Targeted Metabolomics to Assess Exposure to Environmental Chemicals of Concern in Japanese Quail at Two Life Stages
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and Working Solutions
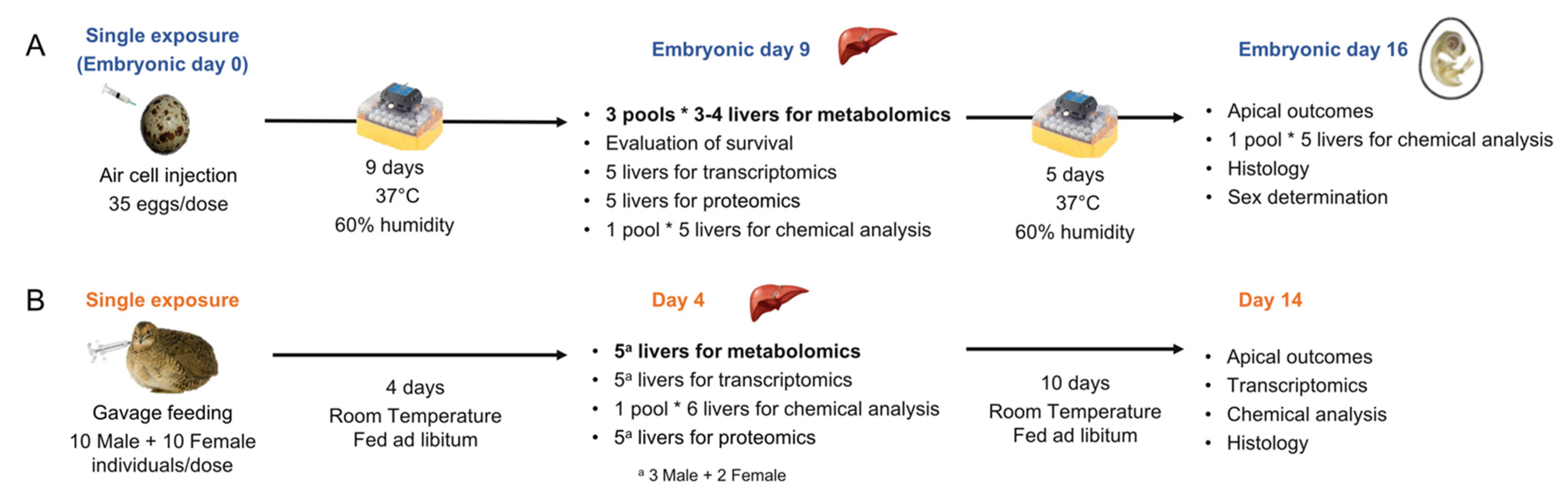
3.2. Egg Injection and Tissue Collection
3.3. Adult Exposure and Tissue Collection
3.4. Targeted Metabolomics
3.4.1. Sample Processing
3.4.2. Mass Spectrometry
3.4.3. Quantification
3.4.4. Quality Control
3.5. Data Visualization and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.-J.; Qian, L.; Ding, L.-Y.; Wang, L.; Wong, M.H.; Tao, H.-C. Ecological and toxicological assessments of anthropogenic contaminants based on environmental metabolomics. Environ. Sci. Ecotechnol. 2021, 5, 100081. [Google Scholar] [CrossRef]
- Viant, M.R.; Sommer, U. Mass spectrometry based environmental metabolomics: A primer and review. Metabolomics 2013, 9, S144–S158. [Google Scholar] [CrossRef]
- Chai, T.; Cui, F.; Yin, Z.; Yang, Y.; Qiu, J.; Wang, C. Chiral PCB 91 and 149 toxicity testing in embryo and larvae (Danio rerio): Application of targeted metabolomics via UPLC-MS/MS. Sci. Rep. 2016, 6, 33481. [Google Scholar] [CrossRef] [PubMed]
- Ziarrusta, H.; Mijangos, L.; Picart-Armada, S.; Irazola, M.; Perera-Lluna, A.; Usobiaga, A.; Prieto, A.; Etxebarria, N.; Olivares, M.; Zuloaga, O. Non-targeted metabolomics reveals alterations in liver and plasma of gilt-head bream exposed to oxybenzone. Chemosphere 2018, 211, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, A.; Kärrman, A.; Pinto, R.; Brunström, B. Metabolic profiling of chicken embryos exposed to perfluorooctanoic acid (PFOA) and agonists to peroxisome proliferator-activated receptors. PLoS ONE 2015, 10, e0143780. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, C.; Wang, P.; Liang, Y.; Zhan, J.; Zhou, Z.; Liu, D. Distribution, metabolism and metabolic disturbances of α-cypermethrin in embryo development, chick growth and adult hens. Environ. Pollut. 2019, 249, 390–397. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, H.; Wu, X.; Zhang, T.; Shen, K.; Li, L.; Peng, Y.; Mehmood, K.; Zhou, D. Metabonomic analysis of the hepatic injury suffer from hexavalent chromium poisoning in broilers. Environ. Sci. Pollut. Res. 2019, 26, 18181–18190. [Google Scholar] [CrossRef]
- Wan, Q.; He, Q.; Deng, X.; Hao, F.; Tang, H.; Wang, Y. Systemic metabolic sesponses of broiler chickens and piglets to acute T-2 toxin intravenous exposure. J. Agric. Food Chem. 2016, 64, 714–723. [Google Scholar] [CrossRef]
- Dorr, B.S.; Hanson-Dorr, K.C.; Assadi-Porter, F.M.; Selen, E.S.; Healy, K.A.; Horak, K.E. Effects of repeated sublethal external exposure to Deep Water Horizon oil on the avian metabolome. Sci. Rep. 2019, 9, 371. [Google Scholar] [CrossRef]
- Geng, D.; Musse, A.A.; Wigh, V.; Carlsson, C.; Engwall, M.; Orešič, M.; Scherbak, N.; Hyötyläinen, T. Effect of perfluorooctanesulfonic acid (PFOS) on the liver lipid metabolism of the developing chicken embryo. Ecotoxicol. Environ. Saf. 2019, 170, 691–698. [Google Scholar] [CrossRef]
- Desforges, J.-P.; Legrand, E.; Boulager, E.; Liu, P.; Xia, J.; Butler, H.; Chandramouli, B.; Ewald, J.; Basu, N.; Hecker, M.; et al. Using transcriptomics and metabolomics to understand species differences in sensitivity to chlorpyrifos in Japanese quail and double-crested cormorant embryos. Environ. Toxicol. Chem. 2021, 40, 3019–3033. [Google Scholar] [CrossRef] [PubMed]
- Basu, N.; Crump, D.; Head, J.; Hickey, G.; Hogan, N.; Maguire, S.; Xia, J.; Hecker, M. EcoToxChip: A next-generation toxicogenomics tool for chemical prioritization and environmental management. Environ. Toxicol. Chem. 2019, 38, 279–288. [Google Scholar] [CrossRef]
- Farhat, A.; Crump, D.; Bidinosti, L.; Boulanger, E.; Basu, N.; Hecker, M.; Head, J.A. An early-life stage alternative testing strategy for assessing the impacts of environmental chemicals in birds. Environ. Toxicol. Chem. 2020, 39, 141–154. [Google Scholar] [CrossRef]
- Boulanger, E.; Farhat, A.; Jeon, Y.S.; Basu, N.; Hecker, M.; Crump, D.; Head, J.A. Evaluation of the Toxic Effects of Eight Environmental Chemicals in Adult Japanese Quail (Coturnix japonica); figshare. Poster 2020. [Google Scholar] [CrossRef]
- Zhao, L.; Xiong, Z.; Lu, X.; Zheng, S.; Wang, F.; Ge, L.; Su, G.; Yang, J.; Wu, C. Metabonomic evaluation of chronic unpredictable mild stress-induced changes in rats by intervention of fluoxetine by HILIC-UHPLC/MS. PLoS ONE 2015, 10, e0129146. [Google Scholar] [CrossRef] [PubMed]
- Ekman, D.R.; Teng, Q.; Villeneuve, D.L.; Kahl, M.D.; Jensen, K.M.; Durhan, E.J.; Ankley, G.T.; Collette, T.W. Profiling lipid metabolites yields unique information on sex- and time-dependent responses of fathead minnows (Pimephales promelas) exposed to 17α-ethynylestradiol. Metabolomics 2009, 5, 22–32. [Google Scholar] [CrossRef]
- McCullough, D.; Webb, R.; Enright, K.J.; Lane, K.E.; McVeigh, J.; Stewart, C.E.; Davies, I.G. How the love of muscle can break a heart: Impact of anabolic androgenic steroids on skeletal muscle hypertrophy, metabolic and cardiovascular health. Rev. Endocr. Metab. Disord. 2021, 22, 389–405. [Google Scholar] [CrossRef]
- Böger, R.H.; Sydow, K.; Borlak, J.; Thum, T.; Lenzen, H.; Schubert, B.; Tsikas, D.; Bode-Böger, S.M. LDL cholesterol upregulates synthesis of asymmetrical dimethylarginine in human endothelial cells: Involvement of S-adenosylmethionine-dependent methyltransferases. Circ. Res. 2000, 87, 99–105. [Google Scholar] [CrossRef]
- Welder, A.A.; Robertson, J.W.; Melchert, R.B. Toxic effects of anabolic-androgenic steroids in primary rat hepatic cell cultures. J. Pharmacol. Toxicol. Methods 1995, 33, 187–195. [Google Scholar] [CrossRef]
- Evrard, P.; Maghuin-Rogister, G. In vitro metabolism of trenbolone: Study of the formation of covalently bound residues. Food Addit. Contam. 1988, 5, 59–65. [Google Scholar] [CrossRef]
- Surugihalli, C.; Porter, T.E.; Chan, A.; Farley, L.S.; Maguire, M.; Zhang, C.; Kattapuram, N.; Muyyarikkandy, M.S.; Liu, H.C.; Sunny, N.E. Hepatic mitochondrial oxidative metabolism and lipogenesis synergistically adapt to mediate healthy embryonic-to-neonatal transition in chicken. Sci. Rep. 2019, 9, 20167. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, J.; Musa, B.B.; Khawar, H.; Yang, X.; Cao, Y.; Yang, X. Developmental changes in hepatic lipid metabolism of chicks during the embryonic periods and the first week of posthatch. Poult. Sci. 2020, 99, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, A.J.; Else, P.L. Membranes as possible pacemakers of metabolism. J. Theor. Biol. 1999, 199, 257–274. [Google Scholar] [CrossRef] [PubMed]
- Zaefarian, F.; Abdollahi, M.R.; Cowieson, A.; Ravindran, V. Avian liver: The forgotten organ. Animals 2019, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ito, S.; Nguyen, H.T.; Yamamoto, K.; Iwata, H. Effects on the hepatic transcriptome of chicken embryos in ovo exposed to phenobarbital. Ecotoxicol. Environ. Saf. 2018, 160, 94–103. [Google Scholar] [CrossRef]
- Jacobsen, A.V.; Nordén, M.; Engwall, M.; Scherbak, N. Effects of perfluorooctane sulfonate on genes controlling hepatic fatty acid metabolism in livers of chicken embryos. Environ. Sci. Pollut. Res. 2018, 25, 23074–23081. [Google Scholar] [CrossRef]
- Farhat, A.; Buick, J.K.; Williams, A.; Yauk, C.L.; O’Brien, J.M.; Crump, D.; Williams, K.L.; Chiu, S.; Kennedy, S.W. Tris(1,3-dichloro-2-propyl) phosphate perturbs the expression of genes involved in immune response and lipid and steroid metabolism in chicken embryos. Toxicol. Appl. Pharmacol. 2014, 275, 104–112. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Ribeiro-Barros, A.I.; Antónion, C. Experimental design and sample preparation in forest tree metabolomics. Metabolites 2019, 9, 285. [Google Scholar] [CrossRef]
- Saccenti, E.; Timmerman, M.E. Approaches to sample size determination for multivariate data: Applications to PCA and PLS-DA of omics data. J. Proteome Res. 2016, 15, 2379–2393. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; De Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Liebisch, G.; Lieser, B.; Rathenberg, J.; Drobnik, W.; Schmitz, G. High-throughput quantification of phosphatidylcholine and sphingomyelin by electrospray ionization tandem mass spectrometry coupled with isotope correction algorithm. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2004, 1686, 108–117. [Google Scholar] [CrossRef] [PubMed]

| Life Stage | Chemical | Doses | Metabolite Class | Metabolite | p-Value | FDR | Log2(FC) | ||
|---|---|---|---|---|---|---|---|---|---|
| HD | MD | LD | |||||||
| ELS | CPF | HD | ABA | Ornithine | 1.00 × 10−2 | 2.87 × 10−2 | −1.62 | ||
| HD | FHB | Arachidonic acid | 7.54 × 10−3 | 2.22 × 10−2 | −1.82 | ||||
| HD | Docosahexaenoic acid | 2.07 × 10−2 | 4.65 × 10−2 | −3.45 | |||||
| HD | FA C22:5n6c | 1.11 × 10−2 | 2.96 × 10−2 | −4.64 | |||||
| MD | LIP | AC C14 | 1.04 × 10−2 | 2.87 × 10−2 | 3.25 | ||||
| MD | AC C16 | 4.99 × 10−3 | 1.70 × 10−2 | 1.90 | |||||
| MD | AC C18:1 | 3.97 × 10−4 | 4.32 × 10−3 | 2.01 | |||||
| MD | AC C18:2 | 3.44 × 10−5 | 1.14 × 10−3 | 2.95 | |||||
| HD | lysoPC a C18:1 | 2.04 × 10−3 | 1.06 × 10−2 | 1.63 | |||||
| HD | lysoPC a C18:2 | 5.92 × 10−3 | 1.87 × 10−2 | 1.60 | |||||
| HD | PC aa C32:2 | 3.36 × 10−4 | 4.29 × 10−3 | 1.76 | |||||
| HD | PC aa C34:1 | 2.07 × 10−4 | 3.01 × 10−3 | 1.56 | |||||
| HD | PC aa C34:2 | 8.09 × 10−4 | 6.71 × 10−3 | 1.71 | |||||
| HD | PC aa C34:3 | 2.76 × 10−3 | 1.17 × 10−2 | 1.82 | |||||
| HD | PC aa C36:1 | 1.16 × 10−5 | 4.83 × 10−4 | 1.85 | |||||
| HD | PC aa C36:2 | 7.35 × 10−5 | 2.03 × 10−3 | 1.90 | |||||
| HD | PC aa C36:3 | 5.05 × 10−4 | 4.65 × 10−3 | 1.62 | |||||
| HD | PC ae C42:2 | 1.59 × 10−3 | 9.25 × 10−3 | 2.04 | |||||
| HD | PC ae C42:4 | 4.16 × 10−4 | 4.32 × 10−3 | −2.04 | |||||
| HD;MD;LD | SM C26:1 | 1.89 × 10−3 | 1.01 × 10−2 | −3.07 | −2.27 | −2.73 | |||
| EE2 | HD;MD;LD | LIP | PC aa C40:2 | 2.23 × 10−7 | 3.71 × 10−5 | −2.60 | −2.34 | −2.74 | |
| Adult | TB | HD; MD; LD | ABA | Asymmetric dimethylarginine | 6.99 × 10−5 | 5.15 × 10−3 | 1.66 | 1.95 | 1.90 |
| Pentose- phosphate | 6.10 × 10−4 | 2.69 × 10−2 | −2.43 | −2.83 | −2.62 | ||||
| Metabolite | Chemicals | Life Stage |
|---|---|---|
| PC ae C36:2 | CPF, EE2 | ELS |
| PC aa C34:1 | CPF, EE2 | ELS |
| PC aa C34:2 | CPF, EE2 | ELS |
| PC aa C36:1 | CPF, EE2 | ELS |
| PC aa C36:2 | CPF, EE2 | ELS |
| PC ae C38:6 | FLX, SeMe | Adult |
| Chemical | ELS | Adult | ||||
|---|---|---|---|---|---|---|
| Administered Concentration (ppm a egg) | Administered Concentration (ppm a) | |||||
| LD | MD | HD | LD | MD | HD | |
| EE2 | 0.54 | 6.3 | 54.2 | 0.05 | 0.5 | 5 |
| CPF | 0.56 | 4.9 | 41.1 | 0.1 | 1 | 10 |
| BaP | 0.01 | 0.05 b | 0.83 | 0.5 | 5 | 50 |
| Pb | 0.07 c (0.11) | 0.7 c (1.1) | 6.7 (10.7) | 35 | 350 | 3500 |
| SeMe | 0.0003 d (0.0007) | 0.002 d (0.005) | 0.03 d (0.07) | 0.1 | 1 | 10 |
| FLX | 0.39 | 4.6 | 32.7 | 1 | 10 | 100 |
| TB | 0.04 | 0.43 | 4.4 | 0.1 | 1 | 10 |
| HBCD | 0.02γ | 0.73γ | 10.5γ | 10 | 100 | 1000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Legrand, E.; Basu, N.; Hecker, M.; Crump, D.; Xia, J.; Chandramouli, B.; Butler, H.; Head, J.A. Targeted Metabolomics to Assess Exposure to Environmental Chemicals of Concern in Japanese Quail at Two Life Stages. Metabolites 2021, 11, 850. https://doi.org/10.3390/metabo11120850
Legrand E, Basu N, Hecker M, Crump D, Xia J, Chandramouli B, Butler H, Head JA. Targeted Metabolomics to Assess Exposure to Environmental Chemicals of Concern in Japanese Quail at Two Life Stages. Metabolites. 2021; 11(12):850. https://doi.org/10.3390/metabo11120850
Chicago/Turabian StyleLegrand, Elena, Niladri Basu, Markus Hecker, Doug Crump, Jianguo Xia, Bharat Chandramouli, Heather Butler, and Jessica A. Head. 2021. "Targeted Metabolomics to Assess Exposure to Environmental Chemicals of Concern in Japanese Quail at Two Life Stages" Metabolites 11, no. 12: 850. https://doi.org/10.3390/metabo11120850
APA StyleLegrand, E., Basu, N., Hecker, M., Crump, D., Xia, J., Chandramouli, B., Butler, H., & Head, J. A. (2021). Targeted Metabolomics to Assess Exposure to Environmental Chemicals of Concern in Japanese Quail at Two Life Stages. Metabolites, 11(12), 850. https://doi.org/10.3390/metabo11120850









