QUICKI | 0.0050 | 0.6166 | |
| GCA | AMH | 0.1590 | 0.2023 |
| FSH | 0.2027 | 0.1026 | |
| LH | 0.1123 | 0.3695 | |
| E2 | 0.0475 | 0.7051 | |
| T | 0.2000 | 0.1074 | |
| AFC | 0.1408 | 0.2594 | |
| QUICKI | 0.0105 | 0.4649 | |
| GCDCA | AMH | 0.1424 | 0.2514 |
| FSH # | 0.3787 | 0.0017 | |
| LH # | 0.2670 | 0.0302 | |
| E2 | 0.1129 | 0.3669 | |
| T | −0.0001 | 0.9991 | |
| AFC | 0.1481 | 0.2353 | |
| QUICKI | 0.0132 | 0.4125 | |
| CDCA-3Gln | AMH | 0.2078 | 0.0940 |
| FSH | −0.1059 | 0.3976 | |
| LH | 0.0869 | 0.4878 | |
| E2 | 0.0170 | 0.8921 | |
| T | 0.1655 | 0.1841 | |
| AFC # | 0.3247 | 0.0078 | |
| QUICKI | 0.0021 | 0.7466 |
| Abbreviation | Full Name | Classification (Primary/Secondary) | Classification (Conjugated/Unconjugated) |
|---|---|---|---|
| TCA | Taurocholic acid | Primary | Conjugated |
| TUDCA | Tauroursodeoxycholic acid | Secondary | Conjugated |
| TCDCA | Taurochenodeoxycholic acid | Primary | Conjugated |
| TDCA | Taurodeoxycholic acid | Secondary | Conjugated |
| HCA | Hyocholic acid | Secondary | Unconjugated |
| CA | Cholic acid | Primary | Unconjugated |
| NorCA | Norcholic acid | Secondary | Unconjugated |
| GHCA | Glycohyocholate | Secondary | Conjugated |
| GCA | Glycocholic acid | Primary | Conjugated |
| GUDCA | Glycoursodeoxycholic acid | Secondary | Conjugated |
| GCDCA | Glycochenodeoxycholic acid | Primary | Conjugated |
| GDCA | Glycodeoxycholic acid | Secondary | Conjugated |
| GLCA | Glycolithocholate | Secondary | Conjugated |
| bUDCA | Isoursodeoxycholic acid | Secondary | Unconjugated |
| UDCA | Ursodeoxycholic acid | Secondary | Conjugated |
| CDCA | Chenodeoxycholic acid | Primary | Unconjugated |
| DCA | Deoxycholic acid | Secondary | Unconjugated |
| LCA | Lithocholic acid | Secondary | Unconjugated |
| LCA_3S | Lithocholic acid 3 sulfate | Secondary | Conjugated |
| 6_ketoLCA | 6-ketolithocholic acid | Secondary | Unconjugated |
| 7_ketoLCA | 7-ketolithocholic acid | Secondary | Unconjugated |
| 12_ketoLCA | 12-ketolithocholic acid | Secondary | Unconjugated |
| 3_DHCA | 3-oxocholic acid | Secondary | Unconjugated |
| CDCA_3Gln | Chenodeoxycholic acid-3-β-d-glucuronide | Primary | Conjugated |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Wu, R.; Qi, D.; Fu, L.; Song, T.; Wang, Y.; Bian, Y.; Shi, Y. Profile of Bile Acid Metabolomics in the Follicular Fluid of PCOS Patients. Metabolites 2021, 11, 845. https://doi.org/10.3390/metabo11120845
Yang X, Wu R, Qi D, Fu L, Song T, Wang Y, Bian Y, Shi Y. Profile of Bile Acid Metabolomics in the Follicular Fluid of PCOS Patients. Metabolites. 2021; 11(12):845. https://doi.org/10.3390/metabo11120845
Chicago/Turabian StyleYang, Xiao, Richao Wu, Dan Qi, Linlin Fu, Tian Song, Ying Wang, Yuehong Bian, and Yuhua Shi. 2021. "Profile of Bile Acid Metabolomics in the Follicular Fluid of PCOS Patients" Metabolites 11, no. 12: 845. https://doi.org/10.3390/metabo11120845
APA StyleYang, X., Wu, R., Qi, D., Fu, L., Song, T., Wang, Y., Bian, Y., & Shi, Y. (2021). Profile of Bile Acid Metabolomics in the Follicular Fluid of PCOS Patients. Metabolites, 11(12), 845. https://doi.org/10.3390/metabo11120845






